Abstract
Pleurobrachia pileus and P. bachei are among the most frequently recorded ctenophores worldwide. Despite their long-standing descriptions dating back nearly 250 years, their taxonomic boundaries remain ambiguous due to the lack of definitive diagnostic features. In Mexican waters, records of both species are primarily based on geographic distribution, with limited morphological evidence supporting their separation. This study aimed to evaluate the morphological diversity of Pleurobrachia spp. along the Mexican coast through a comprehensive morphological approach. Specimens from four sites representing two marine regions (the Gulf of Mexico and the Southern Mexican Pacific coast) were examined for phenotypic variation. A standardized methodology was developed for specimen handling, observation, and digital reconstruction of body plans, enabling the extraction of 38 discrete and continuous morphological traits. Our analysis revealed two distinct and internally consistent phenotypic groups, each corresponding to one of the two regions, with notable differences in tentacular sheath structure, tentacular canals, gastrovascular cavity pigmentation pattern, and pharyngeal morphology. A clinal pattern in body size was observed, with smaller specimens in the north and larger ones in the south. Importantly, neither phenotype matched the diagnostic traits of P. pileus or P. bachei, suggesting that the Mexican populations represent separate, previously unrecognized species. These findings highlight the necessity to re-evaluate the taxonomy of Pleurobrachia in these regions and contribute valuable morphological data toward resolving longstanding questions about species boundaries within this genus.
1. Introduction
The phylum Ctenophora, commonly known as comb jellies, is a group of marine invertebrates that is well-supported by particular synapomorphies, making them a unique and evolutionarily intriguing group within the metazoan [1,2]. The combination of morphological traits displayed by this taxon, together with extensive sequence data, has provided alternative hypotheses about its phylogenetic position [3,4,5,6,7,8,9].
Although the phylum has been the focus of many systematic analyses, its internal classification is debated, and the group presents many problems in alpha taxonomy [2,10,11]. Fragility and the lack of hard structures for identification make their morphological traits difficult to preserve and study [12,13]. Some species disintegrate upon collection, meaning their true morphology is only visible in live observations (e.g., using remotely operated vehicles (ROVs) or deep-sea cameras). Also, standard formalin or ethanol preservation alters their delicate structures, making morphological comparisons unreliable [14,15]. Many species display wide phenotypic variation (size, color, and body structure) due to both environmental (diet or environmental stress) and ontogenetic (body shape based on age) factors, leading to misidentifications [12,16,17,18]. Over time, several authors have described and compared Ctenophora species based on a few specimens that are no longer available for their review in the collections where they were stored [10,17,19,20,21,22,23,24], making comparisons difficult. Some taxa were named multiple times due to poor descriptions or lack of comparative material, leading to synonymy issues within the phylum [2,13]. Current molecular studies (DNA barcoding and genomics) suggest that many Ctenophora species harbor cryptic diversity [25,26,27].
The species delimitation of Ctenophora requires establishing a methodology to obtain replicable and comparable diagnostic traits, defined under standardized conditions, and considering geographical variation. Moreover, the integration of additional biological and molecular data is crucial for refining species boundaries and supporting new species descriptions. These efforts will help to improve the identification of ctenophores worldwide, even by non-specialists, despite the challenges posed by specimen damage and degradation during collection and preservation [12,13,28,29].
In this context, Pleurobrachia (class Tentaculata, order Cydippida, family Pleurobrachiidae) is known to be the most studied comb jelly genus [6,9,30,31]. It was the second added in Ctenophora with the largest number of geographical records worldwide [12,32]. Members of this taxon were first reported as polar organisms [16,33,34] and were characterized by at least five diagnostic attributes: 1. spherical or oval body shapes; 2. eight symmetrical comb rows; 3. two retractile, branched (tentilla), and long tentacles; 4. an apical sensory organ in the aboral region, and 5. a gastrovascular cavity (GVC) elongated towards the oral region [2,17,19,23]. Under this definition, eight species have been compiled and recognized [11,13]: Pleurobrachia pileus Müller (1776), recorded in the northern and southern Atlantic Ocean and Arctic Ocean; P. bachei Agassiz (1860), from the northern Pacific Ocean; P. rhodopis Chun (1879), from the Mediterranean Sea and adjacent seas; P. pigmentata Moser (1903), from the eastern Pacific Ocean; P. cyanea Chun (1889), from the eastern Atlantic Ocean; P. globosa Moser (1903) and P. pigmentata Moser (1903), from the Dutch East Indies marine region; P. striata Moser (1908), from the eastern tropical Pacific Ocean, and P. brunnea Mayer (1912), from the western North Atlantic and eastern Pacific Oceans.
In the past 25 years, many new geographical records based on other biological studies of this genus have been made outside their original distribution, supporting P. pileus and P. bachei as the most commonly recorded species around the world [12,13,30,34,35,36,37,38,39,40,41,42,43,44,45,46]. The distribution of P. pileus is estimated to be extremely wide primarily in the Atlantic and Indian Oceans, ranging from polar regions [10,47,48], cold regions [34,49,50,51], temperate regions [52,53], to both subtropical [46,54] and tropical regions [55,56]. Conversely, P. bachei is recognized as the only species of the genus found in the Pacific Ocean in cold and temperate regions from Canada to California [31,57,58,59].
Although these two species were described almost 250 years ago, their boundaries are unclear, making their recognition difficult. The first description of P. pileus [33] only considered the spherical body shape, the symmetrical presence of the comb rows, and the position of the tentacles, as well as the geographic distribution (polar region of the Atlantic Ocean) as diagnostic attributes, while the description of P. bachei [19] was supported by the general body shape, which is ovoid, a size not larger than 2 cm long, the exit of the tentacular canals further away from the aboral pole, and a shorter gastrovascular cavity compared to P. rhodopis and P. globosa. The validity of these two species remains doubtful due to the lack of clear diagnostic features for their recognition. Since their original descriptions, the most heavily weighted criterion for taxonomic assignment has been geographic distribution. However, morphological attributes have proven to be ambiguous and often lead to questionable identifications, especially when based on poorly preserved specimens, photographs from social media, diver-generated pictures, or individuals of intermediate size in relation to the previously described species [20].
In Mexican coasts, geographical records of ctenophores suggest the presence of at least two species, probably allopatric. In this sense, P. pileus has been recorded in the coastal lagoons of La Mancha, Mandinga, and Ostión in Veracruz into the Gulf of Mexico [60], while P. bachei records are from coasts of Sonora, Sinaloa, Nayarit, and Jalisco in the Pacific Ocean [61,62,63,64]. The identification and distribution of P. pileus and P. bachei in Mexico seems to be related to the Atlantic and Pacific marine regions, respectively, but due to the problem of identifying them, there are many more records only at the genus level [65,66,67,68,69,70,71]. Although the species P. pileus has been recognized with the 18S gene in the northern region of the GoM, Pleurobrachia sp. was also identified in the same area, which could mean that more than one species occurs in this region [6]. Also, there is a record in the Mexican Pacific coast assigned to the species level, using a nomenclatural combination not recognized within the taxonomy of the phylum, which is P. pileus var. bachei, without morphological or molecular evidence to support it [72]. The most recent checklist of Mexican ctenophores adopted a geographical criterion to species assignment, which suggests that all records of Pleurobrachia from the Pacific Ocean and Gulf of California should be considered as P. bachei, while those records from the Gulf of Mexico and Caribbean should be considered as P. pileus [73], all of this without providing morphological evidence of the features shared for these species in the descriptions and taxonomic keys.
In this study, our objective was to evaluate the morphological diversity of Pleurobrachia spp. distributed along the Mexican coast based on a morphological approach. To achieve this, we developed a standardized method for specimen manipulation, observation, and digital reconstruction of different body plans to obtain comparable morphological traits among a comparable number of specimens from various locations. We examined phenotypic variation in specimens from four sites representing two marine regions (Gulf of Mexico and Southern Mexican Pacific coast). Therefore, we proposed a morphological character matrix comprising 38 discrete and continuous attributes to analyze phenotypic variation within and among populations to assess whether this variation supports the existence of at least two distinct morphological species, as previously suggested. In addition, we tested whether the morphological variation observed in Mexican specimens corresponds to either P. pileus or P. bachei.
2. Materials and Methods
A total of 120 specimens of Pleurobrachia spp. from four localities along the Pacific and Gulf of Mexico coasts were examined. Of the total sample, 90 were from the Gulf of Mexico (GoM) and 30 from the Southern Mexican Pacific coast (POcM). Sample sites represented the distribution of the genus in the GoM and a reference at the Pacific coast. The Tamiahua estuary (THVZ) was a sandy beach area with a depth of no more than 1 m, fishing took place in an area of approximately 150 m2 during the morning, and the surface water temperature was 33 °C, while the salinity was 31. Also, several fish larvae, jellyfish, and drifting algae were observed. In the Tampamachoco lagoon (TCVZ), fishing was carried out on a rocky and muddy beach at a depth of less than 1.3 m, many oyster shells were on the bottom, the fishing area was approximately 120 m2 and near the junction with the Tuxpan River, and this sampling was carried out in the afternoon with a surface water temperature of 28 °C and salinity of 29. Some gelatinous competitors were observed, although there were fewer fish or other zooplankton members. In Balzapote bay (BZVZ), fishing was carried out in a sandy and rocky bay, near a dock, in an area of approximately 200 m2, and at a depth of less than 1.5 m during the morning. The surface water temperature was 33 °C, while the salinity was 35; the water was clear, with some fish, eggs, mollusk larvae, sciphozoans, and salps. Finally, in Madero port (MTCH), fishing was carried out on a sandy tourist beach near an abandoned dock with a depth of less than 1.2 m and in an area of around 200 m2, where the water was turbid with the presence of scifomedusae and hydromedusae. There were some fish and annelids; the surface water temperature was 32 °C, while the salinity was 34 (Table 1). Specimens were collected with a fish hand net (1 m long, 50 cm wide, 500 µm mesh size) near the intertidal zone as described above.

Table 1.
Regions, localities, dates, sample size, and keys of collected Pleurobrachia specimens.
The taxonomic identification to the genus level of field-collected specimens was carried out using available descriptions, whereby all specimens collected were spherical, with eight rows of combs arranged symmetrically around the body, a statocyst in the aboral region, an elongated gastrovascular cavity (GVC) in the oral region, and two branched tentacles [10,16,21,23]. This identification was carried out before fixation by taking photographs and making observations in the field with a camera with a microscopic lens of 2 MP, f/3.3; FOV 65°; lens 3P + IR; FF.
Individuals were fixed in formalin/2% seawater and stored at 4 °C until their manipulation in the laboratory, where we developed a method for the observation of the specimens using handmade silicone pools in a microscope slide to maintain an aqueous medium (Formalin/2% seawater–glycerin) to manipulate ctenophores for pictures. This medium reduced the flux of liquid and avoided the movement of the specimens. Using this method, reconstructions from oral/aboral and tentacular/esophageal planes were obtained from each specimen. Plane reconstructions corresponded to single sharp images obtained from at least 30 focal depths of the respective planes, automatically combined successively (i.e., focus stacking), using a digital RisingCam 16 MP attached to a RisingTech UrCMOS stereoscope and the RisingView software V2. The external and internal morphologies of specimens were exhaustively examined for both plan reconstructions, and measurements were taken using the same program. Morphological terminology was adapted from previous ctenophore descriptions [10,17,19,22,23].
To analyze morphological variation in the specimens, 38 discrete and continuous morphological traits were considered. We integrated previous attributes from the available literature (n = 10) and evaluated 14 new ones. A total of 24 discrete attributes were considered, 6 from the external structures (comb rows, tentacles, tentilla, and pharyngeal folds) and 18 from internal structures (gastrovascular cavity, internal canals, statocyst, stomach and tentacle sheaths). Total traits were coded as double- or triple-state, and the first four were analyzed in situ, while the others were analyzed after fixation. Attributes 1–3, 5–7, 10, 12, 13, 24 have been commonly used in the literature for describing species within this genus [10,17,19,22,23], while the rest (4, 8, 9, 11, 14–19, 20–23) are newly proposed in this study (Figure 1 and Figure 2). These characters are as follows:
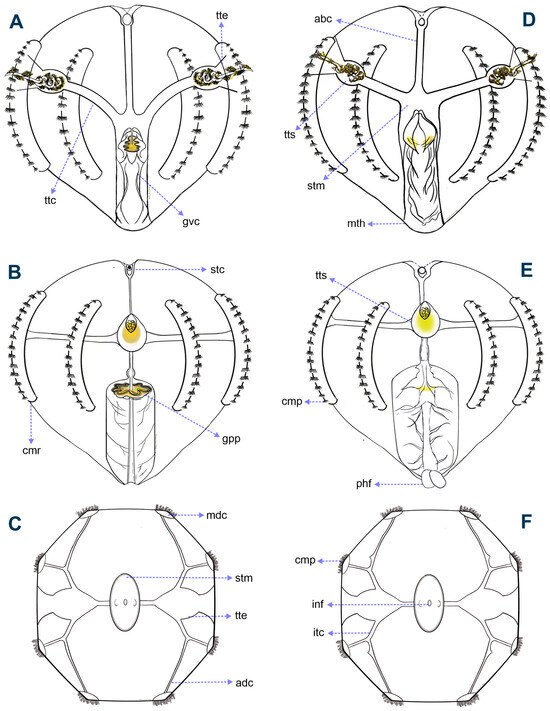
Figure 1.
Schemes and principal morphological features of Mexican Pleurobrachia individuals observed in the Southern Mexican Pacific coast (POcM) region (A–C) and in the Gulf of Mexico (GoM) region (D–F). Side tentacular view (A,D). Side esophageal view (B,E). Aboral view (C,F). Abbreviations: abc, aboral canal; adc, adradial canal; cmp, comb plate; cmr, comb row; gpp, gastrovascular pigmentation pattern; gvc, gastrovascular cavity; inf, infundibulum; itc, interradial canal; mdc, meridional canal; mth, mouth; phf, pharyngeal folds; stc, statocyst; stm, stomach; ttc, tentacle canal; tte, tentacle sheath exit; and tts, tentacle sheath.
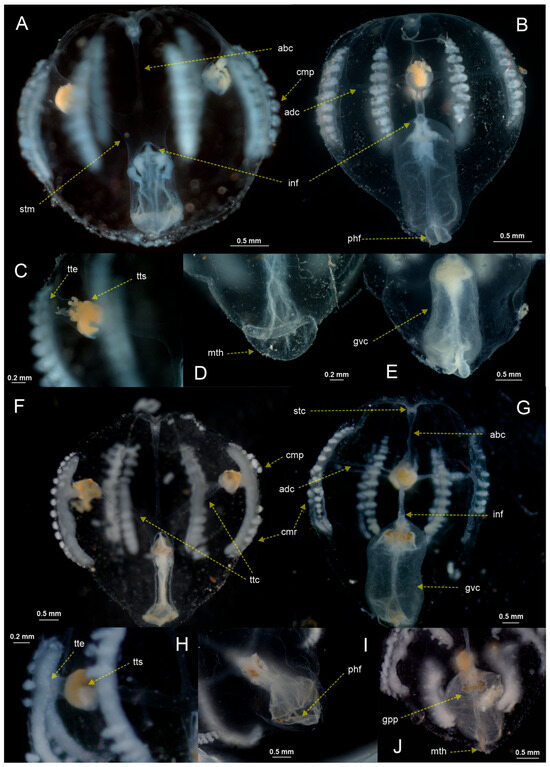
Figure 2.
Discrete morphological attributes showing the distinctive character states of the individuals analyzed in the Gulf of Mexico (GoM) region (A–E) and in the Southern Mexican Pacific coast (POcM) region (F–J). Side tentacular view (A,F). Side esophageal view (B,G). Zoom to the tentacle sheath in the side tentacular view (C,H). Zoom to the gastrovascular cavity in the side tentacular view (D,E,I,J). Abbreviations: abc, aboral canal; adc, adradial canal; cmp, comb plate; cmr, comb row; gpp, gastrovascular pigmentation pattern; gvc, gastrovascular cavity; inf, infundibulum; mth, mouth; phf, pharyngeal folds; stc, statocyst; stm, stomach; ttc, tentacle canal; tte, tentacle sheath exit; and tts, tentacle sheath.
- Tentacle length (TNL). From the opening of the tentacle sheath. (1) Less than 3 times, (2) more than 3 times, or (3) more than 5 times the body length [19,23].
- Tentilla length (TTL). Secondary tentacles or branches. (1) Less than half or (2) more than half the body length [22].
- Stomach tissue margin (STM). Observable in the tentacular plane. (0) Absent or (1) present (Figure 2).
- Adradial canal length (ADL). Observable in aboral view, from the interradial canal attachment. (1) Symmetrical or (2) asymmetrical (Figure 1).
- Adradial canal attachment (ADA). Observable in the esophageal plane, to the meridional canals or comb rows. (1) Aboral region or (2) oral region of the body (Figure 1).
Due to the difficulty of preserving the specimens, quantitative attributes had not been considered by previous authors to identify differences within the species of the genus Pleurobrachia [10,17,19,22,23]. Here, we applied 14 continuous measurements, of which attributes 25 and 26 have already been used in other studies [45,60], while the remainder 27–38 are proposed in this study as follows (Figure 3). These characters are as follows:
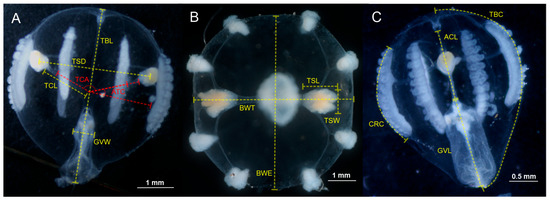
Figure 3.
Measurements of continuous quantitative attributes regarding the internal and external morphological structures of Pleurobrachia specimens distributed along the coast of Mexico. Tentacular plane (A). Oral plane (B). Esophageal side plane (C). Abbreviations: ACL, aboral canal length; ATC, tentacular canal angle; BWE, total body width in the esophageal plane; BWT, total body width in the tentacular plane; CRC, comb row circumference; GVL, gastrovascular cavity length; GVW, gastrovascular cavity width; TBC, total body circumference; TBL, total body length; TCA, angle between tentacular canals; TCL, tentacular canal length; TSD, horizontal distance between tentacle sheaths; TSL, tentacle sheath length; and TSW, tentacle sheath width.
- 25.
- Total body length (TBL). From the statocyst to the mouth opening, in the tentacular plane [45,60] (Figure 3).
- 26.
- 27.
- Total body width (BWE). At the middle of the stomach, in the esophageal plane (Figure 3).
- 28.
- Gastrovascular cavity length (GVL). From the infundibulum to the mouth opening, in the tentacular plane (Figure 3).
- 29.
- Gastrovascular cavity width (GVW). At the widest region, in the tentacular plane (Figure 3).
- 30.
- Tentacular canal length (TCL). From the middle of the stomach to the tentacular sheath base, in the tentacular plane (Figure 3).
- 31.
- Aboral canal length (ACL). From the middle of the stomach to the statocyst base, in the tentacular plane (Figure 3).
- 32.
- Horizontal distance between tentacle sheaths (TSD). At the tentacular sheath middle level, in the tentacular plane (Figure 3).
- 33.
- Tentacle sheath length (TSL). From the sheath base to the end, in the tentacular plane (Figure 3).
- 34.
- Tentacle sheath width (TSW). At the widest region, in the tentacular plane (Figure 3).
- 35.
- Comb row circumference (CRC). Along lateral comb rows, measured by one side and duplicated for symmetry, in the tentacular plane (Figure 3).
- 36.
- Total body circumference (TBC). From the statocyst to the pharyngeal fold, measured by one side and duplicated for symmetry, in the tentacular plane (Figure 3).
- 37.
- Angle between tentacular canals (TCA). At the middle of the stomach, observable in the tentacular plane (Figure 3).
- 38.
- Tentacular canal angle (ATC). At the middle of the stomach, from the horizonal to the tentacular canal, observable in the tentacular plane (Figure 3).
The frequency of character states was examined for each discrete morphological character. Based on the frequency calculated for each locality, we built a contingency table to evaluate if the differences in frequency among character states were associated with different localities or regions. Contingency tables were analyzed with the chi-square test and the contingency coefficient [74]. The normality of the distribution of the quantitative continuous features was tested by the Shapiro–Wilk test, in the case of non-normal distribution, bias was shown. For all variables, the mean and standard deviation were calculated to determine whether the measurements differed between locations, where a one-way analysis of variance (ANOVA) and a Kruskal-Wallis test were performed according to the distribution of the variables [74].
To evaluate whether the phenotypic variation in Mexican Pleurobrachia supports the existence of at least two distinct morphological species as previously suggested [60,61,62,63,64], we conducted a series of multivariate ordination analyses. First, we conducted a principal components analysis (PCA) using pairwise covariance matrices to explore geographic patterns of variation among specimens based on the continuous characters. To approximate a classification and reduce the dimensionality of the analyzed features, a linear discriminant analysis (LDA) was performed with the same attributes. Then, a principal coordinates analysis (PCoA) was performed using a Gower pairwise distance matrix based on 120 specimens and a combination of 24 discrete and 14 continuous morphological traits. Generalized linear models (GLMs) were performed to determine if the size of the organisms is related to latitude or to the marine regions. Body size was analyzed using the PC1 from the analysis of continuous attributes described above. Due to the type of morphometric datasets (especially with linear measurements), it is established that the greatest source of variation is quantified by a new variable PC1, often interpreted as a general representation of body size [75].
Given that taxonomic assignment of Pleurobrachia in Mexico has historically relied on geographic criteria, we conducted an additional analysis to test whether the observed morphological variation among Mexican specimens corresponds to that of P. pileus and P. bachei [60,61,62,63,64], as previously registered. For this purpose, we characterized the reference species using our discrete character set in conjunction with their seminal species descriptions [11,19,33]. The resulting coded matrices for P. pileus and P. bachei were analyzed alongside the matrix of 120 Mexican specimens using a non-metric multidimensional scaling (NMDS). Lastly, to observe the clustering tendency of operational taxonomic units (OTUs), a cluster analysis was performed using the same database with a bootstrap of 500 replicates, a Gower similarity index, and the UPGMA algorithm. Multivariate analyses were performed considering each specimen as an OTU. For all analyses, the alpha significance level was 0.05. All analyses were performed in PAST 4.03 [76].
3. Results
3.1. Discrete Morphological Characters
Of the 24 discrete morphological attributes analyzed, 15 showed the same character state in all individuals observed (Table S1). Meanwhile, the other six related to the tentacular sheath, tentacular canals, gastrovascular cavity (GVC), mouth, and infundibulum presented unique and consistent character states among all analyzed individuals from the Gulf of Mexico (GoM) localities: SOP tentacle sheath opening, SXT tentacle sheath exits, GPP pigmentation pattern of the GVC, GPC pigmentation pattern color of the GVC, TCS tentacular canal shape, and PFS pharyngeal fold size, and these same attributes showed a unique and consistent alternative state in the Southern Mexican Pacific coast (POcM) region locality. The attributes GPT proportion of the GVC and STM stomach tissue margin showed different character states indistinctly in the different specimens analyzed from all localities. Finally, the ABS aboral canal shape attribute presented one character state in all GoM localities, while the POcM locality presented both character states, although the state observed in the GoM localities predominated (Table 2) (Figure 4).

Table 2.
Frequencies of multistate or binary characters used to differentiate Pleurobrachia specimens among Mexican localities. Abbreviations: ABS, aboral canal shape; GPC, pigmentation pattern color of the GVC; GPP, pigmentation pattern of the GVC; GPT, proportion of the GVC; PFS, pharyngeal fold size; SOP, tentacle sheath opening; STM, stomach tissue margin; SXT, tentacle sheath exit; and TCS, tentacular canal shape.
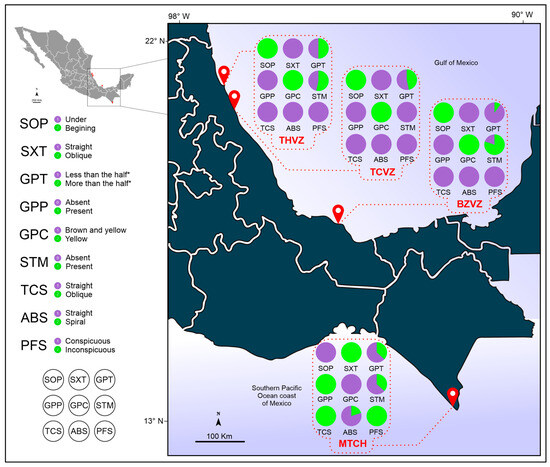
Figure 4.
Distribution of distinctive morphological attributes of Pleurobrachia sp. from several locations in Mexican coast localities. Localities: THVZ, Tamiahua estuary; TCVZ, Tampamachoco lagoon; BZVZ, Balzapote bay; and MTCH, Madero port. Abbreviations: ABS, aboral canal shape; GPC, pigmentation pattern color of the GVC; GPP, pigmentation pattern of the GVC; GPT, proportion of the GVC; PFS, pharyngeal fold size; SOP, tentacle sheath opening; STM, stomach tissue margin; SXT, tentacle sheath exit; and TCS, tentacular canal shape.
According to the above, all specimens of Mexican Pleurobrachia presented the following: a tentacle length (TBL) 3 times larger than the total body length; a tentilla length (TTL) less than the half of the total body length; a tentilla yellow coloration (TTC): GVC coloration (GCL) present; a comb row proportion (CRP) less than three-quarters of the total body circumference; the same comb row length (CRL); tentacle sheath spacing (SSP) far from the oral/aboral axis; a spherical tentacle sheath shape (SSH); a tentacle sheath angle (SAN) less than 45°; a tentacle sheath location (SLC) above the stomach; a star-shaped stomach (STS); an infundibulum location (IFL) under the half of the total body length; a tentacular canal direction (TCD) in the aboral region; an asymmetrical adradial canal length (ADL), and adradial canal attachment (ADA) in the aboral region. Meanwhile, the differences in the other attributes could be grouped into two morphotypes according to the region where they are distributed, which are as follows:
GoM region: all specimens from localities THVZ, TCVZ, and BZVZ showed tentacular sheath opening (SOP) at the beginning of comb rows, a straight tentacular sheath exit (SXT), an absent GVC pigmentation pattern (GPP), a yellow GVC pigmentation (GPC), a straight tentacular canal shape (TCS), a straight aboral canal shape (ABS), and conspicuous pharyngeal folds (PFS); on the other hand, the proportion of the GVC (GPT) in most specimens was less than half of the body size, while the stomach tissue margin (STM) was mostly present in the THVZ and BZVZ localities and totally absent in TCVZ individuals (Table 2) (Figure 1, Figure 2 and Figure 4).
POcM region: all specimens from the MTCH locality showed a tentacular sheath opening (SOP) below the comb rows, an oblique tentacular sheath exit (SXT), a present GVC pigmentation pattern (GPP), a yellow and black GVC pigmentation (GPC), an oblique tentacular canal shape (TCS), and inconspicuous pharyngeal folds (PFS); on the other hand, the proportion of the GVC (GPT) in most specimens was less than half of the body size, the margin of the stomach tissue (STM) was mostly absent, and the shape of the aboral canal (ABS) was mostly straight (Table 2) (Figure 1, Figure 2 and Figure 4).
3.2. Continuous Quantitative Morphological Characters
We observed a body clinal trend with respect to the size of the organisms and the latitude of the localities; the smallest specimens were in the northern localities and the largest corresponded to those from the southern locality (MTCH) (Table 3). Size differences among localities were supported by Kruskal–Wallis and ANOVA tests in the following attributes: BWE (H = 70.01, p < 0.001), ACL (H = 68.60, p < 0.001), and TBC (H = 68.79, p < 0.001) (Table 3). There were also significant differences between the THVZ and MTCH locations for the attributes TBL (H = 64.15, p < 0.001), BWT (F = 47.96, p < 0.001), GVL (H = 57.93, p < 0.001), and TSD (F = 41.20, p < 0.001), CRC (H = 50.47, p < 0.001). In addition, there were significant differences between groups of localities: THVZ and TCVZ were significantly different from BZVZ and MTCH for the attributes GVW (F = 47.27, p < 0.001), TSL (F = 25.98, p < 0.001), TSW (F = 17.98, p < 0.001), and ATC (F = 8.23, p < 0.001). Finally, only one attribute showed no significant differences among all specimens of the analyzed locations: TCA (H = 7.60, p > 0.05) (Table 3).

Table 3.
Measurements of continuous quantitative morphological attributes as mean ± standard deviation (min, max) analyzed to evaluate differences in Pleurobrachia specimens among Mexican localities. Abbreviations: ACL, aboral canal length; ATC, tentacular canal angle; BWE, total body width in the esophageal plane; BWT, total body width in the tentacular plane; CRC, comb row circumference; GVL, gastrovascular cavity length; GVW, gastrovascular cavity width; TBC, total body circumference; TBL, total body length; TCA, angle between tentacular canals; TCL, tentacular canal length; TSD, horizontal distance between tentacle sheaths; TSL, tentacle sheath length; and TSW, tentacle sheath width.
3.3. Multivariate Analyses
The principal components analysis (PCA) corresponding to the fourteen continuous quantitative attributes, including specimens from four localities, explained more than 98% of the total variation in the first two principal components (PC1 = 89.63%, PC2 = 9.04%). The operational taxonomic units (OTU’s) were partially segregated in the multivariate space of the first two PC’s. The linear discriminant analysis, LDA, also explained 86.89% of the variation in the first two axes (Axis1 = 57.4%, Axis2 = 29.49%) (Figure 5A). The first two principal coordinates (PCoA) of the continuous and discrete attributes explained 59.68% of the variations (PCo1 = 48.54%, PCo2 = 11.14%). The respective scatter plot showed that individuals from the GoM and POcM marine regions reside in discrete phenotypic groups in the multivariate space analysis, where north localities of the GoM (THVZ = 93.33%, TCVZ = 93.33%) had a higher percentage of self-assignment than those in the south of the GoM (BZVZ = 76.67%) and in the POcM region (MTCH = 80.00%) in the confusion matrix, although the value of χ2 = 74 evidenced statistically significant differences (Figure 5B). Regarding the generalized linear models (GLM’s), the first one (Figure 5C) showed a deviance of 47.23%, while the second one (Figure 5D) presented a higher deviance of 64.31%, indicating a greater trend in differences in the size of the organisms with respect to the marine regions in which the sampled specimens are located than between localities analyzed independently.
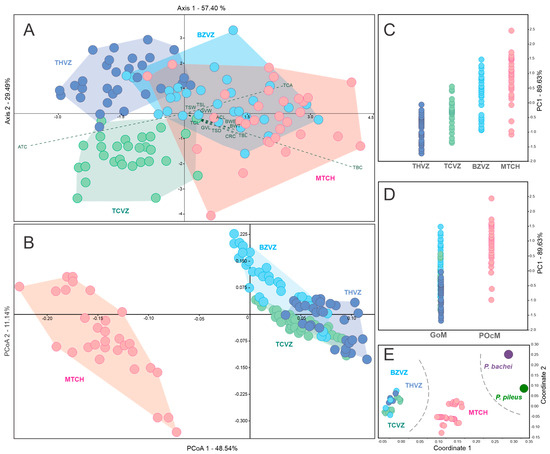
Figure 5.
Scatter plots of ordination analyses to evaluate the morphological variation in Mexican Pleurobrachia specimens. Linear discriminant analysis (LDA) using 14 continuous quantitative characters, (A). Principal coordinate analysis (PCoA) using the 24 discrete and 14 continuous features of all specimens (B). Generalized linear model (GLM) using principal component 1 (PC1) vs. localities (C) and generalized linear model using PC1 vs. regions (D). Non-metric multidimensional scaling (NMDS) using only the 24 discrete attributes and adding the species P. pileus and P. bachei (E). In the center of the scatter plot, vectors correspond to the contribution of the traits in the multivariate space (A). Abbreviations: GoM, Gulf of Mexico; POcM, Southern Mexican Pacific coast; THVZ, Tamiahua estuary; TCVZ, Tampamachoco lagoon; BZVZ, Balzapote bay; MTCH, Madero port; ACL, aboral canal length; ATC, tentacular canal angle; BWE, total body width in the esophageal plane; BWT, total body width in the tentacular plane; CRC, comb row circumference; GVL, gastrovascular cavity length; GVW, gastrovascular cavity width; TBC; total body circumference; TBL, total body length; TCA, angle between tentacular canals; TCL, tentacular canal length; TSD, horizontal distance between tentacle sheaths; TSL, tentacle sheath length; and TSW, tentacle sheath width.
The non-metric multidimensional scaling (NMDS) based on the matrix of all discrete attributes allowed us to identify two clearly differentiated groups within the Pleurobrachia specimens collected in Mexican waters. Equally, this analysis evidenced the distant location in multivariate space of the morphotypes of the species P. pileus and P. bachei (Figure 5E). The resulting dendrogram of the clustering analysis showed two main clades with high statistical support, corresponding to specimens from different oceanic regions, which were different from the previously recorded species (Figure 6). A first clade, identified as the “Southern Pacific Ocean coast of Mexico Cluster,” grouped specimens collected on the southern coast of the Mexican Pacific (MTCH). A second clade corresponded to the “Gulf of Mexico Cluster” and grouped specimens collected in THVZ, TCVZ, and BZVZ. These two clades were supported as sister groups with 99% support, and they were also associated with 100% support to a basal clade comprising both P. pileus and P. bachei. These latter species were associated with 96% support, marking a considerable distance from the Mexican specimens (CCC = 0.99) (Figure 6).
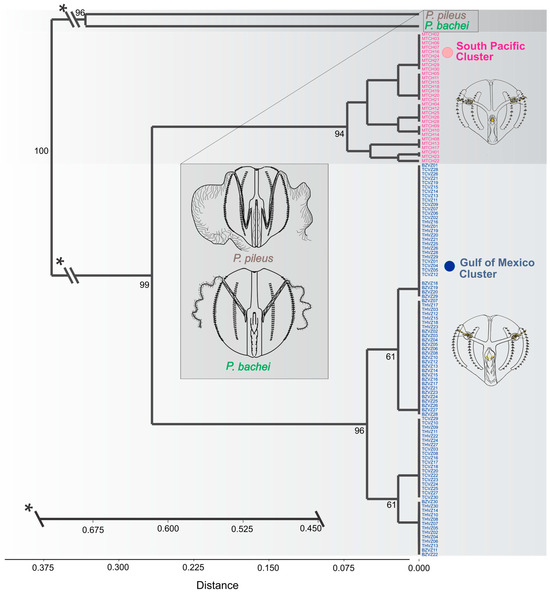
Figure 6.
Cluster by distance using the 24 discrete attributes of all Mexican Pleurobrachia specimens and adding the species P. pileus and P. bachei with a bootstrap of 500 replicates (only bootstrap values of nodes above 50% are shown). Abbreviations: THVZ, specimens from Tamiahua estuary; TCVZ, specimens from Tampamachoco lagoon; BZVZ, specimens from Balzapote bay; and MTCH, specimens from Madero port. *Axis brake.
4. Discussion
The taxonomy of the genus Pleurobrachia in Mexico is a clear example of the global challenges facing the systematics of ctenophores [12,13,51,77]. Existing records of P. pileus in the Gulf of Mexico and P. bachei in the Pacific are well documented but are based predominantly on geographic criteria [73], following the seminal descriptions of both species [72,78]. However, the results of this study suggest that the Mexican populations attributed to P. pileus and P. bachei may in fact represent distinct species that have not yet been formally described. The presence of clearly defined regional morphological patterns and the lack of overlap with traditional diagnostic traits demonstrate the need to reconsider the taxonomy of these organisms in the region. Our findings open the door to future research integrating morphological, genetic, and ecological data, which are essential for accurately delimiting species boundaries and obtaining a better understanding of the biodiversity and biogeography of ctenophores, as has been developed in other organisms [79,80,81,82]. Based on Darwin’s morphological species criterion [83], this study proposes the existence of two distinct morphological species of Pleurobrachia. The recognition of these two entities is based on an analysis of key morphological structures that were already used in the definition of species of the genus, which validates the proposed classification [84].
Over the last two centuries, Pleurobrachia species have been described based on morphological features such as the position and shape of the tentacular sac, the tentacular sheath exit, the proportion of the gastrovascular cavity, and the pigmentation of the tentacles [10,17,19,22,23]. However, the most recent records and studies of the most studied species, P. pileus and P. bachei, have focused on physiological, ecological, and evolutionary aspects, omitting crucial details about the morphology of the species in the regions where they were studied [30,40,42,43,45,46,47,53]. This has led to their identification in Mexico using outdated descriptions [72,78].
Furthermore, the taxonomy of ctenophores is notoriously difficult due to the extremely fragile nature of these organisms; specimens are easily damaged during collection, and their gelatinous bodies are poorly preserved in traditional fixatives such as formaldehyde. This problem not only hides detailed morphological analysis, but also degrades DNA, complicating molecular studies [12,15,34]. As a result, in Mexico, many database records or zooplankton studies have only identified “Pleurobrachia sp.” [65,66,67,68,69,70,71], underestimating the true species richness and making it difficult to trace the biogeography of the group. In our study, the incorporation of new handling and fixation techniques was essential to properly identify morphological attributes and outperform traditional observations, which on several occasions has proven to be insufficient for clear identification of species in this genus [51,66,77].
We found solid evidence for the existence of two well-defined morphotypes. One of them, the Gulf of Mexico (GoM) morphotype, differs from P. pileus in several diagnostic characteristics: the latter has elongated and curved tentacular sheaths located below the stomach; the sheath exits are curved and open to the outside below the end of the comb rows in the aboral region; the stomach is reduced and elongated towards the pharyngeal canals; the GVC covers more than half the body length; the pharyngeal folds are short; and the tentacles have longer tentilla than the body length [10,11,17,19,21] (Figure 5E and Figure 6).
Conversely, the Southern Mexican Pacific coast (POcM) morphotype differs from P. bachei in that this species has elongated tentacular sheaths located laterally at the level of the infundibulum below the stomach; the sheath opening is curved towards the interior of the body but has a straight exit below the end of the comb rows in the aboral region; the stomach is small and elongated towards the pharyngeal canals and the aboral region; the GVC is thin and covers more than half of the total body length; the pharyngeal folds and mouth are reduced and not very prominent; and the tentacles have short tentilla less than half the body length [11,16,19,23] (Figure 5E and Figure 6).
The morphological evidence obtained and compared supports the idea that the specimens found in the GoM and POcM regions belong to two unique morphotypes with evident and diagnosable characteristics. No correspondence was found with the descriptions of the species previously assigned as P. pileus and P. bachei [10,11,16,17,19,21,22,23] (Figure 5E and Figure 6).
Statistical analysis of morphometric variation showed a clinal trend in organism size, with smaller sizes in northern locations and larger sizes in southern locations, regardless of marine region. This trend differs from Bergmann’s rule [85,86,87], highlighting that size, a plastic trait, is not a reliable character for identification to the species level in this genus. The importance of assessing geographic variation in attributes and determining discrete diagnostic characters that are not susceptible to eco-geographic variation is perfectly illustrated by our approach. Changes in size and coloration often reflect phenotypic plasticity in response to environmental conditions and not necessarily reflect a difference at the species level, as has been showed in studies with other marine organisms [88,89,90].
Our study demonstrated the effectiveness of 24 discrete morphological attributes and showed that 9 of them (ABS, aboral canal shape; GPC, pigmentation pattern color of the GVC; GPP, pigmentation pattern of the GVC; GPT, proportion of the GVC; PFS, pharyngeal fold size; SOP, tentacle sheath opening; STM, stomach tissue margin; SXT, tentacle sheath exit; and TCS, tentacular canals shape) were adequate and consistent for identifying two morphotypes of Pleurobrachia. The anatomical structures that allowed these diagnostic differences to be established were the opening and exit of the tentacular sheath, the pattern and color of the GVC pigmentation, the shape of the tentacular canal, and the pharyngeal folds, among others (Figure 2). These findings demonstrate that combining discrete and continuous quantitative data provides a robust approximation of inter- and intraspecific differences [91].
The necessity to carry out modern and integrative taxonomic revisions is of utmost importance in this phylum. Currently, there are 185 species described [11], and it is estimated that there are around a similar number of species waiting to be discovered [2]. In addition, many of the current species have undergone various taxonomic reassignments [2,13]. Despite problems with the use of traditional taxonomy in these species, fossil ancestors of this group have been recognized by identifying unique sclerotized anatomical structures [92,93]. In the case of Pleurobrachia species, it is crucial to continue incorporating studies on ecological, ontogenetic, and molecular aspects, along with morphological studies (such as this one), to elucidate the boundaries between the eight currently recognized species and the two we propose.
5. Conclusions
This study achieved a promising approximation of the possible diversity of Pleurobrachia species by finding two new morphological species distributed along the coasts of Mexico. Previous records of P. pileus in the Gulf of Mexico and P. bachei in the Mexican Pacific Ocean, although well documented morphologically, should be considered provisional and await further analysis in more locations in Mexican marine regions. The geographical separation of these coasts suggests that the populations are distinct evolutionary lineages, a pattern of allopatric speciation observed in other marine organisms [94,95].
Consequently, the incorporation of molecular data from these Mexican populations would allow for the definitive validation of their identity and confirmation of their taxonomic status. The fragility and poor preservation of ctenophores have created a methodological barrier that has stopped significant progress in their systematics, perpetuating a cycle in which both the lack of adequate morphological descriptions and the lack of genetic reference data hide the validation of records and naming of these species. Integrative taxonomy is the way to solve this problem and reveal the true species richness in the genus Pleurobrachia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17100713/s1, Supplementary Table S1. Frequencies of multistate or binary characters used to differentiate Pleurobrachia specimens among Mexican localities.
Author Contributions
Conceptualization, E.A.R. and F.A.-T.; methodology, J.L.N.-S., E.A.R., Z.G.-L., F.A.-T., and A.C.-R.; software, F.A.-T. and J.L.N.-S.; validation, E.A.R., Z.G.-L., A.C.-R., and A.C.-R.; formal analysis, E.A.R., F.A.-T., and J.L.N.-S.; investigation, E.A.R., Z.G.-L., J.J.F.-M., and J.L.N.-S.; resources, E.A.R., F.A.-T., and Z.G.-L.; data curation, F.A.-T. and J.L.N.-S.; writing—original draft preparation, E.A.R., Z.G.-L., F.A.-T., and J.L.N.-S.; writing—review and editing, E.A.R., Z.G.-L., A.C.-R., J.J.F.-M., and J.L.N.-S.; visualization, E.A.R., Z.G.-L., and F.A.-T.; supervision, E.A.R. and Z.G.-L.; project administration, E.A.R. and Z.G.-L.; funding acquisition, E.A.R. and Z.G.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto Politécnico Nacional (IPN), with projects SIP20240531 and SIP20254283 granted to E.A.R. and projects SIP20240526 and SIP20253515 to Z.G.-L.
Institutional Review Board Statement
This study complies with Mexican regulations regarding the ethical treatment of research subjects. The Dirección General de Vida Silvestre does not consider this species a priority, given its large population size, wide distribution in Mexico, and because it is not listed or included within any of the threat categories under NOM-059-SEMARNAT-2010.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We would like to thank all those who directly or indirectly contributed to the collection and processing of specimens, the members of the ENCB—IPN Ecology Laboratory, the members of the CNIN—IBUNAM, and the members of the Biodiversity Microscopy and Photography Laboratory II (LANABIO)—IBUNAM. E.A.R. and Z.G.-L. would like to thank the Secretaria de Investigación y Posgrado for its financial support. This work was part of J.L.N.-S.’s PhD dissertation. J.L.N.-S. was a fellow of Beca de Estimulo Institucional de Formación de Investigadores del Instituto Politécnico Nacional (BEIFI-IPN) and Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI). Finally, we would like to thank the anonymous reviewers and the journal’s editorial team for their comments which helped to improve this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brusca, R.C.; Moore, W.; Shuster, S.M. Invertebrates, 3rd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2016. [Google Scholar]
- Mills, C. Ctenophores. 2010. Available online: https://faculty.washington.edu/cemills/Ctenophores.html (accessed on 15 August 2025).
- Podar, M.; Haddock, S.H.; Sogin, M.L.; Harbison, G.R. A molecular phylogenetic framework for the phylum Ctenophora using 18S rRNA genes. Mol. Phylogenet. Evol. 2001, 21, 218–230. [Google Scholar] [CrossRef]
- Philippe, H.; Derelle, R.; Lopez, P.; Pick, K.; Borchiellini, C.; Boury-Esnault, N.; Vacelet, J.; Renard, E.; Houliston, E.; Quéinnec, E.; et al. Phylogenomics revives traditional views on deep animal relationships. Curr. Biol. 2009, 19, 706–712. [Google Scholar] [CrossRef]
- Simion, P.; Philippe, H.; Baurain, D.; Jager, M.; Richter, D.J.; Di Franco, A.; Roure, B.; Satoh, N.; Quéinnec, É.; Ereskovsky, A.; et al. A large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Curr. Biol. 2017, 27, 958–967. [Google Scholar] [CrossRef]
- Whelan, N.V.; Kocot, K.M.; Moroz, T.P.; Mukherjee, K.; Williams, P.; Paulay, G.; Moroz, L.L.; Halanych, K.M. Ctenophore relationships and their placement as the sister group to all other animals. Nat. Ecol. Evol. 2017, 1, 1737–1746. [Google Scholar] [CrossRef]
- Li, Y.; Shen, X.-X.; Evans, B.; Dunn, C.W.; Rokas, A. Rooting the animal tree of life. Mol. Biol. Evol. 2021, 38, 4322–4333. [Google Scholar] [CrossRef]
- Schultz, D.T.; Haddock, S.H.D.; Bredeson, J.V.; Green, R.E.; Simakov, O.; Rokhsar, D.S. Ancient gene linkages support ctenophores as sister to other animals. Nature 2023, 618, 110–117. [Google Scholar] [CrossRef]
- Moroz, L.L. Brief History of Ctenophora, in Ctenophores: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–26. [Google Scholar]
- Greve, W. Phylum Ctenophora. Fiches d’identification du zooplacton. Conseil International pour l’Exploration de la Mer 1975, 146, 1–6. [Google Scholar]
- Moroz, L.L.; Collins, R.; Paulay, G. Ctenophora: Illustrated Guide and Taxonomy. In Ctenophores: Methods and Protocols; Springer: New York, NY, USA, 2024; pp. 27–102. [Google Scholar]
- Majaneva, S. Understanding the biodiversity and ecological importance of ctenophores lessons from arctic and Baltic Mertensia ovum. Sci. Rep. 2014, 41, 1–74. [Google Scholar]
- Mills, C. Phylum Ctenophora: List of All Valid Species Names. 2017. Available online: http://faculty.washington.edu/cemills/Ctenolist.html (accessed on 15 August 2025).
- Yip, S.Y. A note on the effect of preserving ctenophores in formaldehyde-seawater. Irish. Nat. J. 1982, 20, 416–419. [Google Scholar]
- Thibault-Botha, D.; Bowen, T. Impact of formalin preservation on Pleurobrachia bachei (Ctenophora). J. Exp. Mar. Biol. Ecol. 2004, 303, 11–17. [Google Scholar] [CrossRef]
- Rathbun, M.J. Reports on the scientific results of the expedition to the tropical Pacific, in charge of Alexander Agassiz, by the US Fish Commission Steamer Albatross, from August, 1899, to March, 1900, Commander Jefferson F. Moser, USN, commanding. XXVI. Mem. Mus. Comp. Zool. Harvard Coll. 1907, 35, 369–404. [Google Scholar]
- Mortensen, T. Ctenophora. In The Danish Ingolf-Expedition; Bianco Luno: Copenhagen, Denmark, 1912; pp. 1–195. [Google Scholar]
- Ringvold, H.; Shiganova, T.A.; Knott, K.E.; Galil, B.S. First record of Beroe gracilis Künne, 1939 (Ctenophora: Beroida: Beroidae) from Norway, found in a Mnemiopsis leidyi A. Agassiz, 1865 bloom. Mar. Biodivers. Rec. 2015, 8, 5. [Google Scholar] [CrossRef]
- Agassiz, A. Illustrated Catalogue of the Museum of Comparative Zoology: North American Acalephae; Cambridge University Press: Cambridge, UK, 1865; Volume 2, pp. 29–34. [Google Scholar]
- Bigelow, H. X The Ctenophores. In Reports on the Scientific Results of the Expedition to the Eastern Tropical Pacific 1904–1905. Steamer “Albatross”; Agassiz, A., Ed.; Bulletin of the Museum of Comparative Zoology at Harvard College: Cambridge, MA, USA, 1912; pp. 369–404. [Google Scholar]
- Mayer, A.G. Ctenophores of the Atlantic Coast of North America; Carnegie institution of Washington: Washington, DC, USA, 1912; Volume 162. [Google Scholar]
- Mianzan, H. Ctenophora. In South Atlantic Zooplankton; Boltovskoy, D., Ed.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 561–573. [Google Scholar]
- Mills, C.; Haddock, S. Ctenophora. In The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon; Carlton, J., Ed.; University of California Press: Los Angeles, CA, USA, 2007; pp. 189–199. [Google Scholar]
- Oliveira, O.M.; Miranda, T.P.; Araujo, E.M.; Ayón, P.; Cedeño-Posso, C.M.; Cepeda-Mercado, A.A.; Córdova, P.; Cunha, A.F.; Genzano, G.N.; Haddad, M.A.; et al. Census of Cnidaria (Medusozoa) and Ctenophora from south American marine waters. Zootaxa 2016, 4194, 1–256. [Google Scholar] [CrossRef]
- Johansson, M.L.; Shiganova, T.A.; Ringvold, H.; Stupnikova, A.N.; Heath, D.D.; MacIsaac, H.J. Molecular insights into the ctenophore genus Beroe in Europe: New species, spreading invaders. J. Hered. 2018, 109, 520–529. [Google Scholar] [CrossRef]
- Christianson, L.M.; Johnson, S.B.; Schultz, D.T.; Haddock, S.H.D. Hidden diversity of Ctenophora revealed by new mitochondrial COI primers and sequences. Mol. Ecol. Resour. 2022, 22, 283–294. [Google Scholar] [CrossRef]
- Shiganova, T.A.; Abyzova, G.A. Revision of Beroidae (Ctenophora) in the southern seas of Europe: Systematics and distribution based on genetics and morphology. Zool. J. Linn. Soc. 2022, 194, 297–322. [Google Scholar] [CrossRef]
- Yip, S.Y. The feeding of Pleurobrachia pileus Müller 1776 (Ctenophora) from Galway Bay. In Royal Irish Academy. Section B: Biological, Geological, and Chemical Science; Proceedings of the Royal Irish Academy: Galway, Ireland, 1984; pp. 109–122. [Google Scholar]
- Kazmin, A.; Shiganova, T.; Alekseenko, E.; Lüskow, F.; Abyzova, G. Ctenophores Beroe cucumis and newly discovered Beroe pseudocucumis: Global distribution and background abiotic conditions. Mar. Environ. Res. 2025, 211, 107460. [Google Scholar] [CrossRef] [PubMed]
- Møller, L.F.; Canon, J.M.; Tiselius, P. Bioenergetics and Growth in the ctenophore Pleurobrachia pileus. Hydrobiologia 2010, 645, 167–178. [Google Scholar] [CrossRef]
- Whelan, N.V.; Kocot, K.M.; Moroz, L.L.; Halanych, K.M. Error, signal, and the placement of Ctenophora sister to all other animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5773–5778. [Google Scholar] [CrossRef] [PubMed]
- Harbison, G.; Madin, L.; Swanberg, N. On the natural history and distribution of oceanic ctenophores. Deep Sea Res. 1978, 25, 233–256. [Google Scholar] [CrossRef]
- Müller, O.F. Zoologiae Danicae Prodromus, Seu, Animalium Daniae et Norvegiae Indigenarum Characteres, Nomina, et Synonyma Imprimis Popularium; Smithsonian Institution: Washington, DC, USA, 1776. [Google Scholar]
- Lüskow, F.; Stevens, H. Ctenophore diversity of Atlantic Canada: A synthesis. Can. J. Zool. 2025, 103, 1–8. [Google Scholar] [CrossRef]
- Tamm, S.L. Mechanisms of ciliary coordination in ctenophores. J. Exp. Biol. 1973, 59, 231–245. [Google Scholar] [CrossRef]
- Alvariño, A. Depredadores Planctónicos y la Pesca. In Memorias del II Simposio Latinoamericano sobre Oceanografía Biológica; Universidad de Oriente en Cumaná: Cumaná, Venezuela, 1975. [Google Scholar]
- Arai, M.N. Behaviour of Planktonic Coelenterates in Temperature and Salinity Discontinuity Layers. In Coelenterate Ecology and Behavior; Mackie, G.O., Ed.; Springer: New York, NY, USA, 1976; pp. 211–218. [Google Scholar]
- Freeman, G. The establishment of the oral-aboral axis in the ctenophore embryo. J. Embryol. Exp. Morphol. 1977, 42, 237–260. [Google Scholar] [CrossRef]
- Franc, J.-M. Organization and function of ctenophore colloblasts: An ultrastructural study. Biol. Bull. 1978, 155, 527–541. [Google Scholar] [CrossRef]
- Moss, A.G.; Tamm, S.L. Electrophysiological control of ciliary motor responses in the ctenophore Pleurobrachia. J. Comp. Physiol. 1986, 158, 311–330. [Google Scholar] [CrossRef]
- Hernandez-Nicaise, M. Ctenophora. In Microscopic Anatomy of Invertebrates, Placozoa, Porifera, Cnidaria and Ctenophora; Harrison, F.W., Michael, L., Eds.; Wiley-Liss: New York, NY, USA, 1991; pp. 359–418. [Google Scholar]
- Moss, A.G. The physiology of feeding in the ctenophore Pleurobrachia pileus. Hydrobiologia 1991, 216, 19–25. [Google Scholar] [CrossRef]
- Wang, Z.; Thiébaut, E.; Dauvin, J. Spring abundance and distribution of the ctenophore Pleurobrachia pileus in the Seine estuary: Advective transport and diel vertical migration. Mar. Biol. 1995, 124, 313–324. [Google Scholar] [CrossRef]
- Costello, J.H.; Coverdale, R. Planktonic feeding and evolutionary significance of the lobate body plan within the Ctenophora. Biol. Bull. 1998, 195, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; Bingel, F. Distribution and abundance of ctenophores, and their zooplankton food in the Black Sea. I. Pleurobrachia pileus. Mar. Biol. 1999, 135, 589–601. [Google Scholar] [CrossRef]
- Gibbons, M.; Buecher, E.; Thibault-Botha, D. Observations on the ecology of Pleurobrachia pileus (Ctenophora) in the southern Benguela ecosystem. Afr. J. Mar. Sci. 2003, 25, 253–261. [Google Scholar] [CrossRef][Green Version]
- Van Der Veer, H.; Sadée, C. Seasonal occurrence of the ctenophore Pleurobrachia pileus in the western Dutch Wadden Sea. Mar. Biol. 1984, 79, 219–227. [Google Scholar] [CrossRef]
- Shiganova, T.; Malej, A. Native and non-native ctenophores in the Gulf of Trieste, Northern Adriatic Sea. J. Plankton Res. 2009, 31, 61–71. [Google Scholar] [CrossRef]
- Haddock, S.H.; Case, J.F. Not all ctenophores are bioluminescent: Pleurobrachia. Biol. Bull. 1995, 189, 356–362. [Google Scholar] [CrossRef]
- Esser, M.; Greve, W.; Boersma, M. Effects of temperature and the presence of benthic predators on the vertical distribution of the ctenophore Pleurobrachia pileus. Mar. Biol. 2004, 145, 595–601. [Google Scholar] [CrossRef]
- Gershwin, L.A.; Zeidler, W.; Davie, P.J. Ctenophora of australia. Mem. Queensl. Mus. 2010, 54, 1–45. [Google Scholar]
- Frid, C.; Newton, L.; Williams, J. The feeding rates of pleurobrachia (ctenophora) and sagitta (chaetognatha), with notes on the potential seasonal role of planktonic predators in the dynamics of north sea zooplankton communities. Neth. J. Aquat. Ecol. 1994, 28, 181–191. [Google Scholar] [CrossRef]
- Mianzan, H.W.; Guerrero, R.A. Environmental patterns and biomass distribution of gelatinous macrozooplankton. Three study cases in the South-western Atlantic Ocean. Sci. Mar. 2000, 64, 215–224. [Google Scholar] [CrossRef]
- Ralph, P. Ctenophores from the waters of Cook Strait and Wellington Harbour. In Transactions of the Royal Society of New Zealand; Royal Society of New Zealand: Wellington, New Zealand, 1950. [Google Scholar]
- Sharaf, G.M.; Al-Ghais, S.M. Distribution of zooplankton in offshore waters of the west coast of the United Arab Emirates. Kuwait J. Sci. Eng. 1997, 24, 131–144. [Google Scholar]
- Srichandan, S.; Srichandan, S.; Lotliker, A.A.; Kumar, T.S.; Sahu, K.C. Zooplankton distribution in coastal water of the North-Western Bay of Bengal, off Rushikulya estuary, east coast of India. Indian J. Geo-Mar. Sci. 2015, 44, 519–527. [Google Scholar]
- Arai, M.N.; Welch, D.W.; Dunsmuir, A.L.; Jacobs, M.C.; Ladouceur, A.R. Digestion of pelagic Ctenophora and Cnidaria by fish. Can. J. Fish. Aquat. Sci. 2003, 60, 825–829. [Google Scholar] [CrossRef]
- Gewant, D.S.; Bollens, S.M. Macrozooplankton and micronekton of the lower San Francisco Estuary: Seasonal, interannual, and regional variation in relation to environmental conditions. Estuaries 2005, 28, 473–485. [Google Scholar] [CrossRef]
- Arafat, H.; Alamaru, A.; Gissi, C.; Huchon, D. Extensive mitochondrial gene rearrangements in Ctenophora: Insights from benthic Platyctenida. BMC Evol. Biol. 2018, 18, 65. [Google Scholar] [CrossRef]
- Ocaña-Luna, A.; Mecalco-Hernández, Á.; Sánchez-Ramírez, M.; Castillo-Rivera, M. Nuevos registros y morfometría de Pleurobrachia pileus (Phylum Ctenophora) en el golfo de México. Rev. Mex. Biodivers. 2017, 88, 442–445. [Google Scholar] [CrossRef]
- Gómez-Aguirre, S.; Santoyo-Reyes, H. Plancton de lagunas costeras XI: Transporte en tres estuarios del noroeste de México (noviembre 1973). Rev. Latinoam. Microbiol. 1975, 17, 175–183. [Google Scholar]
- Signoret de Brailovsky, J. Plancton de lagunas costeras: XIII. Pleurobrachia bachei Agassiz, de la Laguna de Agiabampo. Rev. Latinoam. Microbiol. 1975, 17, 249–254. [Google Scholar] [PubMed]
- Alvarez-León, R.; Wedler, E. Hidroides de tres esteros adyacentes a Mazatlán, costa noroeste de México. An. Inst. Investig. Mar. Punta Betín. 1982, 12, 19–32. [Google Scholar] [CrossRef]
- Gómez-Aguirre, S. Contribución al estudio faunístico de celenterados y ctenóforos del plancton estuarino del noroeste de México. An. Inst. Biol. Univ. Nac. Autón. México. 1991, 62, 1–10. [Google Scholar]
- Gómez-Aguirre, S. Memorias II in Simposio Latino-Americano de Oceanografía Biológica; Universidad De Oriente: Cumaná, Venezuela, 1977. [Google Scholar]
- Cooley, N.R. An inventory of the estuarine fauna in the vicinity of Pensacola, Florida. Fla. Mar. Res. Publ. 1978, 31, 2–8. [Google Scholar]
- Biggs, D.; Smith, D.E.; Bidigare, R.R.; Johnson, M.A. In situ estimation of the population density of gelatinous planktivores in Gulf of Mexico surface waters. Mem. Univ. Nfld. Occas. Pap. Biol. 1984, 9, 17–34. [Google Scholar]
- Lalana, R.; Ortiz, M.; Varela, C. Lista actualizada y bibliografía de los celenterados (Cnidaria) y los ctenóforos (Ctenophora), de aguas cubanas. Rev. Biol. 2001, 15, 158–169. [Google Scholar]
- Ruíz, M.; López-Portillo, J. Los Invertebrados. In Entornos Veracruzanos: La Costa de La Mancha; Moreno-Casasola, P., Ed.; Instituto de Ecología: Xalapa/Veracruz, Mexico, 2006; pp. 341–361. [Google Scholar]
- Ordóñez-López, U.; Ornelas-Roa, M.; Uicab-Sabido, R.A.; Escamilla-Sánchez, J.B.; Durán, R.; Méndez, M. Fauna Zooplanctónica en el Litoral Yucateco. In Biodiversidad y Desarrollo Humano en Yucatán; Durán, R., Méndez, M., Eds.; CONABIO, SEDUMA: Mérida, México, 2010; pp. 150–154. [Google Scholar]
- Ocaña-Luna, A.; Sánchez-Ramírez, M.; Aguilar-Duran, R. Macromedusas y Ctenóforos del Sistema Arrecifal Veracruzano y Lagunas Costeras Asociadas. In Aportes al Conocimiento del Sistema Arrecifal Veracruzano: Hacia el Corredor Arrecifal del Suroeste del Golfo de México; Granados-Barba, A., Ortiz-Lozano, L., Salas-Monreal, D., González-Gándara, C., Eds.; Universidad Autónoma de Campeche: Campeche, Mexico, 2015; pp. 121–138. [Google Scholar]
- Ruiz-Escobar, F.; Valadez-Vargas, D.K.; Oliveira, O.M. Ctenophores from the Oaxaca coast, including a checklist of species from the Pacific coast of Mexico. Zootaxa 2015, 3936, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Puente-Tapia, F.A.; Gasca, R.; Schiariti, A.; Haddock, S.H. An updated checklist of ctenophores (Ctenophora: Nuda and Tentaculata) of Mexican seas. Reg. Stud. Mar. Sci. 2021, 41, 20. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Pearson Education India: Chennai, India, 1999. [Google Scholar]
- Bookstein, F.L. “Size and shape”: A comment on semantics. Syst. Zool. 1989, 38, 173–180. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Mianzan, H.; Dawson, E.; Mills, C. Phylum Ctenophora: Comb jellies. N. Zeal. Indoor Bowls 2009, 1, 49–58. [Google Scholar]
- Palma, S.; Rosales, S. Composición, distribución y abundancia estacional del macroplancton de la bahía de Valparaíso. Rev. Investig. Mar. 1995, 23, 49–66. [Google Scholar] [CrossRef]
- Fonseca, G.; Derycke, S.; Moens, T. Integrative taxonomy in two free-living nematode species complexes. Biol. J. Linn. Soc. 2008, 94, 737–753. [Google Scholar] [CrossRef]
- Rocha, L.A.; Lindeman, K.C.; Rocha, C.R.; Lessios, H. Historical biogeography and speciation in the reef fish genus Haemulon (Teleostei: Haemulidae). Mol. Phyl. Evol. 2008, 48, 918–928. [Google Scholar] [CrossRef]
- Valerio-Mendoza, O.; García-Román, J.; Becerril, M.; Armendáriz-Toledano, F.; Cuéllar-Rodríguez, G.; Negrón, J.F.; Sullivan, B.T.; Zúñiga, G. Cryptic species discrimination in western pine beetle, Dendroctonus brevicomis LeConte (Curculionidae: Scolytinae), based on morphological characters and geometric morphometrics. Insects 2019, 10, 377. [Google Scholar] [CrossRef]
- Laakmann, S.; Holst, S. Emphasizing the diversity of North Sea hydromedusae by combined morphological and molecular methods. J. Plankton Res. 2014, 36, 64–76. [Google Scholar] [CrossRef]
- Mallet, J. Species, Concepts of, in Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2001; pp. 427–440. [Google Scholar]
- Simpson, G.G. The species concept. Evolution 1951, 5, 285–298. [Google Scholar] [CrossRef]
- McNab, B.K. On the ecological significance of Bergmann’s rule. Ecology 1971, 52, 845–854. [Google Scholar] [CrossRef]
- Meiri, S.; Dayan, T. On the validity of Bergmann’s rule. J. Biogeogr. 2003, 30, 331–351. [Google Scholar] [CrossRef]
- Berke, S.K.; Jablonski, D.; Krug, A.Z.; Roy, K.; Tomasovych, A. Beyond Bergmann’s rule: Size–latitude relationships in marine Bivalvia world-wide. Glob. Ecol. Biogeogr. 2013, 22, 173–183. [Google Scholar] [CrossRef]
- Macpherson, E. Species range size distributions for some marine taxa in the Atlantic Ocean. Effect of latitude and depth. Biol. J. Linn. Soc. 2003, 80, 437–455. [Google Scholar] [CrossRef][Green Version]
- Sánchez, J.A.; Aguilar, C.; Dorado, D.; Manrique, N. Phenotypic plasticity and morphological integration in a marine modular invertebrate. BMC Evol. Biol. 2007, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Mcalister, J.S.; Miner, B.G. Phenotypic Plasticity of Feeding Structures in Marine Invertebrate Larvae; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Felsenstein, J. A comparative method for both discrete and continuous characters using the threshold model. Amer. Naturalist. 2012, 179, 145–156. [Google Scholar] [CrossRef]
- Stanley, G.D., Jr.; Stürmer, W. A new fossil ctenophore discovered by X-rays. Nature 1987, 328, 61–63. [Google Scholar] [CrossRef]
- Tang, F.; Bengtson, S.; Wang, Y.; Wang, X.L.; Yin, C.Y. Eoandromeda and the origin of Ctenophora. Evol. Dev. 2011, 13, 408–414. [Google Scholar] [CrossRef]
- Ekimova, I.; Valdés, Á.; Chichvarkhin, A.; Antokhina, T.; Lindsay, T.; Schepetov, D. Diet-driven ecological radiation and allopatric speciation result in high species diversity in a temperate-cold water marine genus Dendronotus (Gastropoda: Nudibranchia). Mol. Phylogenet. Evol. 2019, 141, 15. [Google Scholar] [CrossRef]
- Hernández-Hernández, T.; Miller, E.C.; Román-Palacios, C.; Wiens, J.J. Speciation across the tree of life. Biol. Rev. 2021, 96, 1205–1242. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).