Abstract
A tandem synthetic sequence involving photo-induced intramolecular [2+2] cycloaddition followed by acid-promoted ring expansion was developed to access the novel bicyclic triketone framework. The process begins with the UV (254 nm) irradiation of cyclic vinylogous ester, affording a highly strained cyclobutane-fused diketone in an 86% yield. This unique intermediate feature is of a fused four- and six-membered ring system with spatially compressed carbonyl groups. Upon acidic hydrolysis in aqueous MeCN, the strained system undergoes retro-aldol ring expansion, delivering [5.3.0] bicyclic triketones bearing a seven- and five-membered fused ring with three strategically oriented carbonyl units in a 75% yield. Structural elucidation was performed using NMR spectroscopy, UV-Vis, HRMS, and single-crystal X-ray crystallography. The method highlights a concise route for constructing a fused bicyclic triketone of relevance to synthetic and medicinal chemistry.
1. Introduction
The [2+2] photocycloaddition has long been recognized as a powerful photochemical transformation for constructing strained cyclobutanes, which are key intermediates in the synthesis of natural products and medicinal scaffolds [1,2,3,4]. Intramolecular versions of this reaction are particularly valuable, enabling high regio- and stereocontrol while minimizing side reactions [5]. The resulting cyclobutanes, due to their ring strain, serve as versatile precursors for downstream transformations including ring opening and rearrangements [6]. Cyclic vinylogous esters, bearing conjugated carbonyl and alkene functionalities, are attractive substrates for such photochemical cyclizations and have been the research interest of several pioneers in this field [7,8,9]. Recent studies have highlighted the strategic use of cyclic vinylogous esters in intramolecular [2+2] photocycloadditions, enabling high regio- and stereoselectivity in the construction of polycyclic scaffolds through Lewis acid catalysis and even proceed by visible light catalysis [10,11,12,13]. Thus, further development to exploit their synthetic potential in constructing complex molecular structures could be especially valuable in organic synthesis. In this study, we report a synthetic approach that employs tandem photo-induced intramolecular [2+2] cycloaddition and acid-promoted ring expansion to construct a [5.3.0] bicyclic triketone. The resulting fused system features multiple carbonyl groups in a compact fused-ring scaffold, offering opportunities for downstream functionalization. The synthetic sequence is operationally simple, uses mild conditions, and allows efficient access to structurally rich polycarbonyl frameworks.
2. Results and Discussion
2.1. Synthesis and Design of Cyclic Vinylogous Esters
As shown in Scheme 1, to access the bis-cyclic vinylogous ester as the substrate for our proposed intramolecular [2+2] cycloaddition, we synthesized compound 1 via the condensation of cyclopentane-1,3-dione and 1,3-propanediol under Dean–Stark conditions. The reaction proceeded smoothly using p-TSA as a catalyst with the azeotropic removal of water in benzene. Compound 1 was obtained in a 67% yield, and its structure was confirmed by spectroscopic characterization (1H NMR, 13C NMR, HRMS, and X-ray. See Supplementary Materials) (Figure 1). UV-Vis analysis showed an absorption maximum near 239 nm, attributed to the π→π* transition of the α,β-unsaturated carbonyl moiety in the vinylogous ester, indicating its potential for selective excitation under UV irradiation.
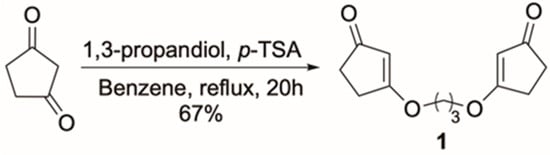
Scheme 1.
Formation of 1 from cyclopentane-1,3-dione.
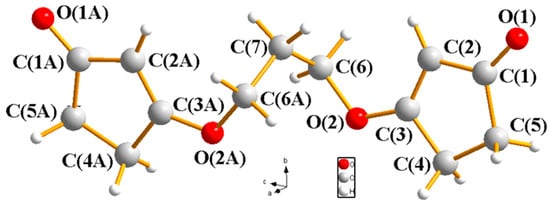
Figure 1.
X-ray structure of 1.
2.2. Photochemical [2+2] Intramolecular Cycloaddition
Irradiation of 1 with 254 nm UV light in methanol resulted in a clean transformation to the tetracyclic product 2. We were pleased to find that the [2+2] cycloaddition exhibited high regio- and stereoselectivity, yielding only a single isomer (Scheme 2). As shown in Figure 2, the crystal structure revealed that formation of the cyclobutane ring proceeded through a head-to-head/endo transition state, establishing the cis-fused 5-4-5 tricyclic core skeleton. To the best of our knowledge, this represents the first example of a [2+2] cycloaddition between two cyclic vinylogous esters, and the inherent ring strain in the newly formed cyclobutane moiety is expected to serve as a driving force for the subsequent ring-opening transformation.
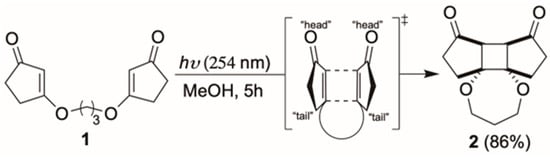
Scheme 2.
Intramolecular [2+2] cycloaddition of 1 to form 2.
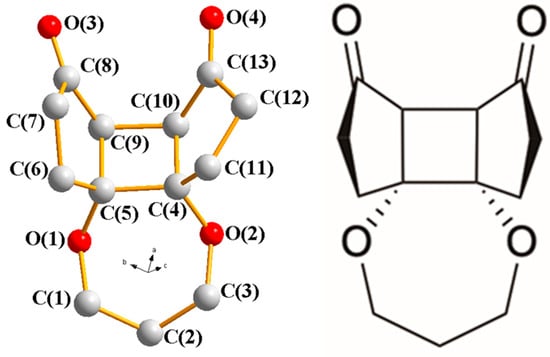
Figure 2.
X-ray structure of 2.
2.3. Acid-Promoted Hydrolytic Ring Expansion
For the subsequent target ring expansion–intramolecular aldol reaction, 2 was treated with p-TSA in a mixture of MeCN and water (2:1). Intriguingly, though the proposed intermediate 3 from the initial ring expansion/hydrolysis was not observed, a bicyclo [5.3.0] triketone 4 was isolated as the sole product, as shown in Scheme 3. The transformation was optimized with 5.0 equivalents of p-TSA at reflux for 40 h, affording 4 in a 75% yield. Single-crystal X-ray diffraction confirmed the [5.3.0] system, which implied the possibility of 3 was initially formed from the ring expansion, and a following intramolecular aldol condensation at the a-b linkage yielded 4. The product exhibits a rigid, fused seven- and five-membered ring framework bearing three carbonyl groups in a well-defined spatial arrangement. Such motifs are rarely accessed through relatively short synthetic routes and are considered synthetically valuable due to their potential reactivity and multiple sites for functionalization.
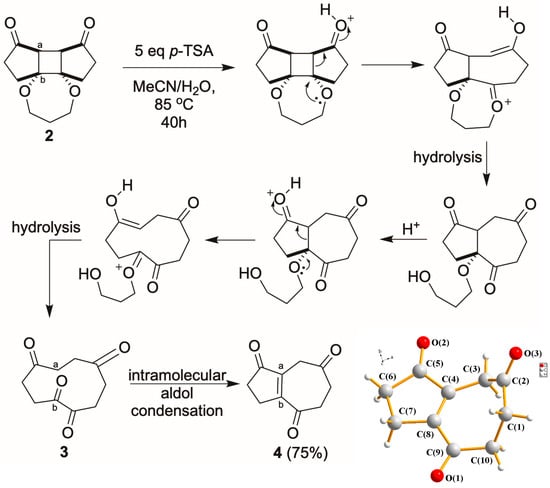
Scheme 3.
Acid-promoted formation of triketone 4 from photo-adduct 2.
3. Materials and Methods
All the reagents were obtained from commercial sources and used without further purification. Reactions were monitored with thin-layer chromatography carried out on 0.25 mm E. Merck silica gel plates (60F-254) using 7% ethanolic phosphomolybdic acid as developing agent. Standard column chromatography was performed using 230–400 mesh silica gel obtained from E. Merck (Rahway, NJ, USA). All NMR spectra were run at 400 MHz (1H NMR) or 100 MHz (13C NMR) in CDCl3 solution at 20 °C with Bruker NEO-400MHz FT-NMR (Bruker Optics, Billerica, MA, USA), and chemical shifts are reported in δ (ppm) using solvent resonance as the internal reference. Bruker AXS SMART APEX II X-ray diffractometer (Bruker Optics, Billerica, MA, USA) was used to collect the experimental data for determining crystal structures, and the resulting data was processed and visualized using Diamond 4.6.8. UV-vis spectrum was taken using JASCO V-750 UV-Visible Spectrophotometer (JASCO, Tokyo, Japan). IR spectrum was taken using Nicolet iS5 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). HRMS data were collected using JEOL JMS-700 (JEOL, Tokyo, Japan). Photoreactions were conducted in Panchum Photochemical Reactor PR-2000 (Panchum, Kaohsiung, Taiwan).
Synthesis
Cyclopentane-1,3-dione (2.000 g, 20.4 mmol, and 2.0 equiv.), 1,3-propanediol (0.744 g, 10.3 mmol, and 1.0 equiv.), and p-TSA (0.039 g, 0.2 mmol, and 0.02 equiv.) were dissolved in benzene (40.0 mL) in a 100 mL single-neck round-bottom flask equipped with a Dean–Stark apparatus. The reaction mixture was refluxed for 20 h and monitored by TLC until complete consumption of the starting material. After completion, the reaction was quenched with 40 mL of saturated aqueous NaHCO3 solution and extracted with dichloromethane (3 × 50 mL). The combined organic layers were washed with 50 mL of brine, dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to afford a crude orange–yellow solid. Purification by gradient column chromatography (CH2Cl2/MeOH = 150:1 to 100:1) afforded pale yellow solid compound 1 (1.305 g, 67%). m.p. 119.0–120.0 °C. TLC: Rf = 0.20 [SiO2; eluent: CH2Cl2/MeOH 10:1 v/v]. 1H NMR (400 MHz, CDCl3, 19.4 °C, δ): 5.34 (s, 2H), 4.13 (t, J = 6.0 Hz, 4H), 2.64–2.62 (m, 4H), 2.48–2.45 (m, 4H), 2.26 (quint, J = 6.0 Hz, 2H). 13C NMR (100 MHz, CDCl3, 19.7 °C, δ): 205.7, 189.8, 104.9, 67.7, 33.9, 28.3, 27.9. HRMS (ESI) m/z calcd. for C13H16O4Na [M + Na]+: 259.0941, found: 259.0941. FTIR (neat, cm−1): 3381, 2943, 2855, 1653, 1579, 1426, 1388, 1305, 1236, 1181, 1042, 859, 668. UV-Vis (MeOH, 22 °C): λmax = 238.8 nm (ε = 1.65 × 102 M−1cm−1). Single crystals of 1 were obtained using a slow evaporation method in CH2Cl2 solution.
3,3′-(Propane-1,3-diylbis(oxy))bis(cyclopent-2-enone) (compound 1, 0.172 g, and 0.7 mmol) was dissolved in methanol (72.0 mL) and transferred into a quartz tube. The tube was sealed with a septa and placed in an ultrasonic bath. A long needle connected to an argon balloon and a short vent needle were inserted, and the solution was degassed by sparging with argon while sonicating for 30 min. The tube was then irradiated with UV light (254 nm) in a Rayonet-type photoreactor for 5 h. After the reaction, the solvent was removed under reduced pressure to give a pale yellow crude product. Purification by column chromatography (CH2Cl2/MeOH = 15:1) afforded compound 2 as a white solid (0.149 g, 86%). m.p. 128.5–129.3 °C. TLC: Rf = 0.09 [SiO2; eluent: CH2Cl2/MeOH 50:1 v/v]. 1H NMR (400 MHz, CDCl3, 19.7 °C, δ): 4.00–3.95 (m, 2H), 3.76–3.69 (m, 2H), 3.09 (s, 2H), 2.71–2.62 (m, 2H), 2.41–2.29 (m, 4H), 2.26–2.14 (m, 3H), 1.83–1.76 (m, 1H). 13C NMR (100 MHz, CDCl3, 19.7 °C, δ): 213.9, 86.7, 66.0, 49.2, 39.0, 33.2, 27.7. HRMS (ESI) m/z calcd. for C13H16O4 [M]+: 236.1050, found: 236.1049. FTIR (neat, cm−1): 2869, 2816, 1739, 1431, 1402, 1379, 1264, 1194, 1067, 945. Single crystals of 2 were obtained using a slow evaporation method in CH2Cl2 solution.
Hexahydro-2H-dicyclopenta [1,4:2,3]cyclobuta [1,2-b][1,4]dioxepine-8,9(8aH,8bH)-dione (compound 2, 0.005 g, 0.21 mmol, and 1.0 equiv.) and p-TSA (0.190 g, 1.10 mmol, and 5.0 equiv.) were dissolved in a mixture of acetonitrile and water (2:1, 3.0 mL) in a 20 mL vial. The reaction mixture was stirred for 40 h at 85 °C and monitored by TLC until completion. After the reaction, it was quenched with saturated aqueous NaHCO3 and extracted with dichloromethane (3×). The combined organic layers were washed with brine (1×), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to give an orange–yellow crude product. Purification by gradient column chromatography (hexane/ethyl acetate = 15:1 to 10:1) afforded 4 as a pale yellow compound (0.032 g, 75%). TLC: Rf = 0.20 [SiO2; eluent: hexane/ethyl acetate 1:1 v/v]. 1H NMR (400 MHz, CDCl3, 20.5 °C, δ): 3.66 (t, J = 2.3 Hz, 2H), 3.06–3.03 (m, 2H), 2.81–2.77 (m, 2H), 2.73–2.70 (m, 2H), 2.57–2.55 (m, 2H). 13C NMR (100 MHz, CDCl3, 20.7 °C, δ): 208.0, 204.4, 198.0, 160.8, 141.9, 39.4, 38.4, 35.3, 33.8, 24.6. HRMS (ESI) m/z calcd. for C10H12O4 [M]+: 196.0739, found: 196.0736. FTIR (neat, cm−1): 3490, 2926, 2766, 1712, 1669, 1622, 1461, 1422, 1344, 1269, 1174. Single crystals of 4 were obtained using a slow evaporation method in CH2Cl2 solution.
4. Conclusions
In summary, we have developed a concise strategy for constructing a tricarbonylated bicyclic compound via sequential photo-induced intramolecular [2+2] cycloaddition, acid-promoted ring expansion, and intramolecular aldol condensation. The transformation leverages a photochemically induced ring strain in a preorganized vinylogous ester framework to access highly strained cyclobutane intermediates. Subsequent thermal activation under acidic conditions initiates fragmentation, leading to ring expansion and the formation of thermodynamically favored [5.3.0] bicyclic triketones. This tandem process demonstrates how conformational control, strain induction, and strain release can be strategically orchestrated to construct structurally complex and functionally dense molecules. The resulting fused-ring skeleton bearing multiple carbonyl groups offer promising synthetic handles for further elaboration in target- or diversity-oriented synthesis.
Supplementary Materials
The following supporting information can be downloaded online. 1H NMR spectrum (400 MHz) of compound 1, 2, 4 in CDCl3; 13C NMR spectrum (100 MHz) of compound 1, 2, 4 in CDCl3; UV-vis absorption spectra of compound 2; X-ray data of compound 1 (CCDC-2482647), 2 (CCDC-2482655), and 4 (CCDC-2482651).
Author Contributions
Conceptualization, G.J.C.; investigation, X.-Y.H.; writing—original draft preparation, G.J.C.; writing—review and editing, G.J.C. and C.C.; supervision, G.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science and Technology Council (NSTC) of Taiwan, grant number NTSC 113-2113-M-033-003 and NTSC 114-2113-M-033-006.
Data Availability Statement
The data are contained within this article and the Supplementary Materials.
Acknowledgments
We gratefully acknowledge the National Science and Technology Council (NSTC) of Taiwan for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bach, T. Stereoselective Intermolecular [2 + 2]-Photocycloaddition Reactions and Their Application in Synthesis. Synthesis 1998, 1998, 683–703. [Google Scholar] [CrossRef]
- Poplata, S.; Tröster, A.; Zou, Y.-Q.; Bach, T. Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions. Chem. Rev. 2016, 116, 9748–9815. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.-Y.; Yan, B.-C.; Sun, H.-D.; Puno, P.-T. Recent advances in the application of [2 + 2] cycloaddition in the chemical synthesis of cyclobutane-containing natural products. Nat. Prod. Bioprospect. 2024, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Bera, N.; Ghosh, S. [2 + 2] Photochemical cycloaddition in organic synthesis. Eur. J. Org. Chem. 2020, 2020, 1310–1326. [Google Scholar] [CrossRef]
- Snider, B.B. Intramolecular cycloaddition reactions of ketenes and keteniminium salts with alkenes. Chem. Rev. 1988, 88, 793–811. [Google Scholar] [CrossRef]
- Winkler, J.D.; Bowen, C.M.; Liotta, F. [2 + 2] Photocycloaddition/Fragmentation Strategies for the Synthesis of Natural and Unnatural Products. Chem. Rev. 1995, 95, 2003–2020. [Google Scholar] [CrossRef]
- Carreira, E.M.; Hastings, C.A.; Shepard, M.S.; Yerkey, L.A.; Millward, D.B. Asymmetric Induction in Intramolecular [2 + 2]-Photocycloadditions of 1,3-Disubstituted Allenes with Enones and Enoates. J. Am. Chem. Soc. 1994, 116, 6622–6630. [Google Scholar] [CrossRef][Green Version]
- Shepard, M.S.; Carreira, E.M. Asymmetric Photocycloadditions with an Optically Active Allenylsilane: Trimethylsilyl as a Removable Stereocontrolling Group for the Enantioselective Synthesis of exo-Methylenecyclobutanes. J. Am. Chem. Soc. 1997, 119, 2597–2605. [Google Scholar] [CrossRef]
- Tedaldi, L.M.; Baker, J.R. In situ Reduction in Photocycloadditions: A Method to Prevent Secondary Photoreactions. Org. Lett. 2009, 11, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Fort, D.A.; Woltering, T.J.; Nettekoven, M.; Knust, H.; Bach, T. Synthesis of Fluorinated Tricyclic Scaffolds by Intramolecular [2+2] Photocycloaddition Reactions. Angew. Chem. Int. Ed. 2012, 51, 10169–10172. [Google Scholar] [CrossRef] [PubMed]
- Brimioulle, R.; Bach, T. [2 + 2] Photocycloaddition of 3-Alkenyloxy-2-cycloalkenones: Enantioselective Lewis Acid Catalysis and Ring Expansion. Angew. Chem. Int. Ed. 2014, 53, 12921–12924. [Google Scholar] [CrossRef] [PubMed]
- Poplata, S.; Bauer, A.; Storch, G.; Bach, T. Intramolecular [2+2] Photocycloaddition of Cyclic Enones: Selectivity Control by Lewis Acids and Mechanistic Implications. Chem.–Eur. J. 2019, 25, 8135–8148. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jandl, C.; Bach, T. Visible-Light-Mediated Enantioselective Photoreactions of 3-Alkylquinolones with 4-O-Tethered Alkenes and Allenes. Org. Lett. 2020, 22, 3618–3622. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).