Abstract
The “solar-powered” Elysia timida (Risso, 1818) is an endemic Mediterranean sacoglossan living in rocky substrates at shallow water. During a scuba dive, one E. timida was photographed and collected. The observation revealed the presence of a small crustacean of the Pinnotheridae family. It was not possible to assign the crustacean to a genus, but it shares typical ecological and external morphological features with other Pinnotherinae species. This is the first report of a pea crab hosted by a non-shelled Heterobranchia and the first case of a symbiotic association between crustaceans and marine Heterobranchia reported in the Mediterranean Sea.
Elysia timida (Risso, 1818) is a “solar-powered” sacoglossan species (Mollusca, Gastropoda) long-studied for its ability to incorporate chloroplasts obtained from the algae it feeds on [1,2]. This species is endemic to the Mediterranean Sea and lives on rocky substrates at shallow water where it is locally common and often quite abundant (Figure 1). During a scuba dive in “Punta del Pecoraro” in Civitavecchia, Latium, Italy (Central Tyrrhenian Sea) on 7 May 2018, two E. timida specimens were observed at 3 m depth.
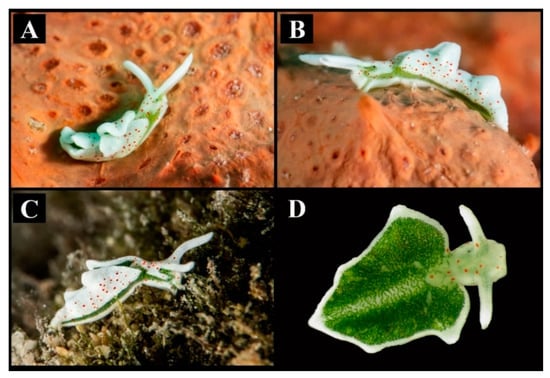
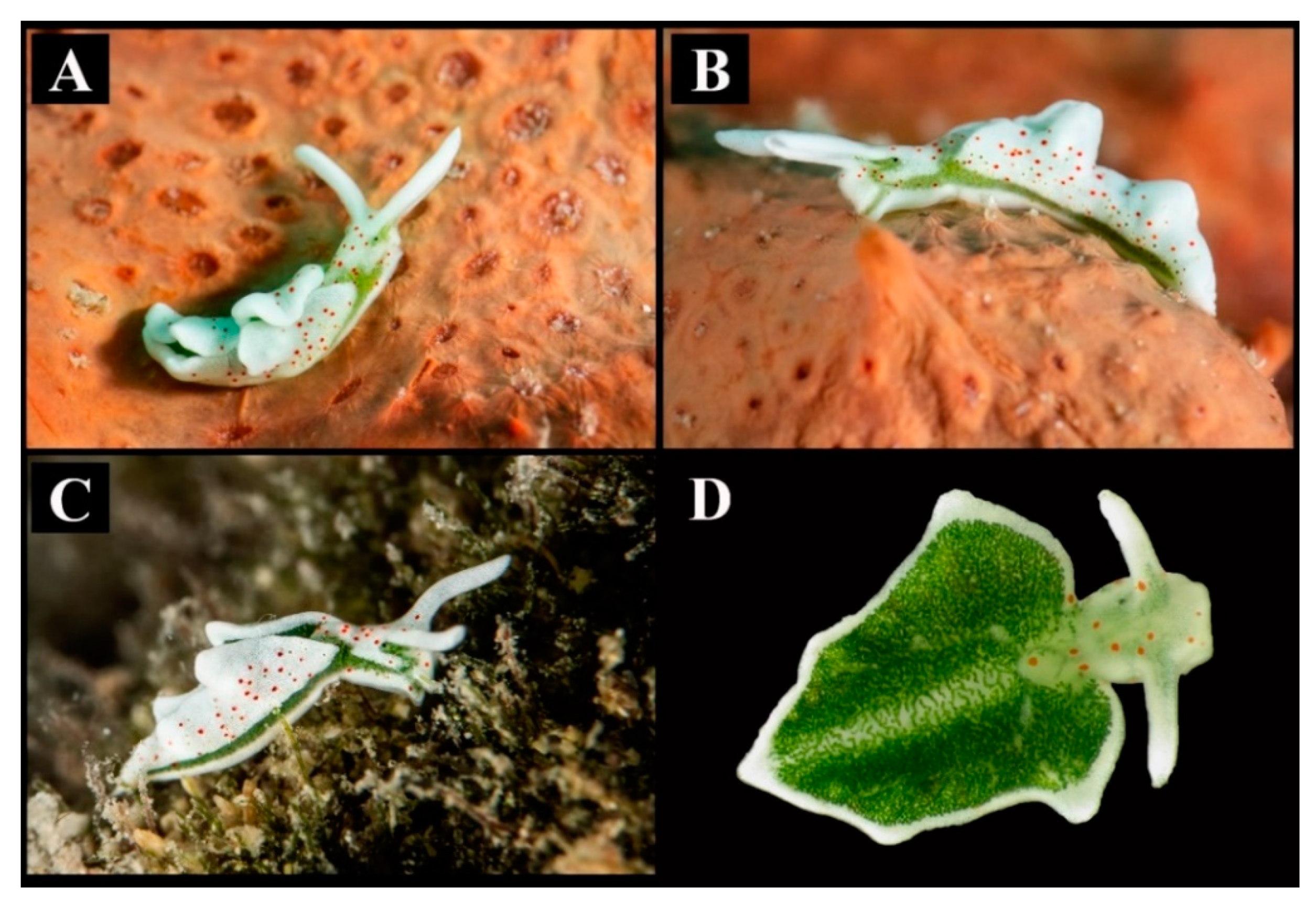
Figure 1.
Elysia timida specimens. (A) Specimen crawling on an orange sponge in Lecce (Apulia, Ionian Sea, Central Mediterranean Sea). (B) Lateral view of the Apulian individual. (C) Elysia timida from Siracusa (Sicily, Tyrrhenian Sea, West Mediterranean Sea). (D) Dorsal view showing the photosynthetic region of the dorsum and of the internal portion of the parapodia.
The two individuals were collected and carefully observed under a stereomicroscope to take photographs of morphological features and then stored in EtOH 96% at the Roma Tre University collection (Vouchers RM3_1384 and RM3_1385) for future molecular analysis. Unexpectedly, while observing one of the two E. timida individuals (Figure 2A), one of the red spot characteristics of the color body pattern of this sacoglossan seemed to move independently along the parapodia of the sea slug, and then disappeared soon after. An in-depth observation allowed us to reveal the presence of a very small decapod crustacean with a white body bearing a single red spot (about half the size of the whole animal) on the dorsal surface of its carapace perfectly matching (both in color and shape) the typical red dots externally decorating the parapodia of E. timida (Figure 2B). Just after a few seconds the small crustacean moved quickly to hole up in the internal surface of the parapodia of its host (Figure 2C). Due to the typical defensive behavior of E. timida to close parapodia when disturbed, it was not possible to take additional pictures of this association.
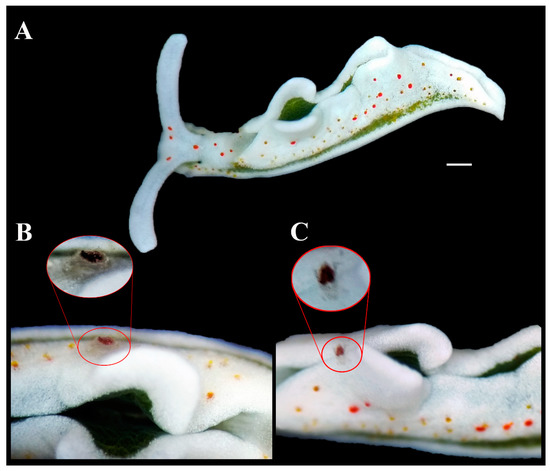
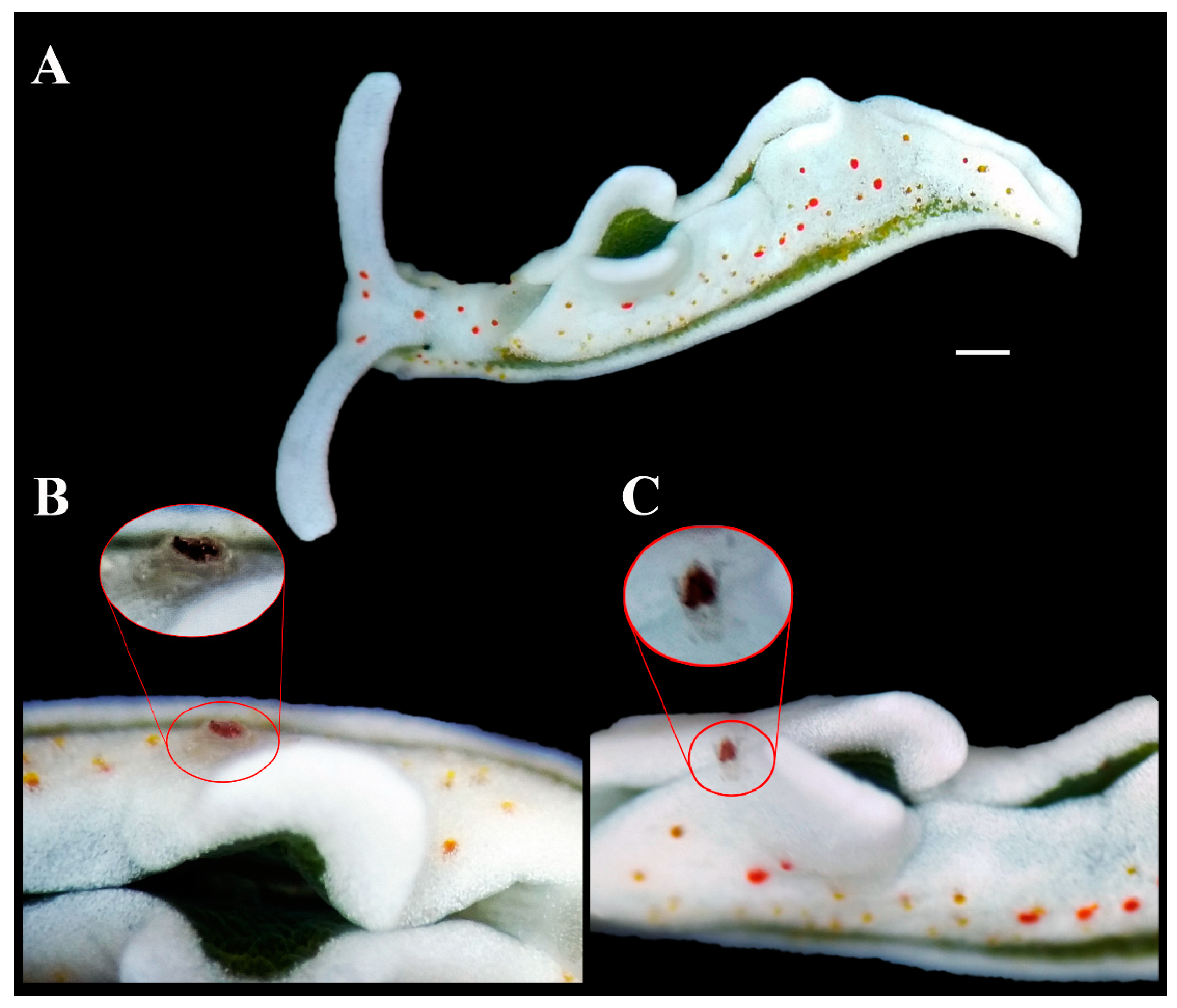
Figure 2.
The new symbiotic relationship. (A) Elysia timida individual (Voucher RM3_1384) hosting the Pinnotheridae crustacean. (B) Frontal view of the pea crab. (C) Dorsal view of the pea crab (red circle) with the red dorsal spot visible on its carapace. Scale bar = 1 mm.
The observation revealed the crustacean as a Decapoda Brachyura belonging to the Pinnotheridae family. In fact, the “pea crabs”, as the members of this latter family are commonly named, comprise around 320 accepted species grouped in four subfamilies: Pinnotherinae, Pinnixinae, Pinnothereliinae, and Pinnixulalinae. While members of the three latter subfamilies are commensal symbionts living inside the holes and tubes of living annelid and sipunculid worms and inside mud shrimp burrows [3,4], members of Pinnotherinae are defined as small symbiotic crabs, living on different hosts, mainly on molluscs [4]. It is reasonable to attribute the small crab here reported to the Pinnotherinae. The reduced body size and the complicated multi-staged life cycles could be the reasons why the biology of most of the Pinnotherinae species is still unknown. Even if most of the Pinnotherinae are hosted by Bivalvia, some genera are known to live in association with Gastropoda with a single case of a pea crab, Opisthopus transversus (Rathbun, 1894), hosted by Heterobranchia species such as Aplysia vaccaria (Wrinkler 1955) [5], Bulla gouldiana (Pilsbry, 1895) and Navanax inermis (Cooper, 1862) [6]. These three heterobranchs belong to basal groups that have conserved a shell, a character lacking in most of the other Heterobranchia. The three species mentioned above are the only cases known until now of symbiotic association between a pea crab and heterobranchs.
Unfortunately, it was not possible to assign the pea crab here investigated at the genus level, due to the limit of the instrument used to observed it, but there are some ecological features that could give interesting insights. The trophic strategy of Pinnotherinae crabs consist of eating the mucus produced by their hosts, which contains organic particles and nutrients [4]. Additionally, some ectosymbionts or species associated with spacious host (like Gastropoda and Echinoidea) or that frequently leave their hosts, show external morphological adaptations such as the presence of eyes sometimes visible through the dorsal surface, for example, Pinnaxodes bipunctatus (Nicolet in Gay, 1849) and Calyptraeotheres camposi (Ayón-Parente and Hendrickx, 2014), a hard carapace and an ornamented body shape. This last feature is functional, making the mucus adhere to the cracks of the carapace and favoring the anchorage, overlaying the external body to conceal the crab or making the crab less palatable [4].
Considering that sacoglossans produce a lot of mucus and that in particular E. timida contains repellent and unpalatable polypropionate compounds [7], it is plausible to hypothesize that the crab here reported is the case of a symbiotic association between a Pinnotherinae species and the sacoglossan E. timida that was never reported before. In fact, the small size of the observed crab, up to 1 mm long, the hard and ornamented carapace, the presence of the eyes visible on the dorsal surface and its ability to mimic the red spot of E. timida are coherent with the typical characteristics showed by other Pinnotherinae species [4]. In particular, the hard carapace and its flattened shape could be a functional adaptation to the particular host it lives on. In fact, E. timida (and consequently the crab) is not protected by a shell but instead chemically defended and the crab could have evolved defensive strategies to camouflage on it and to better adhere to its external body. On the other hand, the presence of the pea crab could be useful for this sacoglossan species in order to avoid the attack of external parasites, which, in fact, are very common in Heterobranchia [8].
Even if commensal relationships are already known between tropical and Indo-Pacific shrimps (Decapoda, Caridea) and shell-less heterobranchs (nudibranchs) [9,10,11], no symbiotic relationship is known to date between shell-less heterobranchs and true crabs. In fact, this is the first report of a pea crab hosted by a non-shelled Heterobranchia and the fourth one regarding a shelled Heterobranchia species [4].
Furthermore, this is the first case of symbiotic association between crustaceans and marine Heterobranchia reported in the Mediterranean Sea.
Author Contributions
Conceptualization, G.F.; methodology, G.F., P.M. and M.S.; validation, P.M., M.S.; investigation, G.F., P.M. and M.S.; data curation, M.S.; writing—original draft preparation, G.F.; writing—review and editing, G.F., P.M. and M.S.; supervision, P.M.; funding acquisition, G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Education, University and Research grant number MIUR, PON 2014–2020, grant AIM 1848751-2, Linea 2 and The APC was funded by MIUR, PON 2014–2020, grant AIM 1848751-2, Linea 2.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are grateful to Genuario Belmonte (Lecce, Italy), Zdeněk Ďuriš (Ostrava, Czech Republic), Carlo Froglia (Ancona, Italy) and Egidio Trainito (Sardinia, Italy) for their useful suggestions. The authors are grateful to the two anonymous reviewers for their help with improving the text and with the English language editing of the manuscript. G.F. and M.S. wish to thank the Scubalandia Crew for technical underwater support. G.F. thanks the Italian Ministry of Education, University and Research (MIUR, PON 2014–2020, grant AIM 1848751-2, Linea 2) for funds.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Melo Clavijo, J.; Frankenbach, S.; Fidalgo, C.; Serôdio, J.; Donath, A.; Preisfeld, A.; Christa, G. Identification of scavenger receptors and thrombospondin-type-1 repeat proteins potentially relevant for plastid recognition in Sacoglossa. Ecol. Evol. 2020, 10, 12348–12363. [Google Scholar] [CrossRef] [PubMed]
- Cartaxana, P.; Rey, F.; LeKieffre, C.; Lopes, D.; Hubas, C.; Spangenberg, J.E.; Cruz, S. Photosynthesis from stolen chloroplasts can support sea slug reproductive fitness. Proc. R. Soc. B 2021, 288, 20211779. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, W.L.; Mc Cain, J.C.; Davidson, E.S. Crustaceorum Catalogus: Decapoda I-Brachyura I-Fam, Pinnotheridae. In Crustaceorum Catalogus. Pars 3; Gruner, H.-E., Holthuis, L.B., Eds.; Dr. W. Junk B.V.: Den Haag, The Netherlands, 1973; pp. 1–160. [Google Scholar]
- De Gier, W.; Becker, C. A Review of the Ecomorphology of Pinnotherine Pea Crabs (Brachyura: Pinnotheridae), with an Updated List of Symbiont-Host Associations. Diversity 2020, 12, 431. [Google Scholar] [CrossRef]
- Beondé, A.C. Aplysia vaccaria, a new host for the pinnotherid crab Opisthopus transversus. Veliger 1968, 10, 375–378. [Google Scholar]
- Campos, E. The Pinnotheridae of the northeastern Pacific (Alaska to Mexico): Zoogeographical remarks and new bivalve hosts (Crustacea, Brachyura, Pinnotheridae). Zootaxa 2016, 4170, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Gavagnin, M.; Spinella, A.; Castelluccio, F.; Cimino, G.; Marin, A. Polypropionates from the Mediterranean mollusk Elysia timida. J. Nat. Prod. 1994, 57, 298–304. [Google Scholar] [CrossRef]
- Anton, R.F.; Schrödl, M. The gastropod–crustacean connection: Towards the phylogeny and evolution of the parasitic copepod family Splanchnotrophidae. Zool. J. Linn. Soc. 2013, 167, 501–530. [Google Scholar] [CrossRef]
- Anker, A.; Ivanov, Y. A little Z. Rex: On the predatory habits of the emperor shrimp. Mar. Biodivers. 2021, 51, 1–6. [Google Scholar] [CrossRef]
- Chow, L.H.; De Grave, S.; Tsang, L.M. Evolution of protective symbiosis in palaemonid shrimps (Decapoda: Caridea) with emphases on host spectrum and morphological adaptations. Mol. Phylogenet. Evol. 2021, 162, 107201. [Google Scholar] [CrossRef] [PubMed]
- Hoeksema, B.W.; Fransen, C.H. Host switch by the Caribbean anemone shrimp Periclimenes rathbunae in Curaçao. Coral Reefs 2017, 36, 607. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).