Abstract
Acyl chloride alcoholysis is a fundamental and typically high-yielding method for ester synthesis. However, competitive side reactions can occur when the acyl chloride possesses multiple electrophilic sites and the alcohol is a strong nucleophile. We report an example of this phenomenon: the reaction of pentafluorobenzoyl chloride with 1,1,1,3,3,3-hexafluoropropan-2-ol yields not only the expected ester but also a significant quantity of the 1,1,1,3,3,3-hexafluoropropan-2-yl 2,3,5,6-tetrafluoro-4-((1,1,1,3,3,3-hexafluoropropan-2-yl)oxy)benzoate. The formation of the latter results from an effective nucleophilic aromatic substitution (SNAr) at the para-fluorine position of the pentafluorophenyl ring by the hexafluoroisopropoxide anion.
1. Introduction
Acyl halides are extensively employed in chemical synthesis for the preparation of esters [1,2,3,4,5,6], amides [2,7,8,9,10], acid anhydrides [11,12,13], ketones [14,15,16,17], and other derivatives. Depending on the reaction conditions, the target products can be obtained in high yields; however, side reactions may also occur. For instance, the reaction of acyl chlorides with alcohols can lead to the formation of alkyl chlorides in addition to, or sometimes even in place of, the expected esters. In particular, it takes place when the reaction proceeds without the use of a base, so the forming HCl is not neutralized and catalyzes the formation of alkyl chlorides [3]. This paper describes an example of a nucleophilic aromatic substitution reaction observed during the reaction of pentafluorobenzoyl chloride with 1,1,1,3,3,3-hexafluoropropan-2-ol (HFIP) in the presence of triethylamine, yielding 1,1,1,3,3,3-hexafluoropropan-2-yl 2,3,5,6-tetrafluoro-4-((1,1,1,3,3,3-hexafluoropropan-2-yl)oxy)benzoate (2) as a byproduct.
2. Results and Discussion
With the aim of synthesizing 1,1,1,3,3,3-hexafluoropropan-2-yl 2,3,4,5,6-pentafluorobenzoate (1) (Scheme 1), we performed the reaction of pentafluorobenzoyl chloride with HFIP using triethylamine (1.2 equiv.) as a base. It is worth mentioning that triethylamine (pKa = 10.75) is basic enough to deprotonate HFIP (pKa = 9.3 [18]), forming alkoxide ion, which is a stronger nucleophile than HFIP.
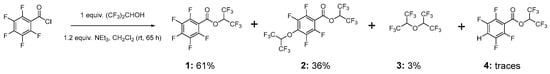
Scheme 1.
Reaction conditions of pentafluorobenzoyl chloride alcoholysis and composition of crude mixture before distillation (mol.% by 1H NMR, Figure S1, Supplementary Material).
A solution of triethylamine and HFIP was added dropwise to pentafluorobenzoyl chloride at 0 °C, forming a turbid mixture. The reaction mixture was allowed to stay at room temperature over a weekend under stirring. After workup with water and 5% aqueous HCl to remove residual triethylamine and acyl chloride, NMR analysis revealed the crude mixture contained 36 mol.% of the 1,1,1,3,3,3-hexafluoropropan-2-yl 2,3,5,6-tetrafluoro-4-((1,1,1,3,3,3-hexafluoropropan-2-yl)oxy)benzoate (2) alongside the target compound 1. Since compound 1 is a major product, we believe that the reaction of pentafluorobenzoyl chloride with HFIP proceeds in a sequential manner: initial esterification forms 1, followed by nucleophilic aromatic substitution at the para-position of the pentafluorophenyl ring in 1 via an SNAr mechanism. So, an increase in HFIP amount up to a 2-fold excess will lead to compound 2 as a major product. Similar in structure to 2, 2,2,2-trifluoroethyl 2,3,5,6-tetrafluoro-4-(2,2,2-trifluoroethoxy)benzoate, was obtained by Nishida et al. as a major product in the reaction of pentafluorobenzoyl fluoride with trimethylsilyl ether (2 equiv.) [19]. Compound 2 was isolated in pure form by distillation and characterized by spectroscopic methods.
3. Materials and Methods
3.1. General Information
1H and 19F spectra were recorded on a Bruker Avance 300 (Bruker Corporation, Billerica, MA, USA) instrument (300 MHz 1H, 282.4 MHz 19F), and 13C NMR spectra were recorded on a Bruker DRX 500 (125.7 MHz) instrument. Chemical shifts (δ) are reported in ppm relative to CCl3F (19F, upfield negative) and TMS (1H, 13C); C6F6 (δF = −162.9 ppm) and CDCl3 (δC = 76.9 ppm) served as internal standards. Coupling constants (J) are reported in Hz. The following abbreviations are used to designate multiplicities: d = doublet, t = triplet, q = quartet, and m = multiplet.
Molecular masses of the compounds were determined by HRMS with a Thermo Electron Corporation DFS (Thermo Fisher Scientific, Waltham, MA, USA) instrument (EI 70 eV). Accurate mass measurements were made relative to the lines of perfluorokerosene used as a standard in the peak matching procedure.
IR and UV-Vis spectra were recorded with a Bruker Vector 22 FT-IR spectrometer and Agilent Cary 5000 spectrophotometer (Agilent Technologies, Santa Clara, CA, USA), respectively.
HPLC analysis was carried out using an HPLC-UV system (Agilent 1100, Agilent Technologies Inc., Santa Clara, CA, USA) including a degasser (G1322A), binary pump (G1312A), autosampler (G1329A), thermostated column compartment (G1316A) with a Zorbax RX C18 column (150 × 4.6 mm with 5.0 µm particle size; Agilent Technologies Inc., Santa Clara, CA, USA), and diode array detector (DAD G1315B). The column was thermostatically controlled at 30 °C. Gradient elution was used as follows: from 30 to 100% (B) for 18 min, from 100 to 30% (B) for 1 min, and at 30% (B) for 3 min, where solvent (A) was bidistilled water and solvent (B) was 100% acetonitrile (for HPLC, gradient grade, ≥99.9%; Concord Technology, Tianjin city, China). The flow rate was 1.0 mL/min. The sample was dissolved in ACN. The elution volume varied depending on the concentration of the prepared solution. Peaks were detected using a maximum wavelength of 210 nm. Peaks from solvents were not included in the purity calculations.
3.2. Synthesis of Pentafluorobenzoyl Chloride
Pentafluorobenzoic acid (63.62 g, 300 mmol) was treated with thionyl chloride (SOCl2, 42.17 g, 787.5 mmol) and DMF (3 drops) at room temperature. The mixture was heated at 90 °C for 12 h. After cooling to room temperature, excess SOCl2 was removed by rotary evaporation. The residue was used in the following step.
3.3. Reaction of Pentafluorobenzoyl Chloride with 1,1,1,3,3,3-Hexafluoro-2-propanol
To the pentafluorobenzoyl chloride (69.15 g, 300 mmol) at 0 °C, a mixture of 1,1,1,3,3,3-hexafluoro-2-propanol (50.40 g, 300 mmol) and triethylamine (36.36 g, 360 mmol) was added. The reaction mixture was stirred at room temperature for 65 h, then quenched with water (300 mL). The organic phase was separated, and the aqueous phase was extracted with CH2Cl2 (2 × 75 mL). The combined organic extracts were washed with 5% aqueous HCl (50 mL) and dried over MgSO4. After solvent removal by rotary evaporation, a crude mixture of products was obtained (102.4 g).
The target compound was purified by vacuum distillation, yielding three distinct fractions based on boiling point ranges.
Fraction 1 (37–40 °C at 1.3 torr, 44.21 g) was a colorless liquid consisting of 1,1,1,3,3,3-hexafluoropropan-2-yl 2,3,4,5,6-pentafluorobenzoate (1) (91.8 mol%) as the primary product and minor impurities: 1,1,1,3,3,3-hexafluoropropan-2-yl 2,3,5,6-tetrafluorobenzoate (4) (4.3 mol.%), 1,1,1,3,3,3-hexafluoropropan-2-yl 2,3,5,6-tetrafluoro-4-((1,1,1,3,3,3-hexafluoropropan-2-yl)oxy)benzoate (2) (1.7 mol%), and 1,1,1,3,3,3-hexafluoro-2-((1,1,1,3,3,3-hexafluoropropan-2-yl)oxy)propane (3) (2.2 mol%) as indicated by 1H NMR (see Figure S2, Supplementary Material).
1,1,1,3,3,3-Hexafluoropropan-2-yl 2,3,4,5,6-pentafluorobenzoate (1)
1H NMR (300 MHz, CDCl3), δ: 5.97 (sept, 1H, 3JHF = 5.8 Hz).
13C{1H} NMR (125.7 MHz, CDCl3), δ: 156.4 (s, C=O), 146.4 (dm, 1JCF = 262.8 Hz), 144.9 (dm, 1JCF = 265.1 Hz), 138.1 (dm, 1JCF = 255.7 Hz), 120.1 (qm, 1JCF = 282.7, CF3), 104.9 (tm, 2JCF = 14.1 Hz), 67.7 (sept, 2JCF = 35.4 Hz, CH).
19F NMR (282.4 MHz, CDCl3), δ: −74.1 (d, 6F, 3JHF = 5.8 Hz, -CO2CH(CF3)2), −136.4 (m, 2F, F-2,6), −145.0 (tt, 1F, 3JFF = 20.8 Hz, 4JFF = 6.7 Hz, F-4), −160.0 (m, 2F, F-3,5).
HRMS: found m/z 361.9802 [M]+; calculated for C10HF11O2 361.9795.
Elemental analysis calculated for C10HF11O2 (%): C, 33.17; H, 0.28; F, 57.71; found (%): C, 33.00; H, 0.33; F, 58.15
Fraction 2 (55–60 °C at 1.3 torr, 23.38 g) was a white solid, consisting mainly of 2 (89 mol.%) and a small amount of 1 (11 mol.%). 1H NMR spectrum is shown in Figure S14, Supplementary Material.
Fraction 3 (73 °C at 1.3 torr, 8.43 g) was a white solid, pure (≥98%, HPLC) 1,1,1,3,3,3-hexafluoropropan-2-yl 2,3,5,6-tetrafluoro-4-((1,1,1,3,3,3-hexafluoropropan-2-yl)oxy)benzoate (2).
Melting point: 35.7–40.2 °C.
UV-Vis (EtOH), λmax/nm (lg ε): 235 (4.00), 283 (3.22).
FT-IR (KBr), ν, cm−1: 3435, 2981, 2927, 2855, 1768, 1650, 1504, 1428, 1385, 1363, 1334, 1295, 1238, 1207, 1115, 1076, 1040, 993, 944, 910, 690.
1H NMR (300 MHz, CDCl3), δ: 5.97 (sept, 1H, 3JHF = 5.8 Hz, -CO2CH(CF3)2), 5.02 (sept, 1H, 3JHF = 5.2 Hz, -OCH(CF3)2).
13C{1H} NMR (125.7 MHz, CDCl3), δ: 156.3 (s, C=O), 146.4 (dm, 1JCF = 265.1 Hz), 140.8 (dm, 1JCF = 253.7 Hz), 138.9 (m), 120.3 (qm, 1JCF = 285.3 Hz, CF3), 120.1 (qm, 1JCF = 285.3 Hz, CF3), 106.0 (t, 2JCF = 14.0 Hz), 67.7 (sept, 2JCF = 35.4 Hz, CH).
19F NMR (282.4 MHz, CDCl3), δ: −74.2 (d, 6F, 3JHF = 5.8 Hz, -CO2CH(CF3)2), −74.3 (m, 6F, -OCH(CF3)2), −136.2 (m, 2F, F-2,6), −154.4 (m, 2F, F-3,5).
HRMS: found m/z 509.9739 [M]+; calculated for C13H2F16O3 509.9743.
Elemental analysis calculated for C13H2F16O3 (%): C, 30.61; H, 0.40; F, 59.59; found (%): C, 30.96; H, 0.70; F, 59.97.
Supplementary Materials
The following supporting information can be downloaded: Figure S1. 1H NMR spectrum of crude mixture of reaction of pentafluorobenzoyl chloride with 1,1,1,3,3,3-hexafluoropropan-2-ol in CDCl3; Figure S2. 1H NMR spectrum of the distillation fraction 1 in CDCl3; Figure S3. 19F NMR spectrum of 1 in CDCl3; Figure S4. 13C NMR spectrum of 1 in CDCl3; Figure S5. MS spectrum of 1; Figure S6. HPLC chromatogram of the distillation fraction 1; Figure S7. 1H NMR spectrum of 2 in CDCl3; Figure S8. 19F NMR spectrum of 2 in CDCl3; Figure S9. 13C NMR spectrum of 2 in CDCl3; Figure S10. MS spectrum of 2; Figure S11. UV-Vis spectrum of 2 in EtOH; Figure S12. FT-IR spectrum of 2 in KBr; Figure S13. HPLC chromatogram of synthetized 2. Purity—98 %; Figure S14. 1H NMR spectrum of the distillation fraction 2 in CDCl3.
Author Contributions
Conceptualization, D.A.P. and A.S.V.; methodology, A.S.V.; validation, A.S.V., S.S.K., and A.V.L.; formal analysis, A.S.V., D.A.P.; investigation, S.S.K., A.S.V., and A.V.L.; resources, D.A.P. and A.S.V.; data curation, S.S.K. and A.V.L.; writing—original draft preparation, D.A.P.; writing—review and editing, D.A.P. and A.S.V.; visualization, S.S.K. and A.V.L.; supervision, D.A.P.; project administration D.A.P.; funding acquisition, D.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 25-23-00445, https://rscf.ru/project/25-23-00445/ (accessed on 14 September 2025).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank the Multi-Access Chemical Research Center of the Siberian Branch of the Russian Academy of Sciences for spectral and analytical measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brandänge, S.; Leijonmarck, H.; Minassie, T. Synthesis of esters from silyl ethers and acyl chlorides: Catalysis by quaternary ammonium chlorides. Acta Chem. Scand. 1997, 51, 953–957. [Google Scholar] [CrossRef][Green Version]
- Strohriegl, P. Esterification and amidation of polymeric acyl chlorides. A new route to polymethacrylates and polymethacrylamides with a variety of different side groups. Die Makromol. Chem. 1993, 194, 363–387. [Google Scholar] [CrossRef]
- Strazzolini, P.; Giumanini, A.G.; Verardo, G. The reaction between acyl halides and alcohols: Alkyl halide vs. Ester. formation. Tetrahedron 1994, 50, 217–254. [Google Scholar] [CrossRef]
- Van Waes, F.E.A.; Drabowicz, J.; Cukalovic, A.; Stevens, C.V. Efficient and catalyst-free condensation of acid chlorides and alcohols using continuous flow. Green. Chem. 2012, 14, 2776–2779. [Google Scholar] [CrossRef]
- Wimmer, Z.; Floro, A.J.F.D.M.; Zarevúcka, M.; Wimmerová, M.; Sello, G.; Orsini, F. Insect pest control agents: Novel chiral butanoate esters (juvenogens). Bioorg. Med. Chem. 2007, 15, 6037–6042. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-M.; Huang, C.-C. Green Conversion of Phenolic Compound to Benzoate Over Polymer-Supported Phase-Transfer Catalysts. Catal. Lett. 2009, 128, 235–242. [Google Scholar] [CrossRef]
- Leggio, A.; Belsito, E.L.; De Luca, G.; Di Gioia, M.L.; Leotta, V.; Romio, E.; Siciliano, C.; Liguori, A. One-pot synthesis of amides from carboxylic acids activated using thionyl chloride. RSC Adv. 2016, 6, 34468–34475. [Google Scholar] [CrossRef]
- Shi, M.; Ye, N.; Chen, W.; Wang, H.; Cheung, C.; Parmentier, M.; Gallou, F.; Wu, B. Simple Synthesis of Amides via Their Acid Chlorides in Aqueous TPGS-750-M. Org. Process Res. Dev. 2020, 24, 1543–1548. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, L.; Liu, Y.; Xiang, J.; Jiang, J. Fe-mediated synthesis of N-aryl amides from nitroarenes and acyl chlorides. RSC Adv. 2021, 11, 15290–15295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.-J.; Wang, J.; Grinberg, N.; Krishnamurthy, D.; Senanayake, C.H. An improved method of amide synthesis using acyl chlorides. Tetrahedron Lett. 2009, 50, 2964–2966. [Google Scholar] [CrossRef]
- Smalley, R.K.; Suschitzky, H. A simple preparation of acid anhydrides. J. Chem. Soc. 1964, 755–757. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Kabalka, G.W. Cobalt(II) chloride catalyzed syntheses of acid anhydrides from acid chlorides. Tetrahedron Lett. 1992, 33, 593–594. [Google Scholar] [CrossRef]
- Trabelsi, I.; Essid, K.; Frikha, M.H. Synthesis of mixed anhydrides of fatty acids: Stability and Reactivity. Ind. Crops Prod. 2017, 97, 552–557. [Google Scholar] [CrossRef]
- Amani, J.; Molander, G.A. Synergistic Photoredox/Nickel Coupling of Acyl Chlorides with Secondary Alkyltrifluoroborates: Dialkyl Ketone Synthesis. J. Org. Chem. 2017, 82, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, P.; Ma, Y.; Szostak, M. Mechanochemical Synthesis of Ketones via Chemoselective Suzuki–Miyaura Cross-Coupling of Acyl Chlorides. Org. Lett. 2022, 24, 2338–2343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Yin, P. Multicomponent Reaction: Pd/Cu-Catalyzed Coupling and Boration of Acyl Chlorides and Alkynes to β-Boryl Ketones. J. Org. Chem. 2023, 88, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Zhang, Y.; Cheng, K. Phosphine-Free Cross-Coupling Reaction of Arylboronic Acids with Carboxylic Anhydrides or Acyl Chlorides in Aqueous Media. J. Org. Chem. 2006, 71, 5725–5731. [Google Scholar] [CrossRef] [PubMed]
- Colomer, I.; Chamberlain, A.E.R.; Haughey, M.B.; Donohoe, T.J. Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 2017, 1, 0088. [Google Scholar] [CrossRef]
- Nishida, M.; Vij, A.; Kirchmeier, R.L.; Shreeve, J.n.M. Synthesis of Polyfluoro Aromatic Ethers: A Facile Route Using Polyfluoroalkoxides Generated from Carbonyl and Trimethysilyl Compounds. Inorg. Chem. 1995, 34, 6085–6092. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).