Abstract
Renicolids are parasites of aquatic birds. Their species identification based on morphological characters is problematic. Here, we revised the composition of Renicola spp. parasitising anatids in nearshore areas of northern seas using integrated morphological and molecular data. We redescribed Renicola somateria and verified the diagnosis of R. mediovitellata. We established that the first intermediate host (FIH) of R. somateria is the mollusc Buccinum undatum, while the FIHs of R. mediovitellata are Nucella spp. molluscs. We described the intramolluscan stages of both species. Renicola somateria and R. mediovitellata formed a separate clade in the molecular trees of the Renicolidae. This finding confirms the existence of three main phylogenetic branches of renicolids, differing in the structure of adults, type of cercariae, and host range. Molecular data demonstrated an amphiboreal distribution of both R. somateria and R. mediovitellata. The former is represented by a single population in Europe and the North Pacific, while the latter forms separate populations in these regions. This may be because R. somateria actually uses not only B. undatum but also some other buccinid species with similar circum-Arctic ranges as the FIH. We discuss the roles played in the formation of digenean ranges by the vagility of the definitive host, the lifespan of the adults, and the distribution of the FIH.
1. Introduction
Digeneans of the family Renicolidae (Plagiorchioidea) are a small but widespread group of dangerous renal parasites of aquatic birds. Species identification of their adults is problematic because the body of mature individuals is almost completely filled with an overgrown uterus densely packed with eggs, which obscure the internal organs, whose size and structure are diagnostic characters. The problems of renicolid taxonomy have been characterized comprehensively in a recent study by Galaktionov et al. [1]. Due to the difficulties associated with the differentiation of morphological characters, the identification of renicolid species heavily relies on the species of the definitive host (DH) from which sexual adults are isolated [2]. This is particularly the case for the renicolids parasitising marine ducks in the nearshore areas of northern Holarctic seas or anatids visiting sea coasts during seasonal migrations and/or wintering. Five Renicola spp. have been described from these birds: R. mediovitellata Bychovskaja-Pavlovskaja, 1950, R. somateria Belopolskaja, 1952, R. mollissima Kulachkova, 1957, R. ovocallosa Reimer, 1971, and R. brantae McIntosh & Farr, 1952 [2,3]. The most common of them are R. somateria and R. mediovitellata, which are particularly difficult to identify because their descriptions are incomplete. In addition, the description of R. somateria by Belopolskaja [4] was actually based on individuals from the common eider, Somateria mollissima (Linnaeus, 1758), belonging to two different species (see below).
The confusion grew even worse after the first molecular studies of Renicola spp. by Skírnisson et al. [5] showed that adult digeneans from the common eider in Iceland identified as R. somateria coincide, by ITS1 rDNA marker, with sporocysts and cercariae from the mollusc Nucella lapillus (Linnaeus, 1758), earlier described by Stunkard [6] as R. thaidus Stunkard, 1964. Galaktionov et al. [1] synonymized the adults of R. thaidus with R. parvicaudatus (Stunkard & Shaw, 1931). However, the intramolluscan stages of R. parvicaudatus develop in Littorina spp. molluscs, and its cercariae are strikingly different from the larvae obtained from N. lapillus [1,7]. Molecular studies have shown that the renicolid intramolluscan stages from N. lapillus form an independent branch in the phylogenetic tree, which differs from the other two branches (Clades I and II) uniting all the other renicolids for which molecular data were available as of 2022 [1]. It seemed logical to suggest that intramolluscan stages from N. lapillus described by Stunkard [6] as R. thaidus actually belonged to R. somateria. This conclusion was made by Galaktionov et al. [1], who attributed isolate 10nIR (GenBank numbers ON650721, ON652707, and ON667891) to R. somateria. However, considering the inaccuracy of the description of the adults of this species in Belopolskaja [4], the identification of individuals used in Skírnisson et al. [5] is doubtful.
The goal of this study was to resolve the status of renicolids parasitising the common eider and other anatids. We used an integrative approach, combining the analysis of morphological and molecular data [8]. As a result, we clearly differentiated the two most common species of renicolids from anatids, R. somateria, and R. mediovitellata. Moreover, we elucidated their life cycles and defined the range of their definitive and intermediate hosts. In addition, we characterized the genetic structure of the populations of these two species and suggested phylogeographic reconstructions and approaches to the taxonomy of Renicolidae.
2. Materials and Methods
2.1. Material Collection and Treatment
The material for this study was collected in 2008–2023 in the Barents Sea, the White Sea, the Sea of Okhotsk, the Sea of Japan, and Iceland. Adult digeneans were extracted from the kidneys of the common eider S. mollissima shot in Southwest Iceland (Reykjavik region) and in the northern part of the Sea of Okhotsk (Shelikhov Bay and Shkiperova Bay) in accordance with the local regulations (Table 1). The ducks were dissected, and the renicolid individuals were extracted from the kidney. The adults were stored in 70 and 95% ethanol for further morphological and molecular analyses, respectively. Some of these adults were fixed in 70% ethanol under the slight pressure of a coverslip and used to make carmine-stained whole mounts for morphological studies and to produce drawings and photographs with the help of a Leica DM2500 compound microscope (Leica Microsystems, Germany) equipped with a TrueChrome 4KPro digital camera (Tucsen, China) in the Zoological Institute of the Russian Academy of Science (ZIN RAS) (St. Petersburg, Russia). We also examined the materials from the Collection of Helminths, section Trematoda, of the ZIN RAS (further abbreviated as ZISP) and from the Collection of Helminths, section Trematoda of the Center of Parasitology of the Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences, Moscow, Russia (further abbreviated as GELAN), including the slides with syntypes of R. somateria and R. mediovitellata.

Table 1.
List of samples used in this study and their GenBank accession numbers.
Individuals of the caenogastropod mollusc Nucella lapillus were collected in the intertidal zone of the Barents Sea (Eastern Murman and Finmark) and SW Iceland (Reykjavik region), N. freycinetii (Deshayes, 1839) in the Sea of Okhotsk (Magadan region), Nucella heyseana (Dunker, 1882) in the Sea of Japan (Vostok Biological station), and Littorina sitkana R.A. Philippi, 1846 in the Sea of Okhotsk (Magadan region) (Table 1). Buccinid gastropods Buccinum undatum Linnaeus, 1758 were sampled by scuba diving in the subtidal zone at a depth of 5–15 m in the Barents Sea (Eastern Murman) and the White Sea (Kandalaksha Bay) (Table 1).
The snails were placed in plastic jars filled with seawater (1 snail per jar) and exposed to sunlight or direct artificial light for 1h. The jars were examined under a stereomicroscope and the individuals that had shed cercariae of Renicola spp. were selected. The infected molluscs were used as a source of cercariae and then dissected under a stereomicroscope. Live sporocysts and cercariae were observed, measured, and photographed using an Olympus CH40 compound microscope (Olympus Optical Co. Ltd., Tokyo, Japan) equipped with an Olympus XC-30 digital camera (Olympus Optical Co. Ltd.) at the “Kartesh” White Sea Biological Station of ZIN RAS, a Leica compound microscope at the Institute of Pathology (Keldur, Iceland), and a Leitz Dialux 20B compound microscope (Leica Microsystems, Wetzlar, Germany) at the Institute of Biological Problems of the North (Magadan, Russia). Sporocysts and encysted metacercariae were measured in vivo. Newly shed cercariae were used for morphological and morphometric studies and for scanning electron microscopy (SEM). Cercariae to be measured were fixed by heating in a drop of seawater on the object slide (until the water started to evaporate) and then gently pressed with a coverslip. Cercariae for SEM examination were fixed and treated as described in Galaktionov et al. [9]. The treated cercariae were viewed under an FEI Quanta 250 (FEI, the Netherlands) scanning electron microscope in the “Taxon” Research Resource Center (http://www.ckp-rf.ru/ckp/3038/, accessed on 20 June 2024) of ZIN RAS. All the measurements presented in the paper are in micrometres, with the mean given in parentheses. The drawings were made with the aid of a camera lucida.
2.2. DNA Extraction, Amplification, and Sequencing
We sequenced fragments of 28S ribosomal RNA (rRNA), cox1, and 12S ribosomal RNA mitochondrial genes for daughter sporocysts, cercariae, and adults of Renicola spp. from infected snails and birds (Table 1). Genomic DNA was extracted from ethanol-fixed isolates using cetrimonium bromide (CTAB) detergent according to the published protocol, with modifications [10]. Fixed samples were rinsed in 1× phosphate-buffered saline for 15 min prior to extraction.
PCR reactions and purification of products were performed according to published protocols [1,11]. The primer sequences are listed in Table 2. DNA sequencing was performed at the Resource Centre for Development of Molecular and Cellular Technologies, St. Petersburg State University. All sequences obtained in this study were deposited in the GenBank database (Table 1).

Table 2.
A list of primers used in this study.
2.3. Alignments and Phylogenetic Analyses
Alignment, trimming, and basic analyses of the newly generated sequences, together with the 28S rRNA, cox1, and 12S rRNA gene partial sequences retrieved from GenBank for other Renicola spp. and other trematodes, were performed in Geneious 7.1.4 (http://www.geneious.com, accessed on 20 June 2024) [15]. Phylogenetic relationships were reconstructed using Bayesian inference (BI) in MrBayes v. 3.2.6 [16] and maximum likelihood (ML) in MEGA 11 [17]. The most suitable evolutionary models were determined by the corrected Akaike information criterion in the Partition Finder program (https://github.com/brettc/partitionfinder, last accessed 10 June 2024). The Hasegawa–Kishino–Yano model with estimates of gamma-distributed among-site rate variation (HKY+ G) was chosen as the one with the best fit for all analyzed genes. Genetic divergences between the taxa were calculated as uncorrected p-distances with 1000 bootstrap iterations for each gene region using MEGA 11 [17]. Tajima’s D neutrality test was calculated in the DNASP 6 program [18]. A median-joining haplotype network was reconstructed with PopART 1.7 [19].
3. Results
Two strikingly different forms of adult digeneans were found on the slides made by Maria M. Belopolskaja and designated as “Renicola somateria type of species” (# 3740 adults from the common eider, Eastern Murman, Barents Sea) deposited in ZISP. One form, represented by 15 individuals, has oval to rounded eggs with a thick eggshell, while the other form, represented by 12 individuals, has elongated-oval eggs with a relatively thin eggshell. It is mentioned in the species description that the egg shape varies and that the eggshell is very thick [4]. Based on this information, we consider that the species R. somateria on the slide is represented by the individuals with rounded eggs and a thick eggshell. Individuals with elongated-oval eggs and relatively thin eggshells correspond to the description of R. mediovitellata by Bychovskaja-Pavlovskaja [20].
In our collections of adult renicolids from the common eider, there were individuals that we identified as R. somateria and R. mediovitellata based on complex morphological characters, including egg structure. The molecular analysis confirmed that the adults in question belonged to two independent species (see Section 3.2). Since the first description of R. somateria in Belopolskaja [4] was based on individuals of two different species, we provide an amended description of R. somateria (see below). The description of adults of R. mediovitellata by Bychovskaja-Pavlovskaja [20] is incomplete. Therefore, we provide more detailed characteristics of the morphology and morphometry of this species (see below). The intramolluscan stages of R. somateria and R. mediovitellata were identified in B. undatum and Nucella spp. caenogastropods, correspondingly, using molecular markers (see Section 3.2). These lifecycle stages are described below.
3.1. Description
Family Renicolidae Dollfus, 1939
Renicola somateria (Belopolskaja, 1952) emend.
Type host (definitive): the common eider Somateria mollissima (Linnaeus, 1758)
Other hosts (definitive): long-tailed duck Clangula hyemalis (Linnaeus, 1758), white-winged scoter Melanitta deglandi (Bonaparte, 1850), velvet scoter M. fusca (Linnaeus, 1758), and King eider Somateria spectabilis (Linnaeus, 1758) (under question) (Anatidae)
Site in definitive host: kidney tubules
Type locality: Eastern Murman (Kola Peninsula, Barents Sea)
Other localities (in the definitive host): White Sea, Barents Sea, Yakutia, Sea of Okhotsk, and Amur River Basin
Type material: 15 syntypes on the slide of M. M. Belopolskaja # 3740 deposited in ZISP.
Representative slides: 3741-1–3741-3 deposited in ZISP. This material represents paragenophores. Slides of V. G. Kulachkova 3742-1–3742-4 deposited in ZISP and slides 2473/Tr, 2477/Tr, 2478/Tr, and 2480/Tr deposited in GELAN.
First intermediate host: Buccinum undatum Linnaeus, 1758 (Caenogastropoda: Buccinidae) (natural)
Site in first intermediate host: gonad
Localities (in the first intermediate host): Barents Sea and White Sea
Second intermediate hosts: B. undatum (infrequent) and unknown bivalves
Representative DNA sequences: cox1 (OR742790, OR742799, OR742794–OR742796, and OR742798), 28S rDNA (OR735485), and 12S rDNA (OR735481 OR735484, and OR735483) (shown in Table 1)

Table 3.
Morphometric parameters of Renicola spp. adults.
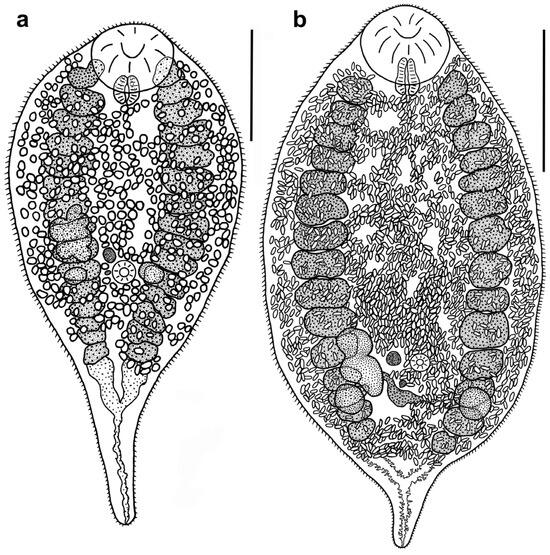
Figure 1.
Sexual adults of Renicola somateria (long-tailed duck, White Sea) (a) and R. mediovitellata (the common eider, Sea of Okhotsk) (b) (ventral view). Scale bars: (a) 300 µm and (b) 500 µm.
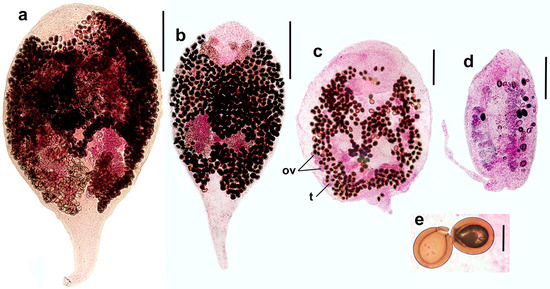
Figure 2.
Representative microphotographs of sexual adults of Renicola somateria (ventral view). Syntype from slide 3740 made by Belopolskaja (the common eider Somateria mollissima, Barents Sea) (a). Specimen from slide 3742-3 made by Kulachkova (long-tailed duck Clangula hyemalis, White Sea) (b). Specimen heavily pressed by a coverslip from slide 3741-1 (the common eider S. mollissima, Sea of Okhotsk) (c). Young specimen from slide 3741-2 (the common eider S. mollissima, Sea of Okhotsk) (d). Eggs of R. somateria from slide 3741-1 (e). Abbreviations: ov: ovary and t: testis. Scale bars: (a) and (b) 300 µm, (c) and (d) 200 µm, and (e) 20 µm.
Adult worms were present in our own material obtained from the common eider at the Sea of Okhotsk (Shkiperova Bay). One of these adults matched renicolid intramolluscan stages from B. undatum sampled in the White Sea and the Barents Sea in the marker DNA sequences (see Section 3.2). In addition, we examined the slides of adult digeneans identified as R. somateria stored in the collections of trematodes in ZISP and GELAN. The description is based on all these materials.
Body ovoid, rounded anteriorly, and attenuated posteriorly, forming a caudal process, its length sometimes reaching 2/3 of body length in young individuals (Figure 2d). Size of worms varying greatly depending on the number of eggs in the uterus. Oral sucker subterminal to terminal, transversely elongated-oval. Ventral sucker small, poorly discernible in large worms with numerous eggs. Prepharynx absent and pharynx small, often deeply embedded in the wall of the oral sucker. Oesophagus short, two caeca, extending to the base of the caudal process. Testes oval and smooth-edged, lying in the posterior third of the body, more or less opposite to each other. Left testis somewhat larger than right testis. Seminal vesicle lying approximately at the level of the posterior to the middle part of the ovary, median or slightly dextral of the body midline. Ovary dextral, pretesticular, larger than testes, and variously lobed. Vitellarium follicular: follicles in two fields passing along the dorsal body side at a considerable distance from body edges and extending from the level of the oral sucker to the base of the caudal process. In young adults, rows of vitelline follicles are almost parallel to each other (Figure 2d). As the uterus fills with eggs, they are displaced laterally in the middle part of the body (Figure 2a–c). Uterus strongly developed, occupying most of the body. Eggs numerous, rounded to oval, and operculate, with a very thick eggshell (Figure 2e). Excretory bladder Y-shaped, with distinct lateral diverticula, and bifurcates approximately at the level of the base of the caudal process, with arms extending into the forebody up to the level of the oral sucker.

Table 4.
Morphometric parameters of cercariae of Renicola spp.
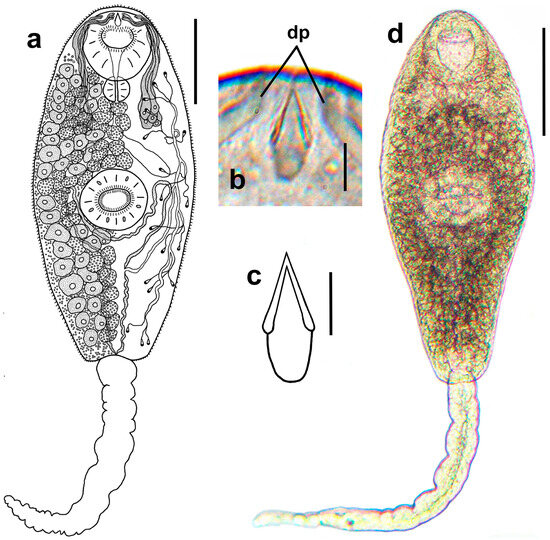
Figure 3.
Cercaria of Renicola somateria. Drawing of the cercaria (ventral view) (a). Phase contrast view of the anterior end of a flattened, live cercaria (ventral view), showing stylet and distal parts of ducts of penetration gland cells (b). Drawing (c) of the stylet (ventral view) and microphotograph of the cercaria (dorsal view) (d). Abbreviations: dp: ducts of penetration gland cells. Scale bars: (a) and (d) 100 µm and (b) and (c) 5 µm.
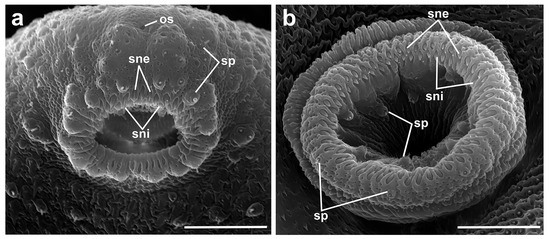
Figure 4.
SEM microphotographs of a cercaria of Renicola somateria, showing spines in the oral (a) and ventral sucker (b). Abbreviations: os: opening of stylet pocket; sne: spines of external row; sni: spines of internal row; and sp: sensory papillae. Scale bars: 10 µm.
Sporocysts occupy the molluscan gonad tissue, forming a tumor-like structure. Sporocyst elongated-oval, 458–1233 × 312–460. Cercariae of xiphidiocercaria type and small, with a highly contractile oval body and body length more than 1.5 times greater than the tail length. Entire body covered with spines. Oral sucker ventro-subterminal, muscular, and approximately the same size as the ventral sucker. Oral sucker armed with two rows of large spines: outer row complete (38–43 spines) and inner row incomplete, comprising 16–20 spines along the anterior and antero-lateral edge of the oral sucker (Figure 4a). Stylet rather small. Its anterior part, making up approximately 2/3 of its length, is shaped like an arrowhead, with light-refracting cutting edges and a pointed tip. Stylet handle rounded (Figure 3b,c).
Ventral sucker equatorial, armed with two alternating rows of 45–48 large spines (Figure 4b). Anteriorly to the external row of spines, the ventral sucker bears six characteristic short sensory papillae (two anterior and four posterior) surrounded by convex tegumental collars. Deeper, behind the posterior row of spines, eight sensory papillae, with a short cilium and high tegumental collar, are arranged along the circumference of the sucker (Figure 4b). There are five pairs of penetration gland cells. Their nucleated bodies arranged symmetrically on either side of the oesophagus, approximately at the level of its middle and posterior. Their ducts pass forward and skirt the oral sucker dorsally; three of them open medially and ventrally near the external opening of the stylet pocket, while two open dorsolaterally to them. Contents of penetration gland cells finely granular.
Entire body of larva densely packed with tegumental cystogenous gland cells. Two types of these cells distinctly seen: cells with coarsely granular contents and cells with granular unstaining contents. Cells of the first type with distinct nuclei and nuclei in cells of the second type indistinguishable. At the final stages of larva formation, gland cells appear to discharge some of the contents into the tegument, with the granular material being visible throughout the body and not only in the cells.
Prepharynx absent, pharynx rounded, and intestine short, bifurcating anteriorly of the ventral sucker. Excretory bladder Y-shaped, with its arms skirting the ventral sucker posteriorly. Main collecting tubes opening at either side into the unpaired part of the bladder, close to its bifurcation. Excretory formula 2[(3 + 3 + 3) + (3 + 3 + 3)] = 36.
Renicola mediovitellata Bychowskaja-Pawlowskaja, 1950
[syn.: cercaria of Renicola thaidus Stunkard, 1964]
Type host (definitive): common pochard Aythya ferina (Linnaeus, 1758)
Other hosts (definitive): gadwalls Anas strepera Linnaeus, 1758, northern shoveler Spatula clypeata (Linnaeus, 1758), common eider Somateria mollissima (Linnaeus, 1758), long-tailed duck Clangula hyemalis (Linnaeus, 1758), harlequin duck Histrionicus histrionicus (Linnaeus, 1758), greater scaup Aythya marila Linnaeus, 1761, Eurasian wigeon A. penelope (Linnaeus, 1758), garganey A. querquedula (Linnaeus, 1758), Baikal teal Sibirionetta formosa (Georgi, 1775), and King eider Somateria spectabilis (Linnaeus, 1758) (under question) (Anatidae)
Site in definitive host: kidney tubules
Type locality: Southwest Siberia
Other localities (in definitive host): Barents Sea, Baltic Sea, Iceland, Kamchatka, Yakutia, Sea of Okhotsk, lower Yenisei, and Amur River Basin
Type material: 20 syntypes (on slides 858-1–858-8, 859-1–859-4, 860, 861-1, 860-2, 862, and 863) deposited in ZISP
Representative slides: 3743-1–3743-3 deposited in ZISP. This material represents paragenophores. 3744-1–3744-3 deposited in ZISP and 2453/Tr and 2479/Tr deposited in GELAN.
First intermediate hosts: Nucella lapillus (Linnaeus, 1758), N. freycinetii (Deshayes, 1839), and N. heyseana (Dunker, 1882) (Caenogastropoda: Muricidae) (natural)
Site in first intermediate host: gonad
Localities (in the first intermediate host): Barents Sea, SW Iceland, Northeast Atlantic, Northwest Atlantic, Sea of Okhotsk, and Sea of Japan
Second intermediate hosts: Mytilus edulis (Linnaeus, 1758), Nucella lapillus, and Argopecten irradians irradians (Lamarck, 1819)
Representative DNA sequences: cox1 (OR742770–OR742779, OR742780–OR742789 OR742791–OR742793, OR742797, and OR742800), 28S rDNA (OR735486 and OR735487), and 12S rDNA (OR735478, OR735479, and OR735480) (shown in Table 1).
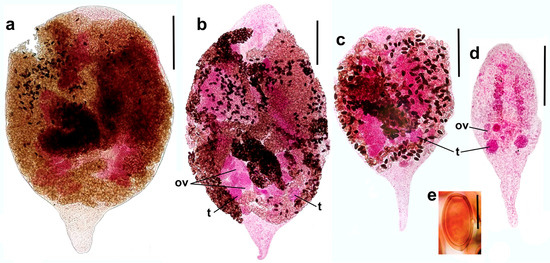
Figure 5.
Representative microphotographs of sexual adults of Renicola mediovitellata (ventral view). Syntype from slide 858-1 made by Bychovskaja-Pavlovskaja (common pochard Aythya ferina, Lake Chany) (a). Specimen heavily pressed by a coverslip from slide 3743-1 (the common eider S. mollissima, Sea of Okhotsk) (b). Specimens from slide 3743-3 (the common eider S. mollissima, Sea of Okhotsk), filled with eggs (c) and juvenile (d). Eggs of R. mediovitellata from slide 3743-3 (e). Abbreviations: ov: ovary and t: testis. Scale bars: (a) and (b) 300 µm, (c) and (d) 200 µm, and (e) 20 µm.
Adult worms were present in our own material from the common eider obtained at the Sea of Okhotsk (Shelikhov Bay, Cape Zubchatyi) and nearshore areas of SW Iceland. The adult worms from both eiders matched renicolid intramolluscan stages from Nucella spp. in the marker DNA sequences (see Section 3.2). In addition, we examined and measured syntypes of R. mediovitellata in the slides of Bychovskaja-Pavlovskaja deposited in ZISP. The description is based on all these materials.
Body ovoid, rounded anteriorly, and attenuated posteriorly. Caudal process pronounced. Size of worms varying greatly depending on the number of eggs in the uterus. Oral sucker subterminal to terminal and transversely elongated-oval. Ventral sucker small and poorly discernible in large worms with numerous eggs. Prepharynx absent and pharynx small, often deeply embedded in the wall of the oral sucker. Oesophagus short, caeca two, extending into the posterior third of the body.
Testes oval and smooth-edged or lightly lobed (Figure 5b–d). In some individuals, one testis is smooth-edged and another is lobed. Testes lying in the posterior third of the body, more or less opposite to each other. Left testis somewhat larger than the right testis. Seminal vesicle lying approximately at the level of the posterior part of the ovary, median or lightly dextral of the body midline. Ovary dextral, pretesticular, larger than testes, and variously lobed. Vitellarium follicular: follicles in two dorsal fields extended from the level of the oral sucker to the base of the attenuated posterior part of the body. In young adults, both rows of vitelline follicles are closer to the body midline and parallel to each other, extending further medially than the caeca and arms of the excretory bladder (Figure 5d). As the worm grows and eggs are accumulated, the vitelline follicles are displaced somewhat more laterally but remain located inward from lateral margins. Uterus strongly developed, occupying most of the body. Eggs numerous, with relatively thin eggshells, elongated-oval, with their length approximately twice larger than their width. Operculum well-pronounced. Small pointed knob present on the opposite egg pole (Figure 5e), more or less pronounced and indiscernible in some eggs. Excretory bladder Y-shaped, with distinct lateral diverticula and bifurcates approximately at the level of the base of the attenuated posterior part of the body, with arms extending into the forebody up to the level of the oral sucker.
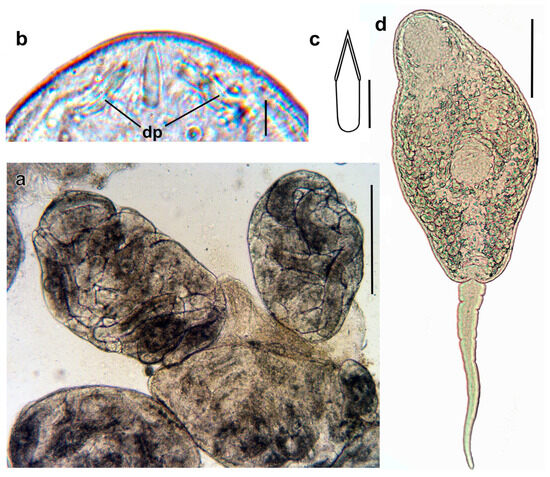
Figure 6.
Intramolluscan stages of Renicola mediovitellata. Sporocysts with embryos and fully-formed cercariae (a). Phase contrast view of the anterior end of a flattened, live cercaria, showing stylet and distal parts of ducts of penetration gland cells (b). Drawing of the stylet (c) and microphotograph of the cercariae (ventral view) (d). Abbreviations: dp: ducts of penetration gland cells. Scale bars: (a) 500 µm, (b) and (c) 5 µm, and (d) 100 µm.
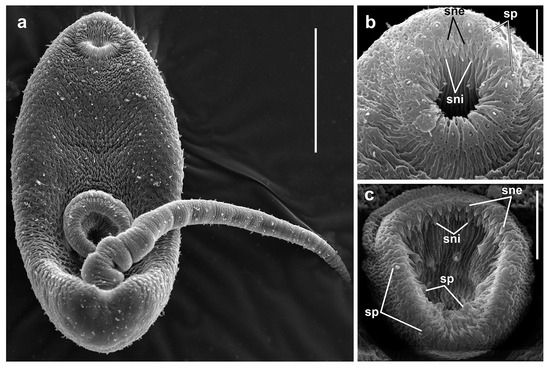
Figure 7.
Cercaria of Renicola mediovitellata. SEM microphotographs showing the cercaria (a) and spines in the oral (b) and ventral suckers (c). Abbreviations: sne: spines of external row; sni: spines of internal row; and sp: sensory papillae. Scale bars: (a) 50 µm, (b) 5 µm, and (c) 10 µm.
The intramolluscan stages of R. thaidus have been described by Stunkard (1964) from the mollusc N. lapillus in the Wood Hole area. Some additions to the description of the cercariae were made by Galaktionov and Skírnisson (2000) [7] using material from Iceland. Sporocysts elongate to oval (Figure 6a) 210–670 × 131–290 (198 ± 6.3 × 413 ± 13.9), replacing gonad tissue and forming a tumor-like structure in its place. It consists of numerous sporocysts separated by layers of host tissue (probably modified hemocytes or fibroblasts). These layers form a three-dimensional network into which sporocysts are embedded.
Cercariae somewhat smaller than the larvae of R. somateria described above. Entire body covered with spines. Stylet with weakly expressed light-refractive layer in the anterior part (Figure 6b,c). Five pairs of penetration gland cells, arranged in the same way as in the larvae of R. somateria. In some specimens, the number of ducts in each of the bundles is reversed, as recorded by Stunkard (1964) [6]. Oral sucker armed with two rows of large spines: outer row complete (26–32 spines) and inner row incomplete, comprising 11–13 spines along the anterior margin of the oral sucker (Figure 7a). Ventral sucker equatorial, armed with two alternating rows of 39–41 large spines (Figure 7b). In other respects, the cercariae of R. mediovitellata do not differ in structure from those of R. somateria.
Metacercaria (Figure 8)
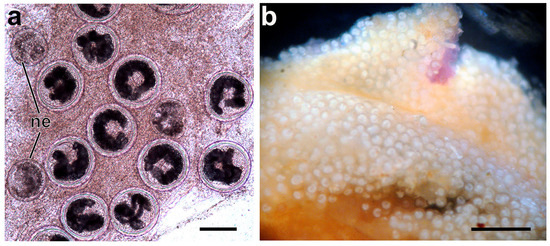
Figure 8.
Metacercariae of Renicola mediovitellata. Metacercariae in the hepatopancreas of blue mussel Mytilus edulis (a). Mass encystment of metacercariae in the same specimen of Icelandic Nucella lapillus where sporocysts develop (b). Abbreviations: ne: newly encysted metacercaria. Scale bars: (a) 100 µm and (b) 1 mm.
The cercariae encyst in gills, walls of suprabranchial chambers, mantle, and foot of M. edulis and P. irradians (Stunkard, 1964). In mussels in the White Sea, the Barents Sea, and Iceland, they were found predominantly in the hepatopancreas (Figure 8a) [21]. Cysts are spherical and 120 × 160 in accordance with Stunkard (1964) [6]. Usually, some encysted metacercariae are found in Nucella spp. molluscs infected by sporocysts with cercariae. In N. lapillus in Iceland, mass encystment of metacercariae in the same mollusc where sporocysts develop has been repeatedly observed (Figure 8b). This phenomenon has not been observed in the Barents Sea and the Sea of Okhotsk. Sporocysts and cercariae from molluscs with mass encystment (isolate 6nImetR on Figure 9, Section 3.2) did not differ from other isolates of R. mediovitellata by the cox1 marker.
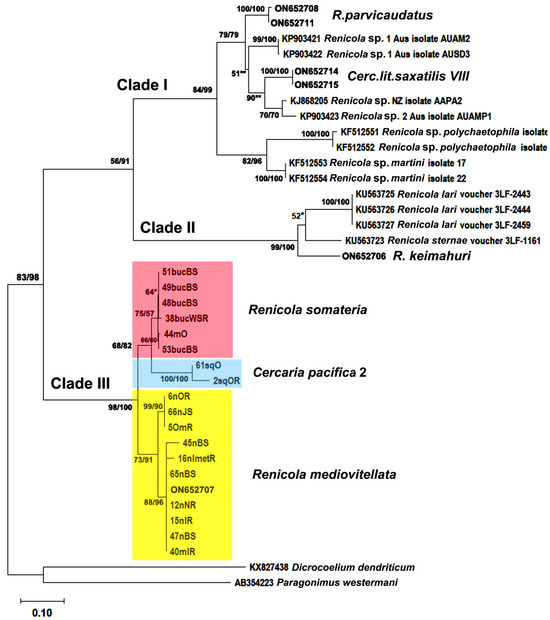
Figure 9.
Phylogenetic relationships between Renicola spp. based on maximum likelihood and Bayesian inference (BI) analyses of the cox1 gene dataset. Maximum likelihood bootstrap support values inferred from 1000 replicates are followed by posterior probabilities from BI analysis; only values ≥ 50 are shown. Asterisks indicate only bootstrap values, and double asterisks indicate only posterior probability values.
3.2. Molecular Results (Figure 9, Figure 10, Figure 11 and Figure 12 and Tables S1–S4 (Supplementary Materials))
We generated 4 new sequences for the partial D1–D3 28S rRNA gene (1159 bp), 32 new sequences for the mitochondrial cox1 gene (318 bp), and 6 new sequences for the 12S mitochondrial rRNA gene (334 bp) of Renicola spp. (Table 1). The phylogeny based on cox1 and D1–D3 28S rRNA sequences showed that the renicolids were divided into three clades (Figure 9 and Figure 10). Two of them (Clade I and Clade II) have been identified in Galaktionov et al. (2023), while Clade III was detected in this study. In Clade III, three separate species were differentiated: R. somateria, R. mediovitellata, and Cercaria pacifica 2. The topology of the trees constructed with the use of the cox1 gene and with the use of the partial 28S gene was somewhat different. In the former tree, Clade III was sister to Clade I + Clade II (Figure 9), while in the latter tree Clade III + Clade I were sister to Clade II (Figure 10). Clade III included both isolates of the intramolluscan stages of Renicola spp. from the first intermediate hosts (FIH) and adults from the DH, i.e., the common eider ducks (Table 1). Sequences of the cox1 gene and partial 28S rRNA gene of the adults from the common eider at the Sea of Okhotsk (Shkiperova Bay, 44mO), which we identified as R. somateria based on morphological characters, matched those of the intramolluscan stages isolated from B. undatum in the Barents Sea and the White Sea (Figure 9 and Figure 10 and Table 1). This means that they are lifecycle stages of the same species, R. somateria. Genetic differences by the cox1 gene (p-distances) between the samples from Europe and those from North Asia were inconsiderable, varying from 0.003 ± 0.003 (between 53BucBS and 44mO) to 0.013 ± 0.007 (between 38bucWSR and 44mO) (Stunkard 1 in Supplementary Materials).
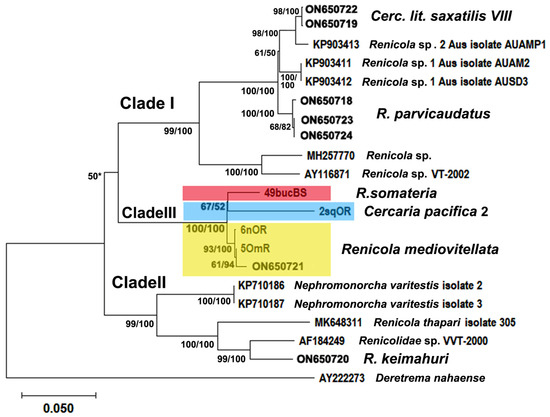
Figure 10.
Phylogenetic relationships between Renicola spp. based on maximum likelihood and Bayesian inference (BI) analyses of the D1–D3 fragments of the 28S rRNA gene dataset: phylogenetic tree reconstructed with D1–D3 fragments of the 28S rRNA gene. Maximum likelihood bootstrap support values inferred from 1000 replicates are followed by posterior probabilities from BI analysis. Asterisks indicate bootstrap support values.
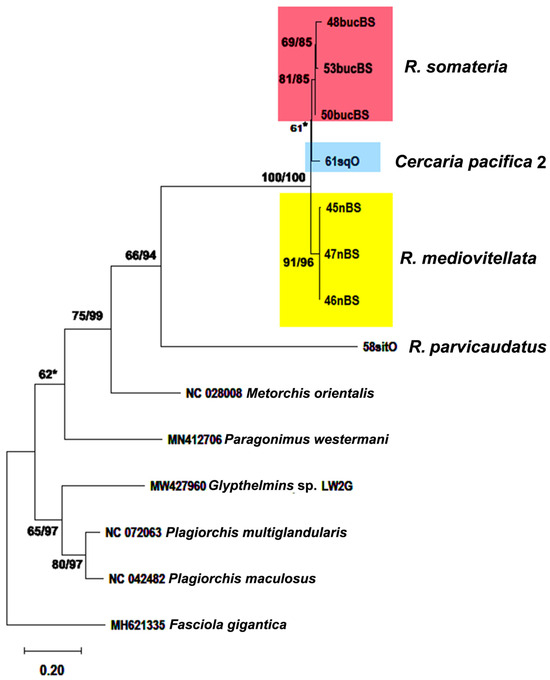
Figure 11.
Phylogenetic relationships between Renicola spp. based on maximum likelihood and Bayesian inference (BI) analyses of the mitochondrial 12S rRNA partial gene dataset. Maximum likelihood bootstrap support values inferred from 1000 replicates are followed by posterior probabilities from BI analysis. Asterisks indicate only bootstrap values.
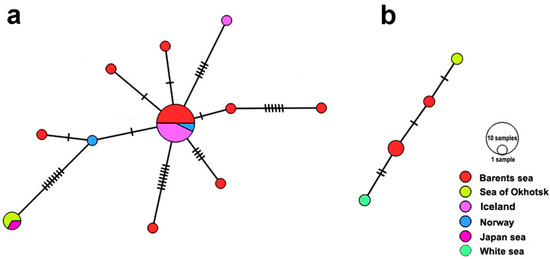
Figure 12.
A median-joining haplotype network for Renicola mediovitellata (a) and R. somateria (b) based on partial cox1 gene sequences. The colours in the haplotype network indicate sampling regions, and the circle size is proportional to the sample size. Hatch marks represent nucleotide substitutions.
Based on the sequences of the same DNA fragments, the adults of R. mediovitellata from the common eider in Iceland (40mIR) and at the Sea of Okhotsk (Cape Zubchatiy, 5OmR) matched the sequences of the intramolluscan stages from Nucella spp. obtained from European and North Asiatic seas (Figure 9 and Table 1). This matching indicates that in this case as well, we are dealing with lifecycle stages of the same species, R. mediovitellata. The pairwise genetic distances of the cox1 gene between North Pacific (NP) and North Atlantic (NA) isolates of R. mediovitellata varied from 0.028 ± 0.009 to 0.047 ± 0.012 (Table S1 in Supplementary Materials), corresponding to the intraspecific level of variation, while the mean intragroup distance in R. mediovitellata was 0.02 ± 0.004 (Table S2 in Supplementary Materials). In the 28S tree (Figure 10), the isolate 6nOR was also separated from 5OmR and 10nIR (ON650721.1 in Figure 10), but the genetic distance of the partial 28S rRNA gene was also very low: 0.011 ± 0.003 for 10nIR and 6nOR and 0.004 ± 0.002 for 6nOR and 5OmR. The phylogeny reconstructed using partial D1-D3 28S rRNA gene (Figure 10) and 12S mitochondrial gene (Figure 11) confirmed the separation of the R. mediovitellata clade. Genetic distances between R. mediovitellata and the other species corresponded to the interspecific level: from 0.029 ± 0.005 to 0.146 ± 0.011 (28S rRNA), 0.080 ± 0.014 to 0.289 ± 0.025 (cox1), and 0.057 ± 0.011 to 0.385 ± 0.027 (12S rRNA).
The third species of Clade III corresponds to the NP isolates of the intramolluscan stages of renicolids from Littorina squalida Broderip & G. B. Sowerby I, 1829. Based on cercarial morphology, these specimens were identified as Cercaria pacifica 2 Pois, Tsimbaljuk & Ardasheva, 1974, which was first described in the Sea of Japan (Pois et al., 1974). Isolates of Cercaria pacifica 2 formed a sister branch to R. somateria, with weak bootstrap values for the 28S rDNA gene and 12S mitochondrial small subunit RNA gene (Figure 10 and Figure 11). The genetic distances between C. pacifica 2 and R. somateria were 0.088 ± 0.016 for cox1, 0.049 ± 0.011 for 12S, and 0.068 ± 0.007 for 28S (Tables S2 and S4 in Supplementary Materials). This means that these species are separate, although closely related. P-distances based on the cox1 gene between 2sqOR (intramolluscan stages from N. freycinetii, northern part of the Sea of Okhotsk) and 61sqO (intramolluscan stages from N. heyseana, the Sea of Japan) isolates (0.032 ± 0.010) corresponded to intraspecific genetic diversity (Table S1 in Supplementary Materials).
Renicola somateria and R. mediovitellata are phylogenetically closer to each other according to the D1-D3 fragments of 28S rRNA (0.029 ± 0.005) (Table S3 in Supplementary Materials) and cox1 (0.080 ± 0.014) (Table S2 in Supplementary Materials) genes. However, according to the 12S mitochondrial rRNA gene, R. somateria is closer to C. pacifica 2, as shown by the genetic distances: 0.049 ± 0.011 between R. somateria and C. pacifica 2 and 0.057 ± 0.011 between R. somateria and R. mediovitellata (Table S4 in Supplementary Materials).
We detected 11 haplotypes in R. mediovitellata, which were arranged in a “star” network (Figure 12). The main dominant haplotype comprised only European isolates from Iceland, Norway, and the Barents Sea. The other European haplotypes were separated from the central haplotype by 1–8 substitutions. All NP isolates represented a single haplotype, which was separated from the closest Norwegian isolate by eight substitutions. The haplotype network of R. somateria was less informative because we had only six isolates at our disposal. We detected four haplotypes, which were separated from each other by 1–4 substitutions. The isolate from the Sea of Okhotsk was separated from the isolate from the Barents Sea by one substitution, while the isolates obtained in geographically nearest areas, the Barents Sea and the White Sea, were separated by 2–3 substitutions. The Tajima’s D value for R. mediovitellata was 102.812 (p = 0), indicating that the population is under the influence of balancing selection. A neutrality test was not performed for R. somateria because of the small sample size.
3.3. Remarks
3.3.1. Adults
Our molecular results provide convincing evidence that R. somateria and R. mediovitellata are independent species and that FIH of the former are caenogastropods B. undatum, while FIHs of the latter are muricid snails Nucella lapillus (Iceland, Barents Sea), N. freycinetii (Sea of Okhotsk), and N. heyseana (Sea of Japan). Previously, Skírnisson et al. [5] have shown, using the molecular marker ITS1 rDNA, that the cercaria of R. thaidus from the Icelandic mollusc N. lapillus is the larva of the adult from the common eider identified as R. somateria. In light of the new information obtained in this study, we can be sure that the adult from Skírnisson et al. [5] belongs to R. mediovitellata.
The adults of R. somateria and R. mediovitellata are morphologically similar, the most distinct differences being in the shape and size of eggs and, especially, in eggshell thickness. In addition, the eggs of R. mediovitellata have a well-pronounced operculum and a knob on the opposite pole. Gubanov and Ryzhikov [22] considered this knob as a characteristic trait of R. mediovitellata. However, the same knob was described in eggs of Renicola pseudosloanei Odening, 1962 and R. ardeolae Khalifa et El-Naffar, 1975 [23,24]. Both these species are otherwise strikingly different from R. mediovitellata. R. pseudosloanei, a parasite of alcids and gaviids, has lobed testes lying close together dorsally of the ventral sucker. Judging by this character, as well as by the range of the DH (sea birds), this species, as well as the closely related R. sloanei Wright, 1954, is likely to belong to Clade II (see [1]). R. ardeolae, which has been reported only from the western cattle egret (Bubulcus ibis [Linnaeus, 1758]), and has two tandem testes, while its vitellaria are arranged in two dorso-lateral fields along the entire length of the caeca up to the level of intestinal bifurcation [24].
The adults of R. brantae McIntosh et Farr, 1952 have been described from the Canada goose (Branta canadensis [Linnaeus, 1758]) in Northern Carolina [25]. The description is incomplete (see Table 3), and no drawing is given. Later, Wright [26] examined paratypes of R. brantae and provided schematic drawings accompanied by a brief description. He noted the similarity of R. mediovitellata and R. brantae and assigned them to the same species group, “mediovitellata” [26]. According to the measurements (Table 3) and the combination of morphological characters (vitellaria rather medial, in the form of two dorsal rows of follicles extending from the oral area to the base of the caudal process, ovary lobed, testes rounded, and eggs elongated, with their length approximately twice greater than width), R. brantae is indistinguishable from R. mediovitellata. In our opinion, the name R. brantae should be considered a junior synonym of R. mediovitellata.
R. mollissima is a parasite of the common eider in the White Sea and Chukotka [27,28]. Its eggs are similar in size to those of R. mediovitellata, and it has two rows of vitelline follicles, which, as in R. mediovitellata, run dorsally along the intestinal caeca. However, in contrast to R. mediovitellata, these rows do not reach the base of the caudal process and end approximately at the level of the testes [27]. In young adults of R. mollissima, vitelline follicles are arranged in a compact group at the sides of the body near the gonads. They do not form two longitudinal lateral rows as they do in young adults of R. mediovitellata and R. somateria [27].
Eggs similar to those described in this paper for R. somateria have been described in only one other species of this genus, R. ovocallosa Reimer, 1971, which has rounded (32–35 × 25–27) eggs with a very thick eggshell (4.5) [29]. Renicola ovocallosa has been recorded in East Germany in various birds, including long-tailed duck (Clangula hyemalis). Renicola somateria has also been reported from these ducks in the White Sea (see species description above). However, R. ovocallosa differs from R. somateria in the shape and position of the vitellaria, which form two compact medio-lateral fields in the equatorial part of the body.
3.3.2. Cercaria
Molecular data are available for several species of renicolid cercariae [1,5,30,31,32,33,34,35]. However, all of them are genetically distinct from the larvae considered in this paper and belong to Clade I or Clade II in accordance with [1] (Figure 9 and Figure 10).
As noted above, the cercariae of R. somateria and R. mediovitellata are morphologically similar, except for the shape and size of the stylet (Figure 3b,c and Figure 6b,c). They can be easily differentiated based on this character as well as the FIH (Nucella spp. for the former and B. undatum for the latter). In addition, the cercariae of these species differ in the number of spines on the edges of the oral and ventral suckers. It seems that the cercaria described by Køie [36] as “Renicola cercaria from B. undatum” by Gullmarsfjorden (Skagerrak strait) is actually the larva of R. somateria (see Table 4). Unfortunately, it is impossible to identify the species of that cercariae because Køie [36] did not provide either a drawing or a description of the stylet and penetration gland cells. Cercaria nordica I Marasaev, 1988 is also similar to that of R. somateria in morphology and size. The intramolluscan stages of this species were recorded in the benthic mollusc Neptunea borealis (R. A. Philippi, 1850) (Buccinidae) in the southeastern part of the Barents Sea [37], i.e., in a host species closely related to B. undatum. While the stylet shape in the cercariae of these two species is almost the same, they differ in the number of penetration gland cells: 3 pairs (3 + 3) or 3 + 4 in Cercaria nordica I and 5 (2 + 3) pairs in the larvae of R. somateria.
The cercaria of Renicola sp. Alda et Martorelli, 2014 from the snail Heleobia australis (A. d’Orbigny, 1835) (Cochliopidae) from the Bahía Blanca estuary, Argentina differs from the cercaria described in this paper by the species of FIH and by the presence of three rows of spines on the anterior border of the oral sucker, four pairs of penetration gland cells with ducts opening on both sides of the stylet, with a common bundle and the stylet whose light-refracting edges almost reach its base [38]. Xiphidiocercaria sp. 2 Etchegoin & Martorelli, 1998 from Heleobia conexa (M. C. Gaillard, 1974) in the Mar Chiquita coastal lagoon, Argentina [39] is similar to Renicola sp. (it differs from it only by the stylet width) and, therefore, cannot belong either to R. somateria or R. mediovitellata.
Cercaria pacifica 2 Pois, Tsimbaljuk et Ardasheva, 1974 from the littoral mollusc Littorina squalida in the Sea of Japan has a similar stylet structure and the same number of penetration gland cells (five pairs) as the larva of R. somateria [40]. Infection of Littorina spp. molluscs with C. pacifica 2 in the Sea of Okhotsk is not uncommon (KG, personal observations). In our molecular trees, C. pacifica 2 isolate is a member of Clade III and appears to be a sister taxon to R. somateria. Genetic differences between C. pacifica 2 and R. somateria by all molecular markers used in our work correspond to the interspecies level. Therefore, there is no doubt that C. pacifica 2 is a larva of some other Renicola sp., not R. somateria.
Rybakov [41] found renicolid cercariae Renicola sp. II and Renicola sp. III in the Sea of Japan in molluscs Batillaria cumingii (Crosse, 1862) and Cryptonatica janthostoma (Deshayes, 1839), respectively. The stylet of these larvae, which is small and has distinct cutting edges, is similar to that of the cercariae of R. somateria. Rybakov [41] did not provide the stylet size, but judging from the figures (Figure 208, p. 323 and Figure 211, p. 324 in [41]), its cutting edges almost reach its base in the cercariae of both species. This distinguishes them from the larvae of R. somateria, in which this part of the stylet occupies about 2/3 of its length. In addition, the FIHs of the two cercariae described by Rybakov [41] are very different from the FIH of R. somateria.
The cercaria of R. mediovitellata has been described by Stunkard [6] as R. thaidus from the mollusc N. lapillus from the Woods Hole region. Galaktionov et al. [1] showed that the adult of R. thaidus described by Stunkard [6] was morphologically identical to R. parvicaudatus (Stunkard & Shaw, 1931) [1], which means that the name R. thaidus is a junior subjective synonym of R. parvicaudatus. The cercaria that Stunkard [6] considered to be the larva of R. thaidus corresponds in all aspects to the larva of R. mediovitellata described in this paper. Thus, the name R. thaidus should be considered as nomen superfluum.
There are also several renicolid xiphidiocercariae on which molecular data are lacking, e.g., Cercaria opaca Holliman, 1961, Cercaria caribbea XXXII Cable, 1956, and C. caribbea XXXIII Cable, 1956 [42,43]. They differ from the larvae described in the paper in the number of penetration gland cells: three pairs in C. caribbea XXXII, six pairs in C. caribbea XXXIII, and numerous in Cercaria opaca [42,43]. The FIH species are also different: Neritina virginea (Linnaeus, 1758) for the former two cercariae and Littoraria irrorata (Say, 1822) for the latter cercaria.
4. Discussion
In this study, we elucidated the composition of Renicola spp. parasitising the common eider and other anatids associated with or occasionally visiting nearshore areas of northern Holarctic seas. Using molecular and morphological criteria, we substantiated the status of R. somateria and supplemented the description of R. mediovitellata. The new information on the latter species allowed us to synonymize R. brantae with it. We described the range of intermediate hosts of R. somateria and R. mediovitellata, which are represented by marine molluscs inhabiting the nearshore areas of Arctic seas and seas of temperate latitudes.
Considering that its intermediate hosts are marine molluscs, it might seem strange that R. mediovitellata was discovered in anatids so far from the sea. Even its first description was based on individuals from ducks taken near Lake Chany in southwestern Siberia [20]. However, these ducks make long-distance seasonal migrations to wintering sites. For instance, the common pochard (Aythya ferina) from southwestern Siberia has been recorded at wintering sites in Western Europe (the British Isles, coasts of Ireland, and Atlantic coast of France) [44,45], and it is from the common pochard that the greatest number of individuals of R. mediovitellata used for the first species description were isolated (26 ind. on 13 slides out of the total 30 ind. on 17 slides, as shown by our examination of the slides made by Bychovskaja-Pavlovskaja and stored as syntypes in ZISP). The northern shoveler (Spatula clypeata), nesting near Lake Chany and listed as a DH of R. mediovitellata by [20], was noted at the wintering site on the Atlantic coast of France and Belgium [44]. Judging from the ringing data, most individuals of gadwall (Anas strepera) nesting near Lake Chany spend the winter in the Caspian Sea, India, and Pakistan [44]. However, there are also wintering sites for gadwall in Western Europe (the British Isles and coasts of Ireland, France, and Spain) [45], and some of the birds nesting in western Siberia apparently reach these sites during seasonal migrations.
The distribution range of the mollusc N. lapillus, which are FIH of R. mediovitellata, extends along the entire European coast from the Barents Sea to the Bay of Biscay [46]. Considering that adult renicolids have a life span of several months [47], this means that the ducks may become infected at the wintering sites and then carry the parasites to the nesting sites. It is noteworthy that there is not a single juvenile or young adult on the slides of R. mediovitellata from ducks taken near Lake Chany made by Bychovskaja-Pavlovskaja and stored in ZISP. All adults are so densely packed with eggs that the internal organs are almost indiscernible (see Figure 5a). This indicates that they were infected a long time ago, apparently at the wintering sites. Other duck species in which adults of R. mediovitellata have been recorded in Chukotka, Yakutia, Kamchatka and the Amur River basin (see above) either spend most of their life in the sea (the common eider, long-tailed duck) or are associated with the sea coast during seasonal migrations and/or wintering [45,48,49].
R. mediovitellata has a broader range of DH (10 species) than R. somateria (4 species). So far, all reliable reports of the latter are from the sea ducks capable of feeding at depths (the common eider can dive down to 60 m), in the subtidal zone [50]. This is the habitat of Buccinum spp. molluscs, FIH of R. somateria. Infected whelks shed cercariae, which then mostly infect bivalve molluscs in the same habitat, the subtidal. This means that the bivalves infected with metacercariae of R. somateria are almost inaccessible for other ducks, which mostly feed at the intertidal. In contrast, muricid Nucella spp. molluscs, FIH of R. mediovitellata, occur in the intertidal zone, and the cercariae shed by them mostly infect bivalves there and in the upper subtidal. In this way, infective metacercariae may reach a fairly broad range of anatids sporadically visiting the coast and foraging in shallow areas.
A curious feature of infection of N. lapillus with the intramolluscan stages of R. mediovitellata in Iceland is the encystment of numerous cercariae in the same mollusc in which they have been formed in the daughter sporocysts (Figure 8b). This phenomenon, very common in Iceland, has never been observed either in the Barents Sea or in the Sea of Okhotsk (KG, personal observations). Based on this feature, Galaktionov and Skírnisson [7] identified two forms of R. mediovitellata (R. thaidus in [7]) in Iceland: form A (almost never encysts in FIHs) and B (encysts in FIHs in large numbers). A comparison of the molecular marker cox1 of these forms (form B—6nImetR and form A—all other isolates from Nucella spp.) did not reveal any differences between them (Figure 9). This feature seems to be an ecological adaptation, which possibly emerged because of the scarcity of mussels, the main second intermediate host (SIH) of R. mediovitellata, in the Icelandic intertidal zone.
4.1. Taxonomy
All renicolids for which molecular data are available are clearly distributed across three large clades in the phylogenetic trees ([1]; Figure 9, Figure 10 and Figure 11). Renicola somateria and R. mediovitellata form Clade III, which also includes C. pacifica 2. The position of Clade III is uncertain: it shows as a sister to Clade I + Clade II in the tree based on the cox1 gene and as a sister to Clade II in the tree based on the 28S rDNA gene (see Figure 9 and Figure 10). An analysis involving more renicolid species is necessary to verify its position.
Renicola somateria and R. mediovitellata differ from the known members of Clade I and Clade II by the medio-lateral position of vitellaria, which form two dorsal longitudinal rows of follicles passing along the caeca and the branches of the excretory bladder or slightly more medially. These two species use aquatic birds associated with the seacoast (and so does, possibly, C. pacifica 2, whose DHs are still unknown) as DHs. A similar combination of characters is found in R. mollissima, which is likely to be included in Clade III after an analysis of molecular markers (at present, unfortunately, we have no material on this species).
It would be premature to assign any taxonomic status to the three clades of Renicolidae identified based on the molecular markers because molecular data are available only on a few members of this group [1,5,30,31,32,33,34,35,51,52,53,54,55]. These clades probably correspond to different genera or even groups of genera. The accumulation of molecular data on more renicolid species would ultimately solve the problem of the taxonomic differentiation of this family, which now contains only two genera: a small genus Nephromonorcha (5 species in accordance with WoRMS) and a large genus Renicola (44 species in accordance with WoRMS). Earlier attempts to divide Renicola on the basis of morphological characters [2,56,57] proved controversial [58].
The known members of the three renicolid clades differ considerably, both in the morphology of adults and cercariae and in the range of the hosts [1]. Taking into account the results of this study, their characters can be summarized as follows:
Clade I (“Parvicaudata” group). Xiphidiocercariae (small styleted cercariae with a simple tail); in adults, testes separate and non-contiguous and vitellaria arranged in a compact group at the base of the caudal process. FIHs—coastal caenogastropods; SIHs—bivalves, occasionally caenogastropods; and DHs—predominantly gulls.
Clade II. Cercariae of the Rhodometopa group (large non-styleted larvae with tail fins) or non-styleted forms of the transitional morphotype from typical xiphidiocercariae to cercariae of the Rhodometopa group; testes contiguous or fused to form a single mass; vitellaria lateral to caeca in the middle third of the body. FIHs—sublittoral caenogastropods, SIHs—fishes and presumably bivalves, DHs—different taxa of seabirds, such as auks, gulls, petrels, shags, penguins, etc.
Clade III. Xiphidiocercariae; in adults, testes separate and non-contiguous and vitellaria arranged medio-laterally, forming two longitudinal rows dorsally of caeca and branches of excretory bladder. FIHs—caenogastropods; SIHs—bivalves, occasionally caenogastropods; and DHs—sea ducks and other anatids visiting sea coasts.
4.2. Distribution and Phylogeography
An analysis of molecular markers showed that the genetic variation of specimens of R. somateria and R. mediovitellata from different geographic areas did not extend beyond the intraspecific level (Table S1 in Supplementary Materials). Both species appear to have a Holarctic distribution. The greatest number of findings has been reported from Eurasia, probably because this region is better explored than North America. Intramolluscan stages of R. mediovitellata (as R. thaidus; see Remarks for synonymy) have been found in mollusc N. lapillus at the Atlantic and the Pacific coasts of North America [6,59], while adults of R. brantae, which we synonymized with R. mediovitellata, have been found in the Canada goose in Northern Carolina [25]. Apparently, R. somateria and/or R. mediovitellata (as Renicola sp.) have been recorded in the common eider in Newfoundland and Labrador by [60].
Since only a few specimens of R. somateria were involved in the haplotype analysis by the mitochondrial cox1 gene, we cannot be entirely sure that this species is represented by the same population in NA and NP (Figure 12b). Nevertheless, it is highly likely that this is the case, considering that the haplotypes from the Barents Sea and the Sea of Okhotsk differed only by one substitution (Figure 12b). At the same time, the haplotype network for R. mediovitellata indicates that this species is clearly differentiated genetically in NA and NP populations (Figure 12a). This differentiation can be also seen in the tree constructed with the use of the more conservative 28S rDNA sequences (Figure 10).
At first glance, this difference between the two sister species might seem strange, considering that they use the same DH and apparently do not differ in the lifespan of adults. If R. somateria is indeed represented by a single circum-Arctic population, there should be a fairly lively gene flow between its parts in NA and NP. At the same time, eastern and western populations of anatids differ both in breeding grounds and in flyways. During breeding, waterfowl in northern East Asia migrate along the East Asian Flyway, a migratory corridor passing along the Asiatic coasts towards the southern limits of Australia and New Zealand [49,61]. Birds nesting in Western Siberia and the European North of Russia make seasonal migrations along the European seaboard towards Africa via the East Atlantic Flyway [62,63]. Other flyways, such as the Central Asian Flyway or the Western Asian–East African Flyway, pass over continental land and tropical seashores, where the intermediate hosts of R. somateria and R. mediovitellata are absent.
Breeding grounds of the waterfowl of western and eastern populations overlap in Taymyr [64,65,66,67]. Provided there are suitable intermediate hosts in the coastal areas of this peninsula, an exchange of parasites brought by birds from NA and NP should be possible. This appears to be the case with R. somateria. Its FIH, the mollusc B. undatum, is an NA Arctic-boreal species not distributed further northeastward than the Barents Sea [46,68,69]. However, our findings for young adults of R. somateria in the Sea of Okhotsk population of the common eider indicate that this parasite is transmitted in the coastal area of the Sea of Okhotsk. There, the role of its FIH is played by some other molluscan species. Various Buccinum spp. occur throughout the Arctic, including the Taymyr coast [68,69], and it is fairly certain that one or more of them serve as the FIH for R. somateria.
A different situation is observed in the case of R. mediovitellata, whose FIHs are boreal Nucella spp. molluscs. As an Atlantic species, N. lapillus is restricted in its northward distribution along the European coast by the Barents Sea and along the North American coast by the southern part of the Labrador Peninsula [46,68]. Pacific Nucella spp. do not advance further northward along the Asian and American coasts than the Bering Strait [46,69]. Therefore, the transmission of R. mediovitellata is currently impossible everywhere in the Siberian seas, along the Arctic coast of Alaska, and in the Canadian Arctic Archipelago. This circumstance must have determined the formation of genetically different populations in NA and NP. They were probably isolated during the Last Glacial Maximum and, in contrast to R. somateria (see above), isolation has persisted after the glacial retreat.
A “star” network of R. mediovitellata in NA, in which the central and most common haplotype is probably the ancestral one [70,71], indicates a relatively recent expansion and/or bottleneck event. The latter may have occurred during the Last Ice Age in the glacial refugium when this species began to disperse into NA after glacial retreat. It is noteworthy that the differences in mutation steps between the central haplotype and some NA haplotypes are of the same level as the differences between the latter and the NP haplotype (Figure 12a). This heterogeneity is probably due to the fact that the population, judging from Tajima’s D value (see Section 3.2), is under the influence of balancing selection. Haplotypes strongly different from the dominant one could have arisen due to the temporary isolation of subpopulation groups or due to intraspecific specialization to the use of different waterfowl species as the DH (see Results). For NP, we have data only for three isolates with matching cox1 haplotypes (Figure 12a). Given that two of them are from the Sea of Okhotsk and one is from the Sea of Japan, we can hypothesize that, similar to NA, there is a dominant haplotype in NP and that it could also have formed in the glacial refugium during the Last Ice Age. The causes of the formation of genetic variation in R. mediovitellata can be elucidated only after the analysis of a greater number of samples from NA and, especially, from NP.
A haplotype network similar to that for R. mediovitellata has been obtained for the notocotylid digenean Tristriata anatis (Notocotylidae) [72,73]. This parasite of anatids has long-living adults, but the distribution of its FIH, i.e., littoral snails Littorina spp., is separated from the Eurasian side by the Siberian seas and from the American side by the Arctic coast of Alaska and the Canadian Arctic Archipelago. Like R. mediovitellata, T. anatis occurs both in NA and NP, where it is represented by genetically distinct populations [73]. At the same time, a haplotype of R. parvicaudatus from the Sea of Okhotsk coincides with the dominant haplotype of this species in NA [1], and R. parvicaudatus also uses Littorina spp. as the FIH. The possibility of genetic exchange between NA and NP groups of R. parvicaudatus may be associated with the behaviour of its DH (various species of gulls), such as their ability for long-distance migrations (see [1] for details). Recent information about trans-Arctic flights of marine and coastal birds [74,75,76,77,78] suggests that trans-Arctic transfer of parasites is possible, particularly for the species with long-living adults capable of surviving a long flight. This is precisely the case of the renicolids discussed here. Trematodes of marine and coastal birds that have short-living adults and use molluscs with disrupted distribution in the Arctic seas (such as microphallids of the “pygmaeus” group (Microphallidae)) as FIHs, form sister species in NA and NP [79]. Sister species in NA and NP are also characteristic of Parvatrema spp. (Gymnophallidae), which have a narrow specificity to the SIH (with only one exception: the FIHs of Parvatrema spp. are currently unknown) [80]. However, if the range of the FIH includes the Arctic seas, species with short-living adults may also have an amphiboreal distribution, as is the case, e.g., with Microphallus pseudopygmaeus Galaktionov, 1980 (Microphallidae) from the “pygmaeus” group of microphallids [79].
We may conclude that the potential for geographic expansion of trematodes parasitising marine and coastal birds is also determined by the most vagile host in the life cycle, which has traditionally been considered a major factor (e.g., [81,82,83,84,85,86,87]). The distribution of FIHs, which are obligatory and the most narrowly specific hosts in the life cycle of these parasites, appears to be equally significant [1,9,73,79,88,89]. In addition, the distribution of the parasites with a narrow specificity to the SIH may also be limited by that of the SIH, such as e.g., in Parvatrema spp., whose parthenogenetic metacercariae develop in the SIH [80]. However, the specificity of digeneans to the SIH is usually much lower than to the FIH, and this role may be played by several animal species with a different distribution. Therefore, the importance of the SIH in limiting the distribution of a particular digenean species is lower than that of the FIH.
Yet another factor affecting the distribution of trematodes is the lifespan of the adults in the DH (birds in the case under consideration). The longer the lifespan, the more likely the expansion of the parasites to new geographic areas, together with their mobile hosts. An extensive distribution area of the parasite may be formed in this way, for example, in the renicolids examined in our study.
5. Conclusions
The use of an integrative approach made it possible to clarify the species composition and elucidate the life cycles of renicolids parasitising ducks in the northern Holarctic. We resolved the ambiguity in the species diagnosis of R. somateria and differentiated this species from the closely related R. mediovitellata. We note that records of R. somateria in faunistic studies of duck parasites should be treated with caution. Our data confirmed an earlier hypothesis that all renicolids for which molecular data are available are distributed across three major clades [1]. Members of these clades differ in the structure of adults and cercariae, the features of transmission, and the taxa of intermediate and definitive hosts involved in their life cycles. The role of the DH for known members of Clade I is played by larids, while within Clade II, there was a transition to the use of fish-eating seabirds as the DH and the involvement of fish as the SIH. Anatids are used as DHs only by species forming Clade III. The transition to parasitism in ducks could be the driver of the evolution of this renicolid group.
Many renicolids, including those discussed in this paper, use migratory birds as the DH. These species have a wide geographical distribution, which, in our opinion, is facilitated by the long life span of adult worms. As a result of the high mobility of migratory birds used as DHs, the parasite can be found in geographical regions and biotopes where its transmission is impossible, e.g., Western Siberia for R. mediovitellata and Yakutia for R. somateria. However, we propose to consider the actual distribution area of the parasite as the geographical region where its transmission is possible, i.e., where all categories of hosts involved in its life cycle are present. This means that the distribution area is primarily determined by sedentary molluscan hosts (FIHs), to which trematodes have a narrow specificity. For example, the distribution area of R. mediovitellata and R. somateria is confined to the coasts of the northern Holarctic seas, while mainland regions such as Western Siberia and Yakutia are best considered as zones of occurrence. By the same token, while Renicola lari Timon-David, 1933 has been recorded in Central and North Europe [2,4,56], the range of this parasite is the Mediterranean Sea, where its FIHs, i.e., Mediterranean molluscs Cerithium lividulum Risso, 1826 and C. vulgatum Brugiuère, 1792are distributed [90].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16070402/s1, Table S1: Table of pairwise genetic divergence between isolates of Renicola, Paragonimus, and Dicrocoelium species. Table S2: Table of mean genetic divergence between renicolid species estimated as the p-distance for the fragment of the cox1 mitochondrial gene. Table S3: Table of mean genetic divergence between Renicola and Nephromonorcha species, estimated as the p-distance for the D1-D3 fragment of the 28S rRNA gene. Table S4: Table of mean genetic divergence between Renicola, Paragonimus, and Plagiorchis species, estimated as the p-distance for the mitochondrial 12S rRNA gene.
Author Contributions
Conceptualization, K.V.G. and A.I.S.; Methodology, K.V.G., A.I.S. and A.A.M.; software, A.I.S., K.V.G., A.A.M. and A.E.R.; validation, K.V.G., A.I.S., K.S., A.A.M. and A.E.R.; writing—original draft preparation, K.V.G. and A.I.S.; writing—review and editing, K.V.G. and A.I.S.; visualization, K.V.G., A.I.S. and K.S.; supervision, K.V.G.; and funding acquisition, K.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Russian Science Foundation (23-14-00329). Sampling at the Barents Sea and the White Sea and work at the “Taxon” Research Resource Center were partly financed by the State Academic Program (122031100260-0). The funders had no role in the study design, data collection, analyses, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available from the authors upon request, and the sequences used in this study are available in the GenBank database: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 10 June 2024), OR742770–OR742800, OR735478–OR735484, and OR735485–OR735487.
Acknowledgments
The authors are grateful to the White Sea Biological Station of the Zoological Institute of the Russian Academy of Sciences (ZIN RAS), Institute of Biological Problems of the North of the Far East Branch of the Russian Academy of Sciences (IBPN RAS) and the Institute for Experimental Pathology, University of Iceland (IEPUI) for providing fieldwork infrastructure. We thank Gennady Atrashkevich, Kira Regel, Kirill Nikolaev, Ivan Levakin, Anastsija Smoljaninova and Georgy Kremnev for their help with sampling and primary treatment of the material. We wish to acknowledge the IEPUI, “Taxon” Research Resource Center (http://www.ckp-rf.ru/ckp/3038/, accesed on 10 June 2024) of ZIN RAS and the research resource Centre “Molecular and Cell Technologies” of St. Petersburg State University for granting access to their facilities. We are grateful to Natalia Lentsman for her help with the manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Galaktionov, K.V.; Solovyeva, A.I.; Blakeslee, A.M.H.; Skírnisson, K. Overview of renicolid digeneans (Digenea, Renicolidae) from marine gulls of northern Holarctic with remarks on their species statuses, phylogeny and phylogeography. Parasitology 2023, 150, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Sudarikov, V.E.; Stenko, R.P. Trematodes of the family Renicolidae. In Helminths of Farming and Hunting Animals; Nauka: Moscow, Russia, 1984; pp. 34–89. (In Russian) [Google Scholar]
- McDonald, M.E. Key to Trematodes Reported in Waterfowl; Resource, Publication 142.; U.S. Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 1981. [Google Scholar]
- Belopolskaja, M.M. Parasites of marine waterfowl. Uch Zap. Leningr. Gos. Univ. Ser. Biol. Nauk. 1952, 141, 127–180. (In Russian) [Google Scholar]
- Skirnisson, K.; Guðmundsdóttir, B.; Andrésdóttir, V.; Galaktionov, K.V. ITS1 nuclear rDNA sequences used to clear the life cycle of the morphologically different larvae and adult renicolid (Renicola, Digenea) parasites found in Iceland. Bull. Scand. Soc. Parasitol. 2003, 12, 50. [Google Scholar]
- Stunkard, H.W. Studies on the Trematode genus Renicola: Observations on the life-history, specificity, and systematic position. Biol. Bull. 1964, 126, 467–489. [Google Scholar] [CrossRef]
- Galaktionov, K.V.; Skirnisson, K. Digeneans from intertidal molluscs of SW Iceland. Syst. Parasitol. 2000, 47, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Costa, I.; Poulin, R. Parasite life-cycle studies: A plea to resurrect an old parasitological tradition. J. Helminthol. 2017, 91, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Galaktionov, K.V.; Solovyeva, A.I.; Miroliubov, A. Elucidation of Himasthla leptosoma (Creplin, 1829) Dietz, 1909 (Digenea, Himasthlidae) life cycle with insights into species composition of the north Atlantic Himasthla associated with periwinkles Littorina spp. Parasitol. Res. 2021, 120, 1649–1668. [Google Scholar] [CrossRef]
- Winnepenninckx, B.; Backeljau, T.; De Wachter, R. Extraction of high molecular weight DNA from molluscs. Trends Genet. 1993, 9, 407. [Google Scholar] [CrossRef]
- Chan, A.H.E.; Saralamba, N.; Saralamba, S.; Ruangsittichai, J.; Thaenkham, U. The potential use of mitochondrial ribosomal genes (12S and 16S) in DNA barcoding and phylogenetic analysis of trematodes. BMC Genom. 2022, 23, 1–13. [Google Scholar] [CrossRef]
- Palm, H.W.; Waeschenbach, A.; Olson, P.D.; Littlewood, D.T.J. Molecular phylogeny and evolution of the Trypanorhyncha Diesing, 1863 (Platyhelminthes: Cestoda). Mol. Phylogenet. Evol. 2009, 52, 351–367. [Google Scholar] [CrossRef]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.; Teslenko, M. Draft MrBayes Version 3.2 Manual: Tutorials and Model Summaries; Institute Pasteur: Paris, France, 2018; p. 180. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Rozas, R. DnaSP, DNA sequence polymorphism: An interactive program for estimating population genetics parameters from DNA sequence data. Comput. Appl. Biosci. 1995, 11, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Bychovskaja-Pavlovskaja, I.E. New species of kidney parasites (genus Renicola) from birds. Dokl. Akad. Nauk SSSR 1950, 71, 415–416. (In Russian) [Google Scholar]
- Galaktionov, K.V.; Bustnes, J.O.; Bårdsen, B.-J.; Wilson, J.G.; Nikolaev, K.E.; Sukhotin, A.A.; Skírnisson, K.; Saville, D.H.; Ivanov, M.V.; Regel, K. V Factors influencing the distribution of trematode larvae in blue mussels Mytilus edulis in the North Atlantic and Arctic Oceans. Mar. Biol. 2015, 162, 193–206. [Google Scholar] [CrossRef]
- Gubanov, N.M.; Ryzhikov, K.M. To trematode fauna of the anserine birds of Verhojan’a. Sci. Rep. Yakutia Branch USSR Acad. Sci. 1958, 1, 109–114. (In Russian) [Google Scholar]
- Wright, C.A. Trematodes of the genus Renicola from birds in British zoos, with descriptions of two new species. Proc. Zool. Soc. Lond. 1954, 124, 51–61. [Google Scholar] [CrossRef]
- Khalifa, R.; El-Naffar, M.K. Renicola ardeolae sp. n. (Renicolidae) a kidney trematode from the buff-backed heron (Ardeola ibis ibis) in the Assiut governorate Egypt. Acta Parasitol. Pol. 1975, 23, 355–360. [Google Scholar]
- McIntosh, A.; Farr, M.M. Renicola brantae n.sp. from the kidney of the Canada goose Branta canadensis. J. Parasilol. 1952, 38, 35–36. [Google Scholar]
- Wright, C.A. Two kidney-flukes from sudanese birds, with a description of a new species. J. Helminthol. 1957, 31, 229–238. [Google Scholar] [CrossRef]
- Kulachkova, V.G. New species of renal trematodes Renicola mollissima sp. nov from Common Eider. Trans. Leningr. Soc. Nat. 1957, 73, 198–203. (In Russian) [Google Scholar]
- Ryzhikov, K.M.; Timofeeva, T.N.; Dudorova, E.N. To cognition of trematodes from the Chukotka eider ducks. Proc. Helminthol. Lab. USSR Acad. Sci. 1966, 17, 157–168. (In Russian) [Google Scholar]
- Reimer, L.W. Neue Cerearien der Ostsee mit einer Diskussion ihrer möglichen Zuordnung und einem Bestimmungsschlüssel. Parasitol. Schriftenr. 1971, 21, 125–149. [Google Scholar]
- Leung Donald, K.M.; Keeney, D.B.; Kohler, A.V.; Peoples, R.C.; Poulin, R. Trematode parasites of Otago Harbour (New Zealand) soft-sediment intertidal ecosystems: Life cycles, ecological roles and DNA barcodes. New Zeal. J. Mar. Freshw. Res. 2009, 43, 857–865. [Google Scholar] [CrossRef]
- Hechinger, Y.R.; Miura, O. Two ‘new’ renicolid trematodes (Trematoda: Digenea: Renicolidae) from the California horn snail, Cerithidea californica (Haldeman, 1840) (Gastropoda: Potamidida). Zootaxa 2014, 3784, 559–574. [Google Scholar] [CrossRef]
- O’Dwyer, K.; Blasco-Costa, I.; Poulin, R.; Faltýnková, A. Four marine digenean parasites of Austrolittorina spp. (Gastropoda: Littorinidae) in New Zealand: Morphological and molecular data. Syst. Parasitol. 2014, 89, 133–152. [Google Scholar] [CrossRef]
- O’Dwyer, K.; Faltýnková, A.; Georgieva, S.; Kostadinova, A. An integrative taxonomic investigation of the diversity of digenean parasites infecting the intertidal snail Austrolittorina unifasciata Gray, 1826 (Gastropoda: Littorinidae) in Australia. Parasitol. Res. 2015, 114, 2381–2397. [Google Scholar] [CrossRef]
- Huston, D.C.; Cutmore, S.C.; Cribb, T.H. Molecular systematics of the digenean community parasitising the cerithiid gastropod Clypeomorus batillariaeformis Habe & Kusage on the Great Barrier Reef. Parasitol. Int. 2018, 67, 722–735. [Google Scholar] [CrossRef]
- Flores, K.; López, Z.; Levicoy, D.; Muñoz-Ramírez, C.P.; González-Wevar, C.; Oliva, M.E.; Cárdenas, L. Identification assisted by molecular markers of larval parasites in two limpet species (Patellogastropoda: Nacella) inhabiting Antarctic and Magellan coastal systems. Polar Biol. 2019, 42, 1175–1182. [Google Scholar] [CrossRef]
- Køie, M. On the endoparasites of Buccinum undatum L. with special reference to the trematodes. Ophelia 1969, 6, 251–279. [Google Scholar] [CrossRef]
- Marasaev, S.F. New renicolid cercaria from the mollusc Neptunea borealis (Prosobranchia, Buccinidae). Parazitologiya 1988, 22, 254–258. (In Russian) [Google Scholar]
- Alda, P.; Martorelli, S.R. Larval trematodes infecting the South-American intertidal mud snail Heleobia australis (Rissooidea: Cochliopidae). Acta Parasitol. 2014, 59, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Etchegoin, J.A.; Martorelli, S.R. Nuevas cercarias en Heleobia conexa (Mollusca: Hydrobiidae) de la albufera. Mar Chiquita. Neotrop. 1998, 44, 41–50. [Google Scholar]
- Pois, N.V.; Tsimbaljuk, A.K.; Ardasheva, N.B. Three new species of marine cercariae from the intertidal zone. Parazitologiya 1974, 53, 413–419. (In Russian) [Google Scholar]
- Rybakov, A.V. Fauna and Ecology of Trematodes of the Mass Species of Molluscs of the Western Part of the Sea of Japan. Ph.D. Thesis, Vladivostok, Leningrad, Russia, 1983. Available online: https://www.dissercat.com/content/fauna-i-ekologiya-trematod-massovykh-vidov-mollyuskov-severo-zapadnoi-chasti-yaponskogo-mory (accessed on 10 June 2024). (In Russian).
- Cable, R.M. Marine cercariae of Puerto Rico. In Scientific survey of Porto Rico and the Virgin Islands; New York Academy of Sciences: New York, NY, USA, 1956; pp. 491–577. [Google Scholar]
- Holliman, R.B. Larval trematodes from the Apalachee Bay area, Florida, with a checklist of known marine cercariae arranged in a key to their super-families. Tulane Stud. Zool. 1961, 9, 1–74. [Google Scholar]
- Veen, J.; Yurlov, A.K.; Delany, S.N.; Mihantiev, A.I.; Selivanova, M.A.; Boere, G.C. An Atlas of Movements of Southwest Siberian Waterbirds; Wetlands International: Wageningen, The Netherlands, 2005. [Google Scholar]
- Johnsgard, P.A. Ducks, Geese, and Swans of the World, Revised Edition [Complete Work]; University of Nebraska-Lincoln: Lincoln, NE, USA, 2010. [Google Scholar]
- Golikov, A.N.; Kusakin, O.G. Shell-bearing gastropods of the intertidal zone of the seas of the USSR; Nauka: Leningrad, Russia, 1978. (In Russian) [Google Scholar]
- Werding, B. Morphologie, Entwicklung und Ökologie digener Trematoden-Larven der Strandschnecke Littorina Littorea. Mar. Biol. 1969, 3, 306–333. [Google Scholar] [CrossRef]
- Krechmar, A.V.; Kondratyev, A.V. Waterfowl Birds of North-East Asia; NESC FEB RAS: Magadan, Russia, 2006. (In Russian) [Google Scholar]
- Takekawa, J.Y.; Newman, S.H.; Xiao, X.; Prosser, D.J.; Spragens, K.A.; Palm, E.C.; Yan, B.; Li, T.; Lei, F.; Zhao, D.; et al. Migration of waterfowl in the East Asian flyway and spatial relationship to HPAI H5N1 outbreaks. Avian Dis. 2010, 54, 466–476. [Google Scholar] [CrossRef]
- Waltho, C.; Coulson, J. The Common Eider.; Bloomsbury Publ.: London, UK; New Delhi, India, 2015. [Google Scholar]
- Matos, A.M.R.N.d.; Lavorente, F.L.P.; Lorenzetti, E.; Meira Filho, M.R.C.; da Nóbrega, D.F.; Chryssafidis, A.L.; de Oliveira, A.G.; Domit, C.; Bracarense, A.P.F.R.L. Molecular identification and histological aspects of Renicola sloanei (Digenea: Renicolidae) in Puffinus puffinus (Procellariiformes): A first record. Rev. Bras. Parasitol. Vet. 2019, 28, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ponce De León, G.; Hernández-Mena, D.I. Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation’ Tree of Life. J. Helminthol. 2019, 93, 260–276. [Google Scholar] [CrossRef]
- Salem, M.A.; Mahdy, O.A.; Shaalan, M.; Ramadan, R.M. The phylogenetic position and analysis of Renicola and Apharyngostrigea species isolated from cattle egret (Bubulcus ibis). Sci. Rep. 2023, 13, 16195. [Google Scholar] [CrossRef]
- Patitucci, K.F.; Kudlai, O.; Tkach, V. V Nephromonorcha varitestis n. sp. (Digenea: Renicolidae) from the American White Pelican, Pelecanus erythrorhynchos in North Dakota, U.S.A. Comp. Parasitol. 2015, 82, 254–261. [Google Scholar] [CrossRef]
- Heneberg, P.; Sitko, J.; Bizos, J.; Horne, E.C. Central European parasitic flatworms of the family Renicolidae Dollfus, 1939 (Trematoda: Plagiorchiida): Molecular and comparative morphological analysis rejects the synonymization of Renicola pinguis complex suggested by Odening. Parasitology 2016, 143, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.A. Studies on the life-history and ecology of the trematode genus Renicola Cohn, 1904. Proc. Zool. Soc. Lond. 1956, 126, 1–50. [Google Scholar] [CrossRef]
- Odening, K. Neue Trematoden aus Vietnamesischen Vogeln des Berliner Tierparks (Mit einer Revision der Familie Renicolidae). Bijdr. Dierkd. 1962, 32, 49–63. [Google Scholar] [CrossRef]
- Gibson, D.I. Family Renicolidae Dollfus, 1939. In Keys to Trematoda 3; CABI Publishing: Wallingford, UK, 2008; pp. 591–594. [Google Scholar]
- Lei Ching, H. Lists of larval worms from marine invertebrates of the Pacific Coast of North America. J. Helminthol. Soc. Washingt. 1991, 58, 57–68. [Google Scholar]
- Bishop, C.A.; Threlfall, W. Helminths parasites of common eider duck, Somateria mollissima (L.), in the Newfoundland and Labrador. Proceeding Helminthol. Soc. Wash. 1974, 41, 25–35. [Google Scholar]
- Miyabayashi, Y.; Mundkur, T. Atlas of Key Sites for Anatidae in the East Asian Flyway; Wetlands International and Wetlands International—Asia Pacific: Kuala Lumpur, Malaysia; Tokyo, Japan, 1999. [Google Scholar]
- Isakov, Y.A. Subfamily Anatinae. In Birds of USSR; Soviet Nauka Publ.: Moscow, Russia, 1952; Volume 4, pp. 344–635. (In Russian) [Google Scholar]
- Newton, I. Bird Migration; Collins: London, UK, 2010. [Google Scholar]
- Petersen, M.R.; Bustnes, J.O.; Systad, G.H. Breeding and moulting locations and migration patterns of the Atlantic population of Steller’s eiders Polysticta stelleri as determined from satellite telemetry. J. Avian Biol. 2006, 37, 58–68. [Google Scholar] [CrossRef]
- Alerstam, T.; Bäckman, J.; Gudmundsson, G.A.; Hedenström, A.; Henningsson, S.S.; Karlsson, H.; Rosén, M.; Strandberg, R. A polar system of intercontinental bird migration. Proceedings. Biol. Sci. 2007, 274, 2523–2530. [Google Scholar] [CrossRef]
- Bustnes, J.O.; Mosbech, A.; Sonne, C.; Systad, G.H. Migration patterns, breeding and moulting locations of king eiders wintering in north-eastern Norway. Polar Biol. 2010, 33, 1379–1385. [Google Scholar] [CrossRef]
- Sokolov, V.; Vardeh, S.; Quillfeldt, P. Long-tailed Duck (Clangula hyemalis) ecology: Insights from the Russian literature. Part 1: Asian part of the Russian breeding range. Polar Biol. 2019, 42, 2259–2276. [Google Scholar] [CrossRef]
- Sirenko, B.I. List of Species of Free-Living Invertebrates of Eurasian Arctic Seas and Adjacent Deep Waters; Russian Academy of Sciences, Zoological Institute: St. Petersburg, Russia, 2001. [Google Scholar]
- Sirenko, B.I. Check-List of Species of Free-Living Invertebrates of the Russian Far Eastern Seas; Russian Academy of Sciences, Zoological Institute: St. Petersburg, Russia, 2013. [Google Scholar]
- Avise, J.C. Phylogeography; Harvard University Press: Cambridge, MA, USA, 2000; ISBN 9780674666382. [Google Scholar]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Gonchar, A.; Galaktionov, K.V. Life cycle and biology of Tristriata anatis (Digenea: Notocotylidae): Morphological and molecular approaches. Parasitol. Res. 2017, 116, 45–59. [Google Scholar] [CrossRef]
- Gonchar, A.; Galaktionov, K.V. New data support phylogeographic patterns in a marine parasite Tristriata anatis Digenea: Notocotylidae. J. Helminthol. 2020, 94, e79. [Google Scholar] [CrossRef] [PubMed]
- Dau, C.P.; Flint, P.L.; Petersen, M.R. Distribution of recoveries ofSteller’s eiders banded on the lower alaska peninsula, Alaska. J. F. Ornithol. 2000, 71, 541–548. [Google Scholar] [CrossRef]
- Davis, S.E.; Maftei, M.; Mallory, M.L. Migratory connectivity at high latitudes: Sabine’s gulls (Xema sabini) from a colony in the Canadian high Arctic migrate to different oceans. PLoS ONE 2016, 11, e0166043. [Google Scholar] [CrossRef]
- Clairbaux, M.; Fort, J.; Mathewson, P.; Porter, W.; Strøm, H.; Grémillet, D. Climate change could overturn bird migration: Transarctic flights and high-latitude residency in a sea ice free Arctic. Sci. Rep. 2019, 9, 17767. [Google Scholar] [CrossRef]
- Ezhov, A.V.; Gavrilo, M.V.; Krasnov, Y.V.; Bråthen, V.S.; Moe, B.; Baranskaya, A.V.; Strøm, H. Transpolar and bi-directional migration strategies of black-legged kittiwakes Rissa tridactyla from a colony in Novaya Zemlya, Barents Sea, Russia. Mar. Ecol. Prog. Ser. 2021, 676, 189–203. [Google Scholar] [CrossRef]
- Able, K.P.; Barron, A.; Dunn, J.L.; Omland, K.E.; Sansone, L. First occurrence of an atlantic common eider (Somateria mollissima dresseri) in the pacific ocean. West. Birds 2014, 45, 90–99. [Google Scholar]
- Galaktionov, K.V.; Blasco-Costa, I.; Olson, P.D. Life cycles, molecular phylogeny and historical biogeography of the pygmaeus microphallids (Digenea: Microphallidae): Widespread parasites of marine and coastal birds in the Holarctic. Parasitology 2012, 139, 1346–1360. [Google Scholar] [CrossRef] [PubMed]
- Galaktionov, K.V.; Gonchar, A.; Postanogova, D.; Miroliubov, A.; Bodrov, S.Y. Parvatrema spp. (Digenea, Gymnophallidae) with parthenogenetic metacercariae: Diversity, distribution and host specificity in the palaearctic. Int. J. Parasitol. 2024, 54, 333–355. [Google Scholar] [CrossRef]
- Criscione, C.D.; Blouin, M.S. Life cycles shape parasite evolution: Comparative population genetics of salmon trematodes. Evolution 2004, 58, 198–202. [Google Scholar] [CrossRef]
- Barrett, L.G.; Thrall, P.H.; Burdon, J.J.; Linde, C.C. Life history determines genetic structure and evolutionary potential of host-parasite interactions. Trends Ecol. Evol. 2008, 23, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Keeney, D.B.; King, T.M.; Rowe, D.L.; Poulin, R. Contrasting mtDNA diversity and population structure in a direct-developing marine gastropod and its trematode parasites. Mol. Ecol. 2009, 18, 4591–4603. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Costa, I.; Waters, J.M.; Poulin, R. Swimming against the current: Genetic structure, host mobility and the drift paradox in trematode parasites. Mol. Ecol. 2012, 21, 207–217. [Google Scholar] [CrossRef]
- Blasco-Costa, I.; Poulin, R. Host traits explain the genetic structure of parasites: A meta-analysis. Parasitology 2013, 140, 1316–1322. [Google Scholar] [CrossRef]
- Feis, M.E.; Thieltges, D.W.; Olsen, J.L.; De Montaudouin, X.; Jensen, K.T.; Bazaïri, H.; Culloty, S.C.; Luttikhuizen, P.C. The most vagile host as the main determinant of population connectivity in marine macroparasites. Mar. Ecol. Prog. Ser. 2015, 520, 85–99. [Google Scholar] [CrossRef]
- Mazé-Guilmo, E.; Blanchet, S.; McCoy, K.D.; Loot, G. Host dispersal as the driver of parasite genetic structure: A paradigm lost? Ecol. Lett. 2016, 19, 336–347. [Google Scholar] [CrossRef]
- Miura, O.; Torchin, M.E.; Kuris, A.M.; Hechinger, R.F.; Chiba, S. Introduced cryptic species of parasites exhibit different invasion pathways. Proc. Natl. Acad. Sci. USA 2006, 103, 19818–19823. [Google Scholar] [CrossRef] [PubMed]
- Thieltges, D.W.; Hof, C.; Borregaard, M.K.; Matthias Dehling, D.; Brändle, M.; Brandl, R.; Poulin, R. Range size patterns in European freshwater trematodes. Ecography 2011, 34, 982–989. [Google Scholar] [CrossRef]
- Prevot, G.; Bartoli, P. Life-cycle of Renicola lari J. Timon-David, 1933 (Trematoda, Renicolidae) (author’s transl). Ann. Parasitol. Hum. Comp. 1978, 53, 561–575. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).