Morphological and Molecular Characterization of Arbuscular Mycorrhizal Fungi from the Rhizosphere of Date Palm (Phoenix dactylifera L.) in the Oasis of Figuig, Morocco
Abstract
1. Introduction
2. Materials and Methods
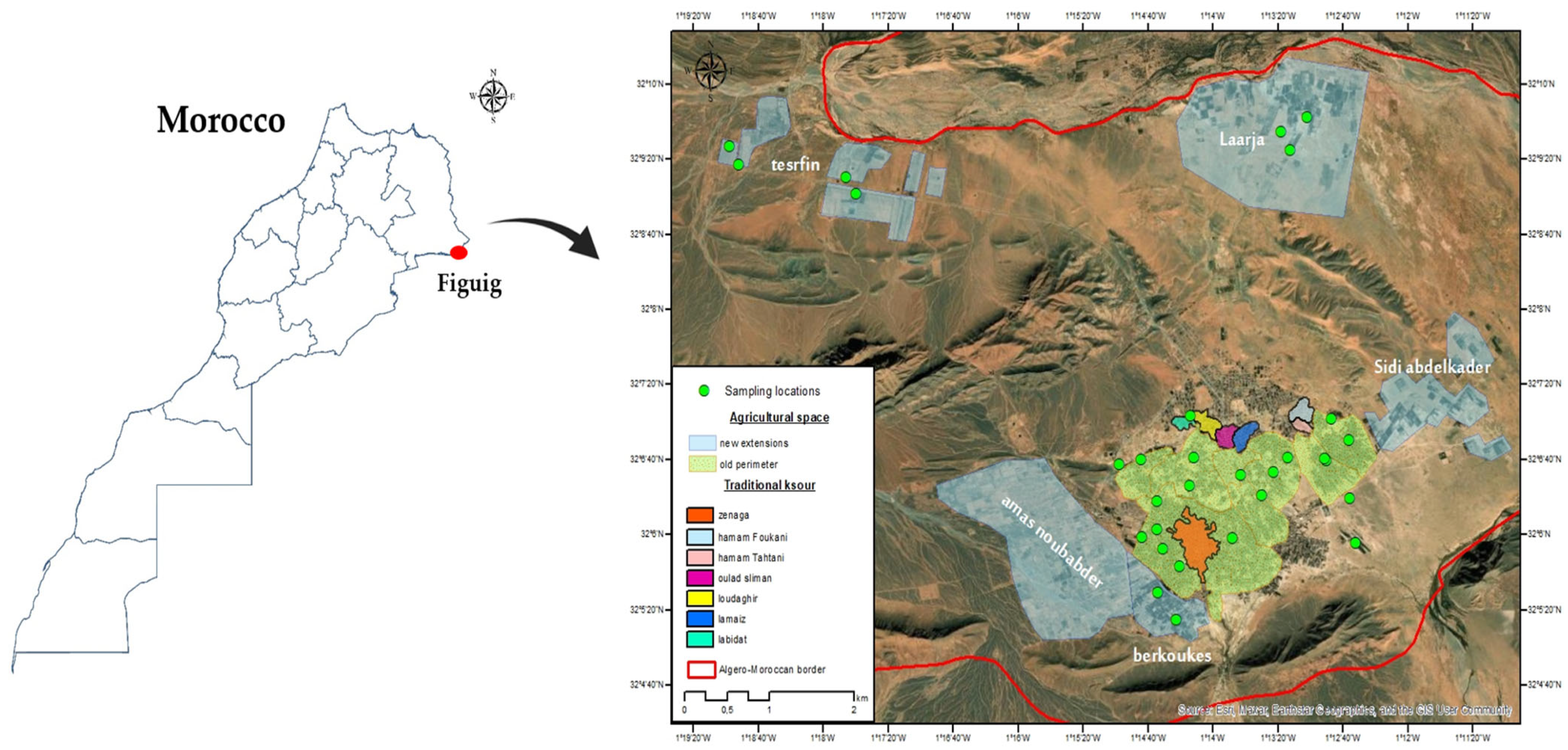
2.1. Soil Sampling
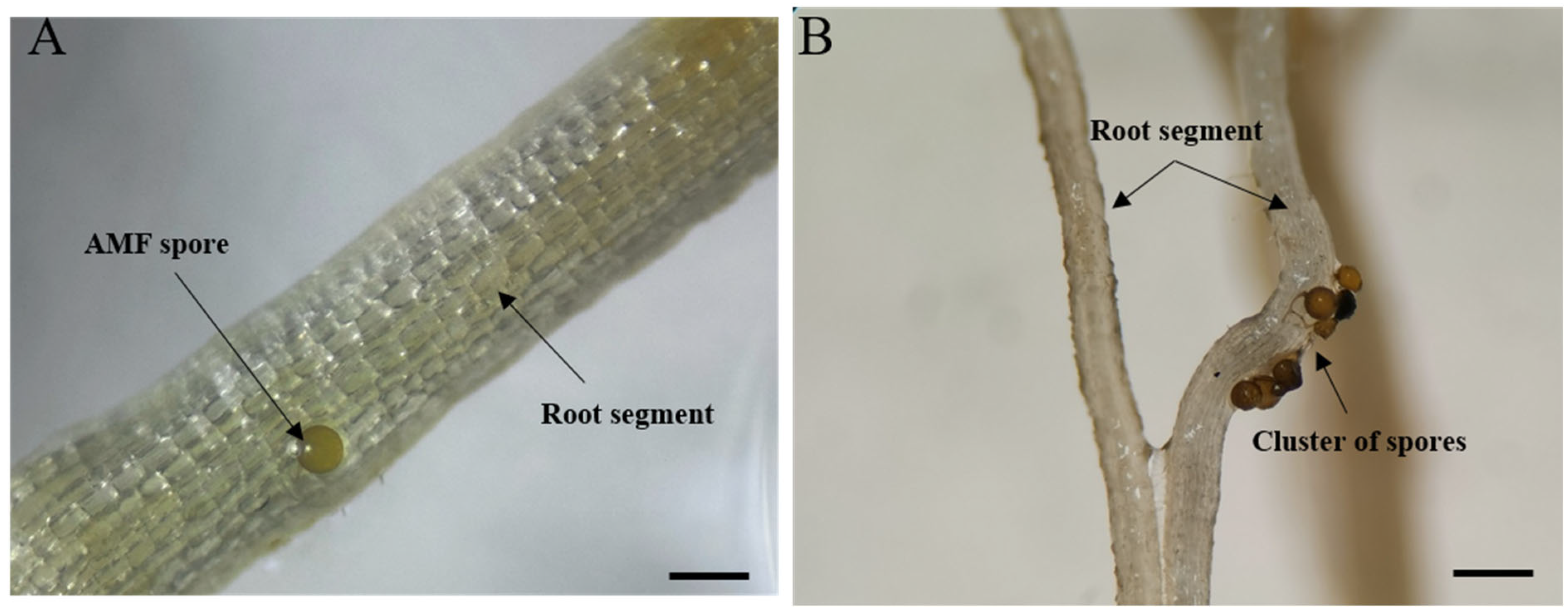
2.2. Establishment of AMF Trap Cultures
2.3. Establishment of AMF Monosporal Cultures
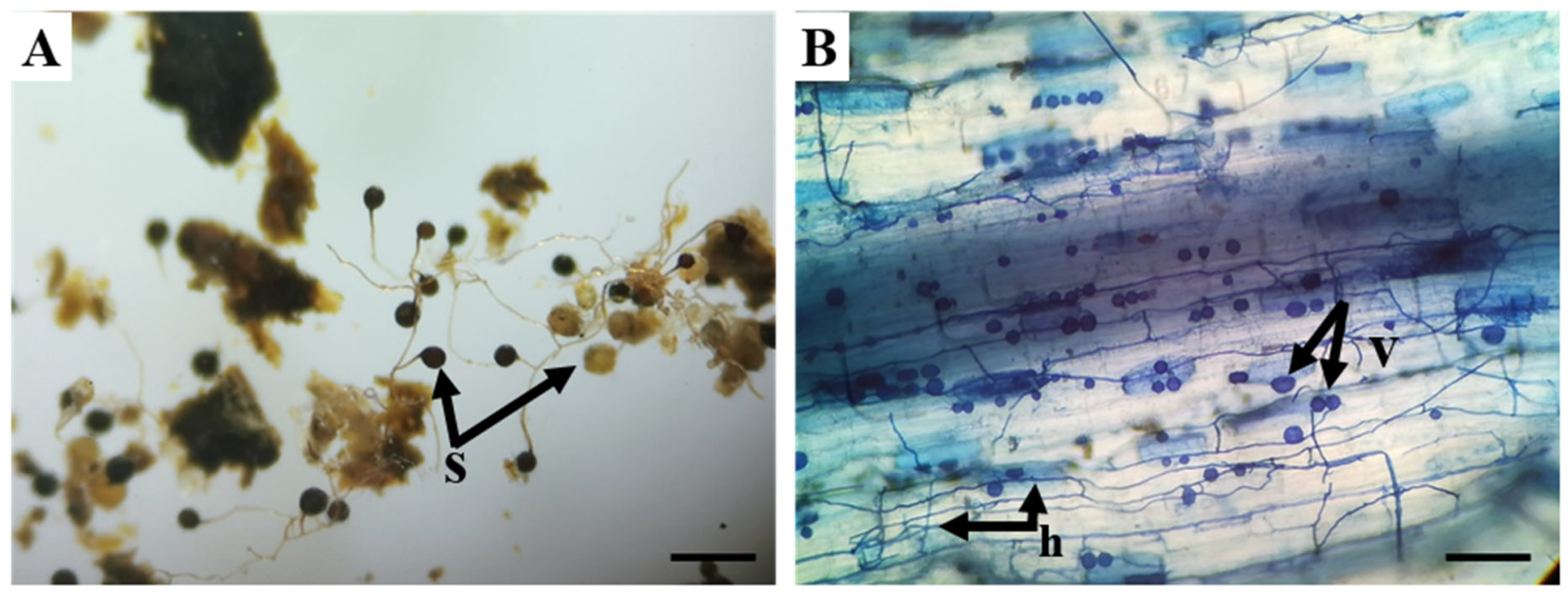
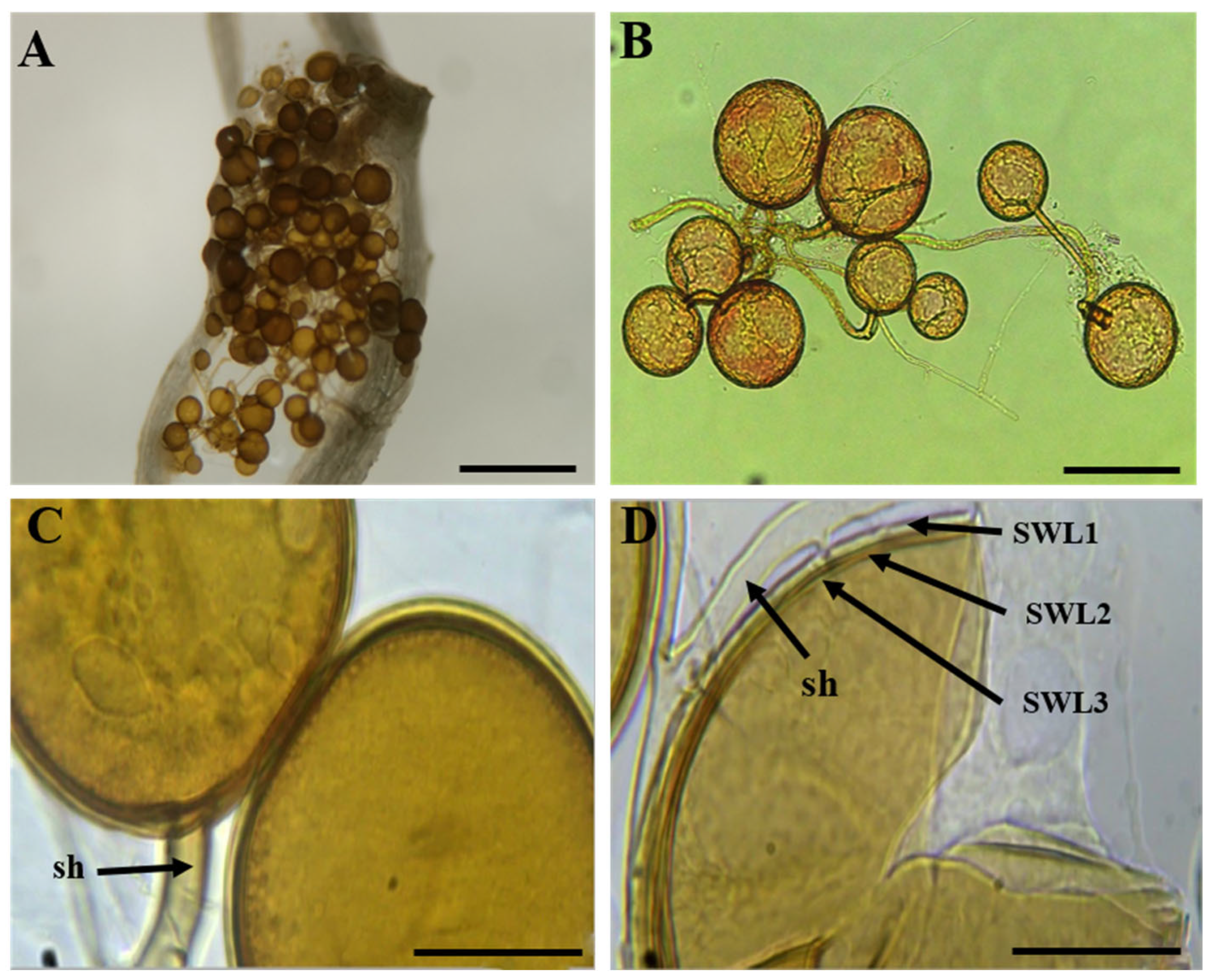
2.4. Morphological Analyses of AMF
2.5. Molecular Analyses and Phylogeny
3. Results
3.1. Establishment of the AMF Culture Collection
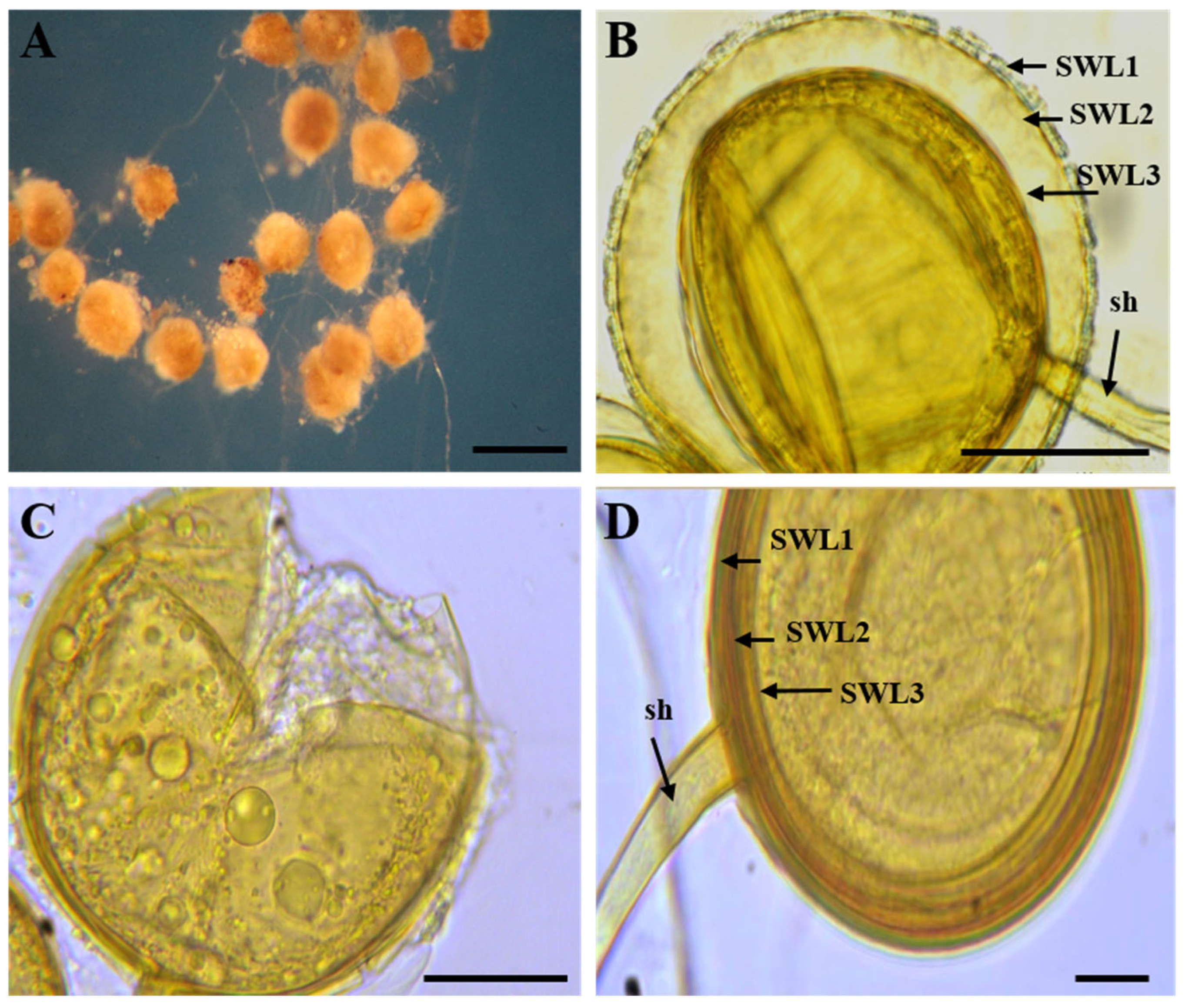
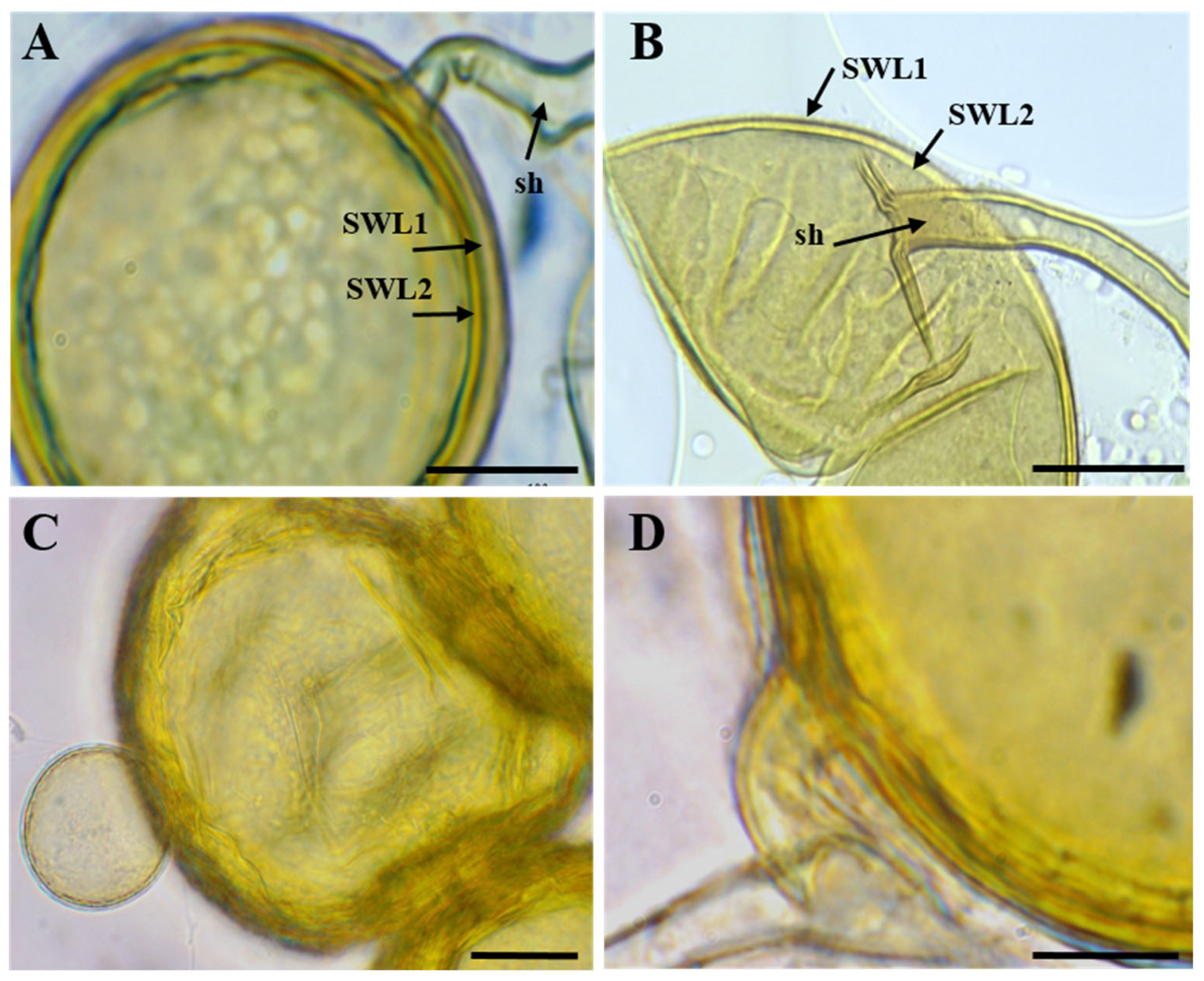
3.2. Morphological Identification of AMF Species from Single-Spore Cultures
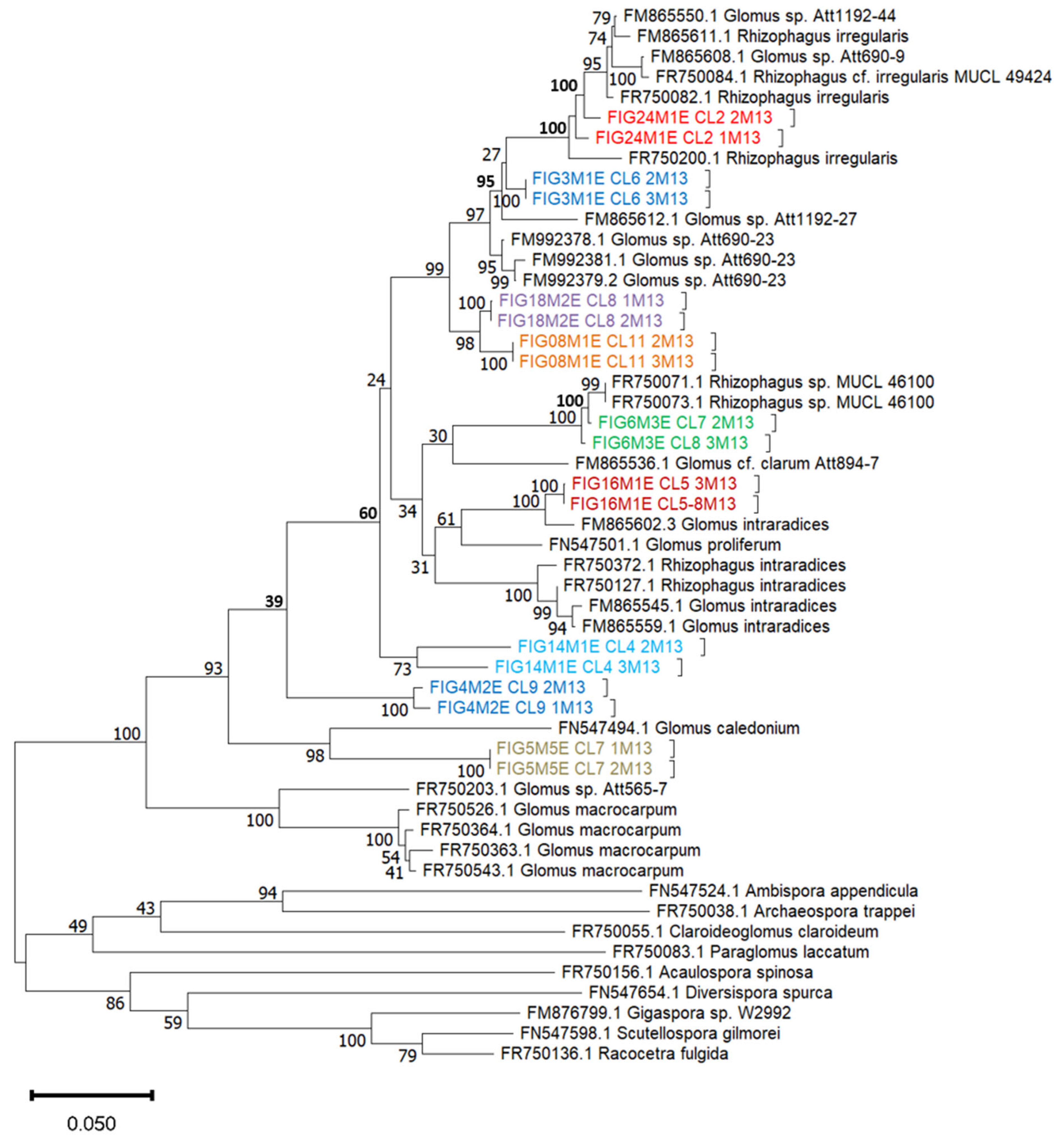
3.3. Molecular Characterization and Phylogenetic Analysis of AMF Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brundrett, M.C.; Tedersoo, L. Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Magkourilou, E.; Bell, C.; Daniell, T.; Field, K. The Functionality of Arbuscular Mycorrhizal Networks across Scales of Experimental Complexity and Ecological Relevance. Funct. Ecol. 2024, 39, 1384–1399. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Mešić, A.; Pošta, A.; Tkalčec, Z.; Thines, M. Outline of Fungi and Fungus-like Taxa–2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Bever, J.D.; Schultz, P.A.; Pringle, A.; Morton, J.B. Arbuscular Mycorrhizal Fungi: More Diverse than Meets the Eye, and the Ecological Tale of Why: The High Diversity of Ecologically Distinct Species of Arbuscular Mycorrhizal Fungi within a Single Community Has Broad Implications for Plant Ecology. Bioscience 2001, 51, 923–931. [Google Scholar] [CrossRef]
- Davison, J.; Moora, M.; Öpik, M.; Adholeya, A.; Ainsaar, L.; Bâ, A.; Burla, S.; Diedhiou, A.; Hiiesalu, I.; Jairus, T. Global Assessment of Arbuscular Mycorrhizal Fungus Diversity Reveals Very Low Endemism. Science 2015, 349, 970–973. [Google Scholar] [CrossRef]
- de Pontes, J.S.; Oehl, F.; Pereira, C.D.; de Toledo Machado, C.T.; Coyne, D.; da Silva, D.K.A.; Maia, L.C. Heterogeneity in Arbuscular Mycorrhizal Fungi and Plant Communities of the Brazilian Cerrado, Transitional Areas toward the Caatinga, and the Atlantic Forest. Microb. Ecol. 2024, 87, 29. [Google Scholar] [CrossRef]
- Větrovský, T.; Kolaříková, Z.; Lepinay, C.; Awokunle Holla, S.; Davison, J.; Fleyberková, A.; Gromyko, A.; Jelínková, B.; Kolařík, M.; Krüger, M. GlobalAMFungi: A Global Database of Arbuscular Mycorrhizal Fungal Occurrences from High-throughput Sequencing Metabarcoding Studies. New Phytol. 2023, 240, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, X.; Wang, J.; Song, J.; Zhu, Z.; Zhao, J.; Liu, M.; Wang, Z. Host Plants Directly Determine the α Diversity of Rhizosphere Arbuscular Mycorrhizal Fungal Communities in the National Tropical Fruit Tree Field Genebank. Chem. Biol. Technol. Agric. 2024, 11, 20. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Rajput, V.; Biswal, B.; Basak, N.; Kumar, R. Impact of Seasonal Dynamics and Agronomic Practices on Soil Health Indicators: Arbuscular Mycorrhizal Fungi, Glomalin-Related Soil Protein, and Ergosterol. Eurasian Soil Sci. 2025, 58, 80. [Google Scholar] [CrossRef]
- Jansa, J.; Wiemken, A.; Frossard, E. The Effects of Agricultural Practices on Arbuscular Mycorrhizal Fungi. Geol. Soc. Lond. Spec. Publ. 2006, 266, 89–115. [Google Scholar] [CrossRef]
- Manoharan, L.; Rosenstock, N.P.; Williams, A.; Hedlund, K. Agricultural Management Practices Influence AMF Diversity and Community Composition with Cascading Effects on Plant Productivity. Appl. Soil Ecol. 2017, 115, 53–59. [Google Scholar] [CrossRef]
- Berza, B. The Bio-Irrigation Concept: The Plant Nutrient and Water Transferring Roles of the Common Mycorrhizal Networks in Arid and Semiarid Agriculture. In Management of Mycorrhizal Symbiosis for Mycoremediation and Phytostabilization; Elsevier: Amsterdam, The Netherlands, 2025; pp. 199–217. [Google Scholar]
- Li, X.; Wu, Y.; Huang, C.; Rahman, M.A.; Argaman, E.; Xiao, Y. Inoculation with Arbuscular Mycorrhizal Fungi in the Field Promotes Plant Colonization Rate and Yield. Eur. J. Agron. 2025, 164, 127503. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil Type and Land Use Intensity Determine the Composition of Arbuscular Mycorrhizal Fungal Communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- FAO. Les Ksour de Figuig: Cultures Osiennes et Pastorales Autour de la Gestion Sociale de Eau et des Terres: Dossier D’Inscription Du Site Figuig Comme Système Ingénieux Du Patrimoine Agricole Mondial (SIPAM); FAO: Rome, Italy, 2021. [Google Scholar]
- Houssni, M.; Kassout, J.; El Mahroussi, M.; Chakkour, S.; Kadiri, M.; Ater, M.; Petrisor, A.-I. Evaluation and Structuring of Agrodiversity in Oases Agroecosystems of Southern Morocco. Agriculture 2023, 13, 1413. [Google Scholar] [CrossRef]
- Gagou, E.; Chakroune, K.; Abbas, M.; Lamkami, T.; Hakkou, A. Evaluation of the Mycorrhizal Potential of Date Palm (Phoenix dactylifera L.) Rhizosphere Soils in the Figuig Oasis (Southeastern Morocco). J. Fungi 2023, 9, 931. [Google Scholar] [CrossRef]
- Al-Karmadi, A.; Okoh, A.I. An Overview of Date (Phoenix Dactylifera) Fruits as an Important Global Food Resource. Foods 2024, 13, 1024. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahib, W.; Marshall, R.J. The Fruit of the Date Palm: Its Possible Use as the Best Food for the Future? Int. J. Food Sci. Nutr. 2003, 54, 247–259. [Google Scholar] [CrossRef]
- Soomro, A.H.; Marri, A.; Shaikh, N. Date Palm (Phoenix dactylifera): A Review of Economic Potential, Industrial Valorization, Nutritional and Health Significance. In Neglected Plant Foods of South Asia: Exploring and Valorizing Nature to Feed Hunger; Springer: Cham, Switzerland, 2023; pp. 319–350. [Google Scholar]
- Kalukuta Mahina, L.; Gagou, E.; Chakroune, K.; Hakkou, A.; El Jaziri, M.; Lamkami, T.; Van Pottelsberghe de la Potterie, B. Turning Waste into Wealth: The Case of Date Palm Composting. Sustainability 2025, 17, 7980. [Google Scholar] [CrossRef]
- El-Juhany, L.I. Degradation of Date Palm Trees and Date Production in Arab Countries: Causes and Potential Rehabilitation. Aust. J. Basic Appl. Sci. 2010, 4, 3998–4010. [Google Scholar]
- Zaid, A. Date Palm Cultivation; Food & Agriculture Organization of the United Nations: Rome, Italy, 2002; ISBN 92-5-138514-9. [Google Scholar]
- Ben Zineb, A.; Lamine, M.; Khallef, A.; Hamdi, H.; Ahmed, T.; Al-Jabri, H.; Alsafran, M.; Mliki, A.; Sayadi, S.; Gargouri, M. Harnessing Rhizospheric Core Microbiomes from Arid Regions for Enhancing Date Palm Resilience to Climate Change Effects. Front. Microbiol. 2024, 15, 1362722. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, B.; Morte, A.; Pérez-Gilabert, M.; Honrubia, M. Autofluorescence Detection of Arbuscular Mycorrhizal Fungal Structures in Palm Roots: An Underestimated Experimental Method. Mycol. Res. 2006, 110, 887–897. [Google Scholar] [CrossRef]
- Gagou, E.; Bouchentouf, H.; Chakroune, K.; Abbas, M.; Lamkami, T.; El Jaziri, M.; Hakkou, A. Indigenous Arbuscular Mycorrhizal Fungi Consortium Enhances Growth and Protects Boufeggous Gharas Date Palm Against Fusarium Oxysporum f. Sp. Albedinis Infection in Figuig Oasis (Morocco). Int. J. Plant Biol. 2025, 16, 20. [Google Scholar] [CrossRef]
- Sabet, Y. Mycorrhisal Habit in the Date Palm (Phoenix dactylifera Linn.). Nature 1940, 145, 782–783. [Google Scholar] [CrossRef]
- Abdelaaziz, N.; Khardi, A.; Aboumadane, H.; Goutoutou, M.; Fatima, J. Enhancing Growth and Nutrient Uptake in Bouffegous Date Palm Variety with Seaweed Extracts and AMF/PGPR Combination in the Field. Agric. Biol. Res. 2024, 40, 1072–1077. [Google Scholar]
- Al-Whaibi, M.H.; Khaliel, A.S. The Effect of Mg on Ca, K and P Content of Date Palm Seedlings under Mycorrhizal and Non-Mycorrhizai Conditions. Mycoscience 1994, 35, 213–217. [Google Scholar] [CrossRef]
- Jaiti, F.; Kassami, M.; Meddich, A.; El Hadrami, I. Effect of Arbuscular Mycorrhization on the Accumulation of Hydroxycinnamic Acid Derivatives in Date Palm Seedlings Challenged with Fusarium Oxysporum f. Sp. Albedinis. J. Phytopathol. 2008, 156, 641–646. [Google Scholar] [CrossRef]
- Jaiti, F.; Meddich, A.; El Hadrami, I. Effectiveness of Arbuscular Mycorrhizal Fungi in the Protection of Date Palm (Phoenix dactylifera L.) against Bayoud Disease. Physiol. Mol. Plant Pathol. 2007, 71, 166–173. [Google Scholar] [CrossRef]
- Meddich, A.; Ait El Mokhtar, M.; Bourzik, W.; Mitsui, T.; Baslam, M.; Hafidi, M. Optimizing Growth and Tolerance of Date Palm (Phoenix dactylifera L.) to Drought, Salinity, and Vascular Fusarium-Induced Wilt (Fusarium oxysporum) by Application of Arbuscular Mycorrhizal Fungi (AMF). In Root Biology; Springer: Cham, Switzerland, 2018; pp. 239–258. [Google Scholar]
- Nzanza, B.; Marais, D.; Soundy, P. Effect of Arbuscular Mycorrhizal Fungal Inoculation and Biochar Amendment on Growth and Yield of Tomato. Int. J. Agric. Biol 2012, 14, 965–969. [Google Scholar]
- Ren, A.-T.; Zhu, Y.; Chen, Y.-L.; Ren, H.-X.; Li, J.-Y.; Abbott, L.K.; Xiong, Y.-C. Arbuscular Mycorrhizal Fungus Alters Root-Sourced Signal (Abscisic Acid) for Better Drought Acclimation in Zea mays L. Seedlings. Environ. Exp. Bot. 2019, 167, 103824. [Google Scholar] [CrossRef]
- Ren, L.; Wang, B.; Yue, C.; Zhou, S.; Zhang, S.; Huo, H.; Xu, G. Mechanism of Application Nursery Cultivation Arbuscular Mycorrhizal Seedling in Watermelon in the Field. Ann. Appl. Biol. 2019, 174, 51–60. [Google Scholar] [CrossRef]
- Antunes, P.M.; Koch, A.M.; Morton, J.B.; Rillig, M.C.; Klironomos, J.N. Evidence for Functional Divergence in Arbuscular Mycorrhizal Fungi from Contrasting Climatic Origins. New Phytol. 2011, 189, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Lekberg, Y.; Koide, R.; Twomlow, S. Effect of Agricultural Management Practices on Arbuscular Mycorrhizal Fungal Abundance in Low-Input Cropping Systems of Southern Africa: A Case Study from Zimbabwe. Biol. Fertil. Soils 2008, 44, 917–923. [Google Scholar] [CrossRef]
- Marulanda-Aguirre, A.; Azcón, R.; Ruiz-Lozano, J.M.; Aroca, R. Differential Effects of a Bacillus megaterium Strain on Lactuca sativa Plant Growth Depending on the Origin of the Arbuscular Mycorrhizal Fungus Coinoculated: Physiologic and Biochemical Traits. J. Plant Growth Regul. 2008, 27, 10–18. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, M.; Wang, Z.; Li, J.; Liu, K.; Huang, D. The Role of Arbuscular Mycorrhizal Symbiosis in Plant Abiotic Stress. Front. Microbiol. 2024, 14, 1323881. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Xiao, D.; Chen, M.; He, X. Higher Colonization but Lower Diversity of Root-Associated Arbuscular Mycorrhizal Fungi in the Topsoil than in Deep Soil. Appl. Soil Ecol. 2024, 194, 105195. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Anandham, R.; Senthilkumar, M.; Sa, T. Diversity and Community Structure of Arbuscular Mycorrhizal Fungi in the Rhizosphere of Salt-Affected Soils. In Rhizosphere Microbes: Soil and Plant Functions; Springer: Singapore, 2020; pp. 453–470. [Google Scholar]
- Krishnamoorthy, R.; Premalatha, N.; Karthik, M.; Anandham, R.; Senthilkumar, M.; Gopal, N.; Selvakumar, G.; Sa, T. Molecular Markers for the Identification and Diversity Analysis of Arbuscular Mycorrhizal Fungi (AMF). In Molecular Markers in Mycology: Diagnostics and Marker Developments; Springer: Cham, Switzerland, 2017; pp. 177–199. [Google Scholar]
- Öpik, M.; Moora, M.; Liira, J.; Zobel, M. Composition of Root-colonizing Arbuscular Mycorrhizal Fungal Communities in Different Ecosystems around the Globe. J. Ecol. 2006, 94, 778–790. [Google Scholar] [CrossRef]
- Sharmah, D.; Jha, D.; Pandey, R. Molecular Approaches in Arbuscular Mycorrhizal Research: A Review. J. Phytol. 2010, 2, 75–90. [Google Scholar]
- Noceto, P.-A.; Durney, C.; Van Tuinen, D.; de Sousa, J.; Wipf, D.; Courty, P.-E. Arbuscular Mycorrhizal Fungal Communities Differ in Neighboring Vineyards of Different Ages. Mycorrhiza 2023, 33, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Noceto, P.-A.; Bettenfeld, P.; Boussageon, R.; Hériché, M.; Sportes, A.; van Tuinen, D.; Courty, P.-E.; Wipf, D. Arbuscular Mycorrhizal Fungi, a Key Symbiosis in the Development of Quality Traits in Crop Production, Alone or Combined with Plant Growth-Promoting Bacteria. Mycorrhiza 2021, 31, 655–669. [Google Scholar] [CrossRef]
- Touré, G.; Nandjui, J.; Koné, A.; Kouadjo, A.; Ebou, A.; Tiho, S.; Zézé, A. Diversité Des Champignons Mycorhiziens à Arbuscules et Interactions Avec le Système Sol-Litière Dans un Écotone Forêt-Savane, Côte d’Ivoire. Côte D’ivoire-Etude Gest. Sols 2021, 28, 93–104. [Google Scholar]
- Rivaton, D. Soil Arbuscular Mycorrhizal Fungi Assessment in Organic Stockless Farming Systems: Focus on Phosphorus Nutrition; Newcastle University: Newcastle upon Tyne, UK, 2016. [Google Scholar]
- Krüger, M.; Krüger, C.; Walker, C.; Stockinger, H.; Schüßler, A. Phylogenetic Reference Data for Systematics and Phylotaxonomy of Arbuscular Mycorrhizal Fungi from Phylum to Species Level. New Phytol. 2012, 193, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Stockinger, H.; Krüger, C.; Schüßler, A. DNA-based Species Level Detection of Glomeromycota: One PCR Primer Set for All Arbuscular Mycorrhizal Fungi. New Phytol. 2009, 183, 212–223. [Google Scholar] [CrossRef]
- Stockinger, H.; Krüger, M.; Schüßler, A. DNA Barcoding of Arbuscular Mycorrhizal Fungi. New Phytol. 2010, 187, 461–474. [Google Scholar] [CrossRef]
- Al-Yahya’ei, M.N.; Oehl, F.; Vallino, M.; Lumini, E.; Redecker, D.; Wiemken, A.; Bonfante, P. Unique Arbuscular Mycorrhizal Fungal Communities Uncovered in Date Palm Plantations and Surrounding Desert Habitats of Southern Arabia. Mycorrhiza 2011, 21, 195–209. [Google Scholar] [CrossRef]
- Symanczik, S.; Błaszkowski, J.; Chwat, G.; Boller, T.; Wiemken, A.; Al-Yahya’ei, M.N. Three New Species of Arbuscular Mycorrhizal Fungi Discovered at One Location in a Desert of Oman: Diversispora omaniana, Septoglomus nakheelum and Rhizophagus arabicus. Mycologia 2014, 106, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Symanczik, S.; Błaszkowski, J.; Koegel, S.; Boller, T.; Wiemken, A.; Al-Yahya’Ei, M.N. Isolation and Identification of Desert Habituated Arbuscular Mycorrhizal Fungi Newly Reported from the Arabian Peninsula. J. Arid Land 2014, 6, 488–497. [Google Scholar] [CrossRef]
- Chebaane, A.; Symanczik, S.; Oehl, F.; Azri, R.; Gargouri, M.; Mäder, P.; Mliki, A.; Fki, L. Arbuscular Mycorrhizal Fungi Associated with Phoenix dactylifera L. Grown in Tunisian Sahara Oases of Different Salinity Levels. Symbiosis 2020, 81, 173–186. [Google Scholar] [CrossRef]
- El Hilali, R.; Symanczik, S.; Oehl, F.; Ouahmane, L.; Bouamri, R. Cultivation, Identification, and Application of Arbuscular Mycorrhizal Fungi Associated with Date Palm Plants in Drâa-Tafilalet Oasis. Rhizosphere 2022, 22, 100521. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Cruz, C.; Mahdhi, M.; Mars, M.; Caeiro, M.F. Arbuscular Mycorrhizal Fungi in Soil, Roots and Rhizosphere of Medicago Truncatula: Diversity and Heterogeneity under Semi-Arid Conditions. PeerJ 2019, 7, e6401. [Google Scholar] [CrossRef]
- Walker, C. Methods for Culturing and Isolating Arbuscular Mycorrhizal Fungi. Mycorrhiza News 1999, 11, 2–4. [Google Scholar]
- Cotuna, O.; Sărăţeanu, V.; Durău, C.C. Influence of Arbuscular Mycorrhizae (AM) Colonization on Plant Growth: Plantago lanceolata L. Case Study. J. Food Agric. Environ. 2013, 11, 2005–2008. [Google Scholar]
- De Deyn, G.B.; Biere, A.; Van der Putten, W.; Wagenaar, R.; Klironomos, J. Chemical Defense, Mycorrhizal Colonization and Growth Responses in Plantago Lanceolata L. Oecologia 2009, 160, 433–442. Oecologia 2009, 160, 433–442. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, X.; Li, S. Effects of Arbuscular Mycorrhizal Fungi on Rice Growth under Different Flooding and Shading Regimes. Front. Microbiol. 2021, 12, 756752. [Google Scholar] [CrossRef]
- Fontana, A.; Reichelt, M.; Hempel, S.; Gershenzon, J.; Unsicker, S.B. The Effects of Arbuscular Mycorrhizal Fungi on Direct and Indirect Defense Metabolites of Plantago lanceolata L. J. Chem. Ecol. 2009, 35, 833–843. [Google Scholar] [CrossRef]
- Romero, F.; Argüello, A.; de Bruin, S.; van der Heijden, M.G. The Plant–Mycorrhizal Fungi Collaboration Gradient Depends on Plant Functional Group. Funct. Ecol. 2023, 37, 2386–2398. [Google Scholar] [CrossRef]
- Walker, C.; Vestberg, M. A Simple and Inexpensive Method for Producing and Maintaining Closed Pot Cultures of Arbuscular Mycorrhizal Fungi. Agric. Food Sci. 1994, 3, 233–240. [Google Scholar] [CrossRef]
- Daniels, B.; Skipper, H. Methods for the Recovery and Quantitative Estimation of Propagules from Soil; American Phytopathological Society: St. Paul, MN, USA, 1982. [Google Scholar]
- Sieverding, E.; Friedrichsen, J.; Suden, W. Vesicular-Arbuscular Mycorrhiza Management in Tropical Agrosystems; Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ): Eschborn, Germany, 1991; ISBN 3-88085-462-9. [Google Scholar]
- Tchabi, A.; Coyne, D.; Hountondji, F.; Lawouin, L.; Wiemken, A.; Oehl, F. Efficacy of Indigenous Arbuscular Mycorrhizal Fungi for Promoting White Yam (Dioscorea rotundata) Growth in West Africa. Appl. Soil Ecol. 2010, 45, 92–100. [Google Scholar] [CrossRef]
- Koskey, R. A Convenient, Permanent Slide Mounting Medium. Newsl. Mycol. Soc. Amer. 1983, 34, 59. [Google Scholar]
- Brundrett, M.C.; Abbott, L. Mycorrhizal Fungus Propagules in the Jarrah Forest: I. Seasonal Study of Inoculum Levels. New Phytol. 1994, 127, 539–546. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Kovács, G.M.; Gáspár, B.K.; Balázs, T.K.; Buscot, F.; Ryszka, P. The Arbuscular Mycorrhizal Paraglomus majewskii Sp. Nov. Represents a Distinct Basal Lineage in Glomeromycota. Mycologia 2012, 104, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Schüßler, A.; Bonfante, P. The Obligate Endobacteria of Arbuscular Mycorrhizal Fungi Are Ancient Heritable Components Related to the Mollicutes. ISME J. 2010, 4, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Schüssler, A.; Walker, C. The Glomeromycota: A Species List with New Families and New Genera; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2010. [Google Scholar]
- Redecker, D.; Schüßler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An Evidence-Based Consensus for the Classification of Arbuscular Mycorrhizal Fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Palenzuela, J.; Ineichen, K.; da Silva, G.A. Advances in Glomeromycota Taxonomy and Classification. IMA Fungus 2011, 2, 191–199. [Google Scholar] [CrossRef]
- Walker, C.; Sanders, F. Taxonomic Concepts in the Endogonaceae: III. The Separation of Scutellospora Gen. Nov. from Gigaspora Gerd. & Trappe. Mycotaxon 1986, 27, 169–182. [Google Scholar] [CrossRef]
- Abbas, Y.; Ducousso, M.; Abourouh, M.; Azcón, R.; Duponnois, R. Diversity of Arbuscular Mycorrhizal Fungi in Tetraclinis Articulata (Vahl) Masters Woodlands in Morocco. Ann. For. Sci. 2006, 63, 285–291. [Google Scholar] [CrossRef]
- Bouamri, R.; Dalpé, Y.; Serrhini, M.; Bennani, A. Arbuscular Mycorrhizal Fungi Species Associated with Rhizosphere of Phoenix dactylifera L. in Morocco. Afr. J. Biotechnol. 2006, 5, 510–516. [Google Scholar]
- Kachkouch, W.; Touhami, A.O.; Filali-Maltouf, A.; El Modafar, C.; Moukhli, A.; Oukabli, A.; Benkirane, R.; Douira, A. Arbuscular Mycorrhizal Fungi Species Associated with Rhizosphere of Olea Europaea L. in Morocco. J. Anim. Plant Sci. 2012, 15, 2275–2287. [Google Scholar]
- Lamaizi, S.; Meddich, A.; Akensous, F.-Z.; Hafidi, M. Arbuscular Mycorrhizal Fungi Application: Selected Case Studies Corroborating Sustainable Drought Mitigation and Enhanced Crop Productivity. In Sustainable Agriculture Under Drought Stress; Elsevier: Amsterdam, The Netherlands, 2025; pp. 385–399. [Google Scholar]
- Meddich, A.; Jaiti, F.; Bourzik, W.; El Asli, A.; Hafidi, M. Use of Mycorrhizal Fungi as a Strategy for Improving the Drought Tolerance in Date Palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Ouahmane, L.; Hafidi, M.; Thioulouse, J.; Ducousso, M.; Kisa, M.; Prin, Y.; Galiana, A.; Boumezzough, A.; Duponnois, R. Improvement of Cupressus Atlantica Gaussen Growth by Inoculation with Native Arbuscular Mycorrhizal Fungi. J. Appl. Microbiol. 2007, 103, 683–690. [Google Scholar] [CrossRef]
- Bencherif, K.; Boutekrabt, A.; Fontaine, J.; Laruelle, F.; Dalpe, Y.; Sahraoui, A.L.-H. Impact of Soil Salinity on Arbuscular Mycorrhizal Fungi Biodiversity and Microflora Biomass Associated with Tamarix articulata Vahll Rhizosphere in Arid and Semi-Arid Algerian Areas. Sci. Total Environ. 2015, 533, 488–494. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular Mycorrhizal Fungi in Alleviation of Salt Stress: A Review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Ouhaddou, R.; Anli, M.; Ben-Laouane, R.; Boutasknit, A.; Baslam, M.; Meddich, A. The Importance of the Glomus Genus as a Potential Candidate for Sustainable Agriculture under Arid Environments: A Review. Int. J. Plant Biol. 2025, 16, 32. [Google Scholar] [CrossRef]
- Bouamri, R.; Dalpé, Y.; Serrhini, M. Effect of Seasonal Variation on Arbuscular Mycorrhizal Fungi Associated with Date Palm. Emir. J. Food Agric. 2014, 26, 977–986. [Google Scholar] [CrossRef]
- Alguacil, M.d.M.; Torrecillas, E.; Hernandez, G.; Roldan, A. Changes in the Diversity of Soil Arbuscular Mycorrhizal Fungi after Cultivation for Biofuel Production in a Guantanamo (Cuba) Tropical System. PLoS ONE 2012, 7, e34887. [Google Scholar] [CrossRef]
- de Novais, C.B.; Pepe, A.; Siqueira, J.O.; Giovannetti, M.; Sbrana, C. Compatibility and Incompatibility in Hyphal Anastomosis of Arbuscular Mycorrhizal Fungi. Sci. Agric. 2017, 74, 411–416. [Google Scholar] [CrossRef]
- Stockinger, H.; Walker, C.; Schüßler, A. ‘Glomus intraradices DAOM197198’, a Model Fungus in Arbuscular Mycorrhiza Research, Is Not Glomus intraradices. New Phytol. 2009, 183, 1176–1187. [Google Scholar] [CrossRef]
- Redecker, D.; Schüßler, A. 9 Glomeromycota. In Systematics and Evolution: Part A; Springe: Berlin/Heidelberg, Germany, 2014; pp. 251–269. [Google Scholar]
- Beaudet, D.; Terrat, Y.; Halary, S.; de la Providencia, I.E.; Hijri, M. Mitochondrial Genome Rearrangements in Glomus Species Triggered by Homologous Recombination between Distinct mtDNA Haplotypes. Genome Biol. Evol. 2013, 5, 1628–1643. [Google Scholar] [CrossRef]
- Nadimi, M.; Daubois, L.; Hijri, M. Mitochondrial Comparative Genomics and Phylogenetic Signal Assessment of mtDNA among Arbuscular Mycorrhizal Fungi. Mol. Phylogenetics Evol. 2016, 98, 74–83. [Google Scholar] [CrossRef]
- Stockinger, H.; Peyret-Guzzon, M.; Koegel, S.; Bouffaud, M.-L.; Redecker, D. The Largest Subunit of RNA Polymerase II as a New Marker Gene to Study Assemblages of Arbuscular Mycorrhizal Fungi in the Field. PLoS ONE 2014, 9, e107783. [Google Scholar] [CrossRef]
- Voříšková, A.; Jansa, J.; Püschel, D.; Krüger, M.; Cajthaml, T.; Vosátka, M.; Janoušková, M. Real-Time PCR Quantification of Arbuscular Mycorrhizal Fungi: Does the Use of Nuclear or Mitochondrial Markers Make a Difference? Mycorrhiza 2017, 27, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Aili, Y.; Ma, X.; Wang, H.; Dawuti, M. Mycorrhizal Fungal Colonization Promotes Apparent Growth and Physiology of Alhagi sparsifolia Seedlings under Salt or Drought Stress at Vulnerable Developmental Stage. Plant Growth Regul. 2024, 102, 267–278. [Google Scholar] [CrossRef]
- Corradi, N.; Ruffner, B.; Croll, D.; Colard, A.; Horák, A.; Sanders, I.R. High-Level Molecular Diversity of Copper-Zinc Superoxide Dismutase Genes among and within Species of Arbuscular Mycorrhizal Fungi. Appl. Environ. Microbiol. 2009, 75, 1970–1978. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, W.; Zhang, Y.; Jiang, S.; Deng, Y.; Christie, P.; Murray, P.J.; Li, X.; Zhang, J. Arbuscular Mycorrhizal Fungi in Soil and Roots Respond Differently to Phosphorus Inputs in an Intensively Managed Calcareous Agricultural Soil. Sci. Rep. 2016, 6, 24902. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Davison, J.; Moora, M.; Zobel, M. DNA-Based Detection and Identification of Glomeromycota: The Virtual Taxonomy of Environmental Sequences. Botany 2014, 92, 135–147. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy; Freeman: San Francisco, CA, USA, 1973. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagou, E.; Guérin, C.; Chakroune, K.; Abbas, M.; Lamkami, T.; El Jaziri, M.; Hakkou, A. Morphological and Molecular Characterization of Arbuscular Mycorrhizal Fungi from the Rhizosphere of Date Palm (Phoenix dactylifera L.) in the Oasis of Figuig, Morocco. Diversity 2025, 17, 710. https://doi.org/10.3390/d17100710
Gagou E, Guérin C, Chakroune K, Abbas M, Lamkami T, El Jaziri M, Hakkou A. Morphological and Molecular Characterization of Arbuscular Mycorrhizal Fungi from the Rhizosphere of Date Palm (Phoenix dactylifera L.) in the Oasis of Figuig, Morocco. Diversity. 2025; 17(10):710. https://doi.org/10.3390/d17100710
Chicago/Turabian StyleGagou, Elmostafa, Claire Guérin, Khadija Chakroune, Mahmoud Abbas, Touria Lamkami, Mondher El Jaziri, and Abdelkader Hakkou. 2025. "Morphological and Molecular Characterization of Arbuscular Mycorrhizal Fungi from the Rhizosphere of Date Palm (Phoenix dactylifera L.) in the Oasis of Figuig, Morocco" Diversity 17, no. 10: 710. https://doi.org/10.3390/d17100710
APA StyleGagou, E., Guérin, C., Chakroune, K., Abbas, M., Lamkami, T., El Jaziri, M., & Hakkou, A. (2025). Morphological and Molecular Characterization of Arbuscular Mycorrhizal Fungi from the Rhizosphere of Date Palm (Phoenix dactylifera L.) in the Oasis of Figuig, Morocco. Diversity, 17(10), 710. https://doi.org/10.3390/d17100710





