Abstract
The interaction of propane-1,3-dithiol with the chiral bis-thioether, which combines two 2(5H)-furanone moieties, bridged through their carbon atoms C(4) by the propane-1,3-dithiol fragment, in DMF in the presence of potassium hydroxide or cesium carbonate resulted in the formation of an optically active fused bicyclic sulfur heterocycle, possessing 1,4-dithiepine and unsaturated γ-lactone moieties. The studied reaction follows an unexpected pathway in a basic medium with the thiolate–thiolate exchange. The structure of the novel heterocycle of the 1,4-dithiepinofuranone series is characterized by single-crystal X-ray diffraction.
1. Introduction
Organosulfur compounds are very diverse and are widely used in chemical synthesis, herbal medicine, pharmacology, new materials development, agriculture, the food industry, modern technology, etc. [1,2,3,4,5,6,7,8]. The sulfur heterocycles of the 2(5H)-furanone series, including optically active ones, are also attractive objects in this area of research. 2(5H)-Furanones are oxygen heterocyclic compounds belonging to the group of α,β-unsaturated γ-lactones that play an important role in organic and medicinal chemistry. It is known that the substances possessing a 2(5H)-furanone ring exhibit a wide range of biological activities, including antitumor, antimicrobial, antifungal, antiviral, anti-inflammatory, and antioxidant [9,10,11,12,13]. The combination of two biologically active fragments in a single molecule, namely the γ-lactone ring and the sulfur-containing moiety, is considered as a promising approach towards the extension of the range of pharmaceutically important compounds and substances with other practically useful properties.
The reactions of furanones with aliphatic, aromatic, and heterocyclic thiols have been well-studied, and various thioethers, possessing an SR substituent at carbon atoms C(3), C(4), or C(5) of the lactone ring, have been selectively obtained. Sulfur-containing binucleophilic reagents have received less attention in contrast to the application of thiols in the reactions with 2(5H)-furanones, carried out under basic or acidic conditions. Thus, the interaction of furanone derivatives with S,O-, S,N-, and S,S-binucleophiles, such as 2-mercaptoethanol [14,15], o-aminothiophenol [16], thioamino acids and their derivatives [17], disodium 2-oxo-1,3-dithiole-4,5-dithiolate [18], ethane-1,2-dithiol [19], propane-1,3-dithiol [20], and 2,2′-oxydiethanethiol [21], are described. The use of these binucleophilic reagents in the reactions with 3,4-dichloroderivatives of 2(5H)-furanone allowed us to observe the structural diversity of the thiylation products on the basis of the unsaturated lactone ring, including thioethers, various bis-thioethers, sulfur-containing fused systems, and spiro bicyclic systems. Moreover, the S,O-macroheterocyclic compound containing an 18-membered oxathiamacrocycle and two 2(5H)-furanone moieties was obtained from the thiol of the furanone series in an alkaline medium [21].
Further research on furanone substrates capable of participating in macrocyclization led us to the discovery of an unexpected reaction pathway that occurs in a basic medium. Here, we report the synthesis and characterization of a novel chiral fused bicyclic sulfur heterocycle, possessing γ-lactone and 1,4-dithiepine moieties, as a trans-thiylation product of the 2(5H)-furanone bis-thioether on the basis of propane-1,3-dithiol.
A literature search revealed very few reported cases of the trans-thiylation or thiolate–thiolate exchange reactions found in the series of 1,4-diisopropylsulfanyl-2-nitrobenzene [22], 5-tert-butylsulfanyl-6-nitroquinoline [23], 2-arylsulfanyl-, and 2-benzothiazolylsulfanyl 1-nitroethenes [24], as well as the bis-thioethers obtained from the reactions of propane-1,3-dithiol with 2-chloronitrobenzene and 2-chloro-3-nitropyridine [25]. A common feature of all these reactions is that the activating nitro group is in an adjacent position to the exchanged sulfur-containing substituent. In contrast to these examples, we show in this paper the possibility of a trans-thiylation reaction in the absence of a nitro group in the molecule of the chlorocontaining bis-thioether of 2(5H)-furanone.
2. Results and Discussion
A compound that has been considered as a possible precursor of thiamacrocycle bearing a lactone ring is an optically active bis-thioether 1 that combines two furanone fragments bridged through their carbon atoms C(4) by the—S(CH2)3S—chain (Scheme 1). The bis-thioether 1 was obtained from stereochemically pure 5(S)-(l-menthyloxy)-3,4-dichloro-2(5H)-furanone and propane-1,3-dithiol under base catalysis according to a previously reported procedure [20].
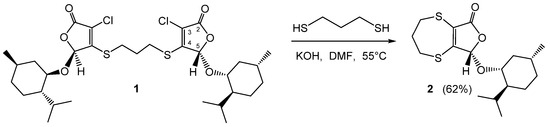
Scheme 1.
Reaction of bis-thioether 1 with propane-1,3-dithiol in the presence of KOH.
We assumed that the interaction of compound 1 with dithiol in the presence of a base would lead to the formation of the product of the nucleophilic substitution of both chlorine atoms at position 3 of two lactone rings in molecule 1 by the propane-1,3-dithiol fragment. However, the reaction of bis-thioether 1 with propane-1,3-dithiol carried out in DMF in the presence of potassium hydroxide resulted in the formation of the novel chiral bicyclic sulfur-containing compound 2 in a yield of 62% (Scheme 1). The same fused heterocycle 2 was isolated in a similar yield (58%) using high dilution conditions with the slow addition of reactants and cesium carbonate as a base, although in this case the reaction required more prolonged heating in DMF at 55 °C.
The structure of compound 2 was confirmed by IR, 1H and 13C NMR spectroscopy, and HRMS (see the Supplementary Materials). The IR spectrum of bicycle 2 exhibits the absorption bands of the stretching vibrations of the C–H bonds (2800–3000 cm−1), a strong doubly split band of the carbonyl group (1770, 1749 cm−1), and a band of medium intensity at 1654 cm−1 assigned to the stretching vibrations of the C=C bond of the lactone ring. In the 1H NMR spectrum of heterocycle 2, there were a singlet for the methine proton at carbon atom C-8 at δ 5.60 ppm, complex multiplets of the diastereotopic SCH2 protons in the range of δ 3.1–3.4 and 3.7–4.1 ppm, and a multiplet (2.2–2.4 ppm) arising from the methylene protons at carbon atom C(3). The monoterpene alcohol fragment is represented in the spectrum by three doublets of three methyl groups in the high field region (δ 0.79, 0.92, and 0.93 ppm), the signal of the methine proton at the carbon atom C(1′) (δ 3.47 ppm), as well as complex multiplets, corresponding to other protons of the l-menthol moiety in the range of δ 0.7–2.3 ppm. The signals in the one-dimensional NMR spectra were assigned by analyzing the HSQC spectrum of heterocycle 2.
The structure of bicyclic compound 2 was also determined by single-crystal X-ray diffraction using colorless crystals obtained from a hexane/benzene solution (Figure 1).
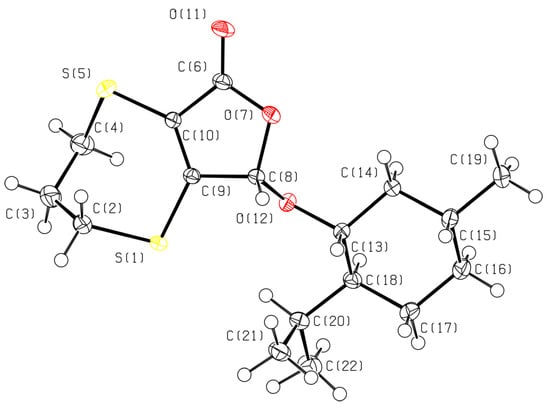
Figure 1.
Molecular structure of bicyclic compound 2 in the crystal.
The structure of compound 2 was solved in the chiral space group P212121, and the asymmetric unit contains a single crystallographically independent molecule (Z′ = 1). According to the X-ray data, the five-membered ring is planar. The seven-membered cycle of 2 in the crystal assumes a “twist” conformation: the S(1)C(9)C(10)S(5) fragment, containing a double bond C(9)=C(10) and the sulfur atoms adjacent to it, is planar. The C(3) atom lies in the same plane, and the C(2) and C(4) atoms are spaced at equal distances on the opposite sides of this plane. The X-ray diffraction data confirm the absolute configuration of the menthyl group and the specified (S)-configuration of the asymmetric carbon atom C(8) of the lactone ring in the crystal. The absolute configuration of the chiral centers has been determined from the anomalous scattering of the heavy sulfur atoms.
The observed formation of heterocycle 2 in the basic medium can be explained by the initial thiolate anion creation from propane-1,3-dithiol and the nucleophilic addition of the thiolate anion to the vinylic carbon atom C(4) of the lactone ring of bis-thioether 1. Presumably, the cleavage of the C–S bond in the intermediate anion adduct leads to the formation of two identical thiolate anions, which cyclisize to produce 1,4-dithiepinofuranone 2.
Since the result of this transformation consists of the replacement of one sulfur-containing group with another, it can be described as a trans-thiylation reaction or thiolate–thiolate exchange. This reaction may be used for the preparation of bicyclic fused sulfur-containing heterocycles possessing the 2(5H)-furanone moiety and similar compounds difficult to access by other methods.
To the best of our knowledge, only one representative of the class of fused heterocycles, possessing γ-lactone and 1,4-dithiepine moieties, has been described in the literature [26]. Namely, 8-cyclohexyl-3,4-dihydro-2H-[1,4]dithiepino[2,3-c]furan-6(8H)-one was obtained in two steps, starting from the alkylation of 2-cyclohexanoyl-1,3-dithiane with methyl bromoacetate. The subsequent treatment of the resulting ester with sodium bis(trimethylsilyl)amide caused an unexpected base-promoted carbanion rearrangement with the formation of the 1,4-dithiepinofuranone system.
3. Materials and Methods
3.1. General Information
4,4′-(Propane-1,3-diylsulfanediyl)bis{5(S)-[(1R,2S,5R)-2-isopropyl-5-methylcyclohexyloxy]-3-chlorofuran-2(5H)-one} (1) was synthesized according to the known method [20]. Propane-1,3-dithiol and Cs2CO3 (both from Acros Organics, China) were used as received without further purification. All solvents were purified and distilled by standard procedures. Analytical thin layer chromatography (TLC) was carried out on Sorbfil PTLC-AF-A-UF plates using UV light (254 nm) as the visualizing agent. Silica gel 60A (Acros Organics; China, 70–230 mesh; 0.060–0.200 mm) was used for open column chromatography. The melting point was measured on a Boetius hot stage and was not corrected. Optical rotation was measured on a Perkin-Elmer model 341 polarimeter at λ 589 nm and at 20 °C in chloroform (concentration c is provided as g/100 mL). IR spectrum was recorded on a Bruker Tensor-27 spectrometer fitted with a Pike MIRacle ATR accessory (diamond/ZnSe crystal plate). IR spectrum was recorded of solid with characteristic absorption wavenumbers (νmax) reported in cm−1. NMR spectra were measured on a Bruker Avance III 400 spectrometer at 400.17 MHz (1H) and 100.62 MHz (13C) at 20 °C in the deuterated chloroform. The chemical shifts (δ) are expressed in parts per million (ppm) and are calibrated using residual undeuterated solvent peak as an internal reference (CDCl3: δH 7.26, δC 77.16). All coupling constants (J) are reported in Hertz (Hz) and multiplicities are indicated as s (singlet), d (doublet), ddd (doublet of doublets of doublets), and m (multiplet). High-resolution mass spectrum (HRMS) was obtained through electrospray ionization (ESI) with positive (+) ion detection on a Bruker micrOTOF–QIII quadrupole time-of-flight mass spectrometer.
The X-ray diffraction (XRD) data for the single crystal of compound 2 were obtained on a Bruker D8 QUEST automated three-circle diffractometer with a PHOTON III area detector and an IμS DIAMOND microfocus X-ray tube: λ(MoKα) = 0.71073 Å, ω/ϕ scanning mode with a step of 0.5°. Data collection and indexing, determination, and refinement of unit cell parameters were carried out using the APEX4 v2021.10-0 software package. Numerical absorption correction based on the crystal shape, additional spherical absorption correction, and systematic error correction were performed using the SADABS-2016/2 software [27]. Using OLEX2 [28], structures were solved by direct methods using the SHELXT-2018/3 program [29] and refined by full-matrix least-squares on F2 using the SHELXL-2018/3 program [30]. Nonhydrogen atoms were refined anisotropically. The positions of hydrogen atoms of methyl groups were inserted using the rotation of the group with idealized bond angles; the remaining hydrogen atoms were refined using a riding model. Most calculations were performed using the WinGX-2021.3 software package [31].
Crystal data for C17H26O3S2 (M = 342.50 g/mol): orthorhombic, space group P212121 (no. 19), a = 8.2365(3) Å, b = 12.4920(6) Å, c = 17.0520(7) Å, V = 1754.49(13) Å3, Z = 4, T = 105 K, μ(Mo Kα) = 0.313 mm−1, Dcalc = 1.297 g/cm3, 54580 reflections measured (2.746° ≤ 2θ ≤ 30.062°), 5021 unique (Rint = 0.0930, Rsigma = 0.0532), which were used in all calculations. The final R1 was 0.0577 (I > 2σ(I)) and wR2 was 0.1147 (all data).
CCDC 2345671 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk). XRD data were obtained from the Collective Spectro-Analytical Center of FRC Kazan Scientific Center of RAS by support of the State Assignment of the Federal Research Center “Kazan Scientific Center”, Russian Academy of Sciences.
3.2. 8(S)-[(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyloxy]-3,4-dihydro-2H-[1,4]dithiepino-[2,3-c]furan-6(8H)-one (2)
To a solution of bis-thioether 1 (0.26 g; 0.4 mmol) in DMF (25 mL) with intense stirring under argon at room temperature was added KOH (0.05 g; 0.9 mmol), and dropwise a solution of propane-1,3-dithiol (0.04 mL; 0.4 mmol) in DMF (5 mL). The reaction mixture was stirred at 55 °C for 6 h, followed by addition of the second portion of KOH (0.05 g; 0.9 mmol) and subsequent mixing for 18 h at the same temperature (monitored by 1H NMR spectroscopy). According to 1H NMR spectroscopy, the reaction mixture contained product 2 and bis-thioether 1 in a ratio of 1:0.13. The solution was then decanted from solid and evaporated to dryness. The resulting brown solid residue was purified by column chromatography on silica gel (eluent CH2Cl2). The main fraction (Rf = 0.60) was recrystallized from a hexane–benzene mixture (5:1) to afford bicycle 2 in 62% (0.17 g) yield. Colorless crystals; mp 128–130 °C; +87.2 (c 0.87, CHCl3); IR (ATR) νmax 2960, 2930, 2875 (C–H), 1770, 1749 (C=O), 1654 (C=C lactone) cm−1; 1H NMR (CDCl3, 400 MHz, ppm) δ 5.60 (1H, s, H-8), 4.02–3.91 (1H, m, SCH2), 3.87–3.76 (1H, m, SCH2), 3.47 (1H, ddd, 3J = 10.7, 4.4 Hz, H-1′), 3.34–3.24 (1H, m, SCH2), 3.24–3.14 (1H, m, SCH2), 2.39–2.18 (4H, m, H-3, H-6′, H-8′), 1.71–1.59 (2H, m, H-3′, H-4′), 1.47–1.26 (2H, m, H-2′, H-5′), 1.15–0.73 (3H, m, H-3′, H-4′, H-6′), 0.93 (3H, d, 3J = 7.0 Hz, CH3 (iPr)), 0.92 (3H, d, 3J = 6.5 Hz, H-7′), 0.79 (3H, d, 3J = 6.9 Hz, CH3 (iPr)); 13C{1H} NMR (CDCl3, 100 MHz, ppm) δ 167.5 (C-6), 153.5 (C-8a), 125.0 (C-5a), 104.0 (C-8), 83.8 (C-1′), 48.1 (C-2′), 42.4 (C-6′), 34.2 (C-4′), 32.4, 31.6 (C-2, C-4), 31.7 (C-5′), 30.8 (C-3), 25.4 (C-8′), 23.0 (C-3′), 22.2, 21.0, 16.0 (C-7′, C-9′, C-10′); HRMS (ESI) m/z 365.1217 (calcd for C17H26NaO3S2 [M + Na]+ 365.1216).
4. Conclusions
The bis-thioether 1 on the basis of 5(S)-(l-menthyloxy)-2(5H)-furanone was converted into a novel sulfur-fused bicyclic compound 2 as a result of the thiolate–thiolate exchange reaction with propane-1,3-dithiol carried out in DMF in the presence of a base. The obtained optically active product 2 contains unsaturated γ-lactone, l-menthol, and 1,4-dithiepine fragments. The observed reaction, accompanied by the replacement of one sulfur-containing group with another, can serve as a convenient method for the preparation of bi- and tricyclic fused S-heterocycles.
Supplementary Materials
The following supporting information can be downloaded online. 1H, 13C{1H}, HSQC NMR, IR spectra, HRMS for compound 2.
Author Contributions
Conceptualization, A.R.K.; methodology, A.M.K. and E.S.R.; investigation, A.M.K., E.S.R. and D.P.G.; resources, L.Z.L.; software, E.S.R. and D.P.G.; writing—original draft preparation, A.M.K. and E.S.R.; writing—review and editing, L.Z.L. and A.R.K.; visualization, A.M.K., E.S.R., and D.P.G.; funding acquisition, L.Z.L.; project administration, A.R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the financial support of the Russian Science Foundation (project No. 23-73-10182, https://rscf.ru/en/project/23-73-10182/, accessed on 12 April 2024).
Data Availability Statement
All data are included in the manuscript and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mustafa, M.; Winum, J.Y. The importance of sulfur-containing motifs in drug design and discovery. Expert Opin. Drug Discov. 2022, 17, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Benkeblia, N.; Xiao, J. Onion (Allium cepa L.) bioactives: Chemistry, pharmacotherapeutic functions, and industrial applications. Food Front. 2022, 3, 380–412. [Google Scholar] [CrossRef]
- Ferro, C.T.B.; Dos Santos, B.F.; da Silva, C.D.G.; Brand, G.; da Silva, B.A.L.; de Campos Domingues, N.L. Review of the syntheses and activities of some sulfur-containing drugs. Curr. Org. Synth. 2020, 17, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Saidhareddy, P.; Jiang, X. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat. Prod. Rep. 2020, 37, 246–275. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, W.; Li, H.; Zhang, Q.; Liu, H. Sulfur-functionalized metal-organic frameworks: Synthesis and applications as advanced adsorbents. Coord. Chem. Rev. 2020, 408, 213191. [Google Scholar] [CrossRef]
- Jia, T.; Wang, M.; Liao, J. Chiral sulfoxide ligands in asymmetric catalysis. Top. Curr. Chem. 2019, 377, 8. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.; Capaccio, V.; Lamparelli, D.H.; Capacchione, C. Metal complexes bearing sulfur-containing ligands as catalysts in the reaction of CO2 with epoxides. Catalysts 2020, 10, 825. [Google Scholar] [CrossRef]
- Kamakura, Y.; Tanaka, D. Metal-organic frameworks and coordination polymers composed of sulfur-based nodes. Chem. Lett. 2021, 50, 523–533. [Google Scholar] [CrossRef]
- León-Rojas, A.F.; Urbina-González, J.M. Las furan-2[5H]-onas (Δα,β-butenolidas), su preparación e importancia biológica. Av. Química 2015, 10, 67–78. [Google Scholar]
- Rossi, R.; Lessi, M.; Manzini, C.; Marianetti, G.; Bellina, F. Synthesis and biological properties of 2(5H)-furanones featuring bromine atoms on the heterocyclic ring and/or brominated substituents. Curr. Org. Chem. 2017, 21, 964–1018. [Google Scholar] [CrossRef]
- Husain, A.; Khan, S.A.; Iram, F.; Iqbal, M.A.; Asif, M. Insights into the chemistry and therapeutic potential of furanones: A versatile pharmacophore. Eur. J. Med. Chem. 2019, 171, 66–92. [Google Scholar] [CrossRef] [PubMed]
- Villamizar-Mogotocoro, A.-F.; León-Rojas, A.-F.; Urbina-González, J.-M. Δα,β-Butenolides [furan-2(5H)-ones]: Ring construction approaches and biological aspects—A mini-review. Mini-Rev. Org. Chem. 2020, 17, 922–945. [Google Scholar] [CrossRef]
- Kayumov, A.R.; Sharafutdinov, I.S.; Trizna, E.Y.; Bogachev, M.I. Antistaphylococcal activity of 2(5H)-furanone derivatives. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms: Current Research and Future Trends in Microbial Biofilms, 1st ed.; Yadav, M.K., Singh, B.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 77–89. [Google Scholar] [CrossRef]
- Gumulka, W.; Kokosinski, J. Derivatives of mucochloric acid. Part I. Pseudoesters and pseudothioesters of mucochloric acid. Pol. Organika 1976, 1, 45–60. [Google Scholar]
- Devyatova, N.F.; Kosolapova, L.S.; Kurbangalieva, A.R.; Berdnikov, E.A.; Lodochnikova, O.A.; Litvinov, I.A.; Chmutova, G.A. Reactions of 2-sulfanylethanol with mucochloric acid and its derivatives. Russ. J. Org. Chem. 2008, 44, 1225–1232. [Google Scholar] [CrossRef]
- Ren, J.; Ma, D.; Sha, Y.; Li, F.; Cheng, M. A new synthesis of novel tricyclic 2(5H)-furanone heterocycles from 3,4,5-trichloro-2(5H)-furanone. Heterocycles 2010, 81, 1427–1434. [Google Scholar] [CrossRef]
- LaLonde, R.T.; Xie, S. A study of inactivation reactions of N-acetylcysteine with mucochloric acid, a mutagenic product of the chlorination of humic substances in water. Chem. Res. Toxicol. 1992, 5, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Hartke, K.; Rauschen, F. Einige ungewöhnliche umsetzungen des dinatrium-2-oxo-1,3-dithiolats mit biselektrophilen. J. Prakt. Chem. 1997, 339, 15–19. [Google Scholar] [CrossRef]
- Kurbangalieva, A.R.; Lodochnikova, O.A.; Devyatova, N.F.; Berdnikov, E.A.; Gnezdilov, O.I.; Litvinov, I.A.; Chmutova, G.A. Structural diversity of interaction products of mucochloric acid and its derivatives with 1,2-ethanedithiol. Tetrahedron 2010, 66, 9945–9953. [Google Scholar] [CrossRef]
- Khabibrakhmanova, A.M.; Rabbanieva, E.S.; Gerasimova, D.P.; Islamov, D.R.; Latypova, L.Z.; Lodochnikova, O.A.; Kurbangalieva, A.R. Optically active bisthioethers and disulfones derived from furan-2(5H)-one and dithiols: Synthesis and structure. Russ. J. Org. Chem. 2022, 58, 1160–1169. [Google Scholar] [CrossRef]
- Kurbangalieva, A.R.; Hoang, L.T.; Lodochnikova, O.A.; Kuzmicheva, M.Y.; Pradipta, A.R.; Tanaka, K.; Chmutova, G.A. First example of synthesis of S,O-macroheterocycle on the basis of 2(5H)-furanone and 2,2′-oxydiethanethiol. Russ. Chem. Bull. 2016, 65, 1278–1284. [Google Scholar] [CrossRef]
- Cogolli, P.; Testaferri, L.; Tingoli, M.; Tiecco, M. Alkyl thioether activation of the nitro displacement by alkanethiol anions. A useful process for the synthesis of poly[(alky1thio)benzenes]. J. Org. Chem. 1979, 44, 2636–2642. [Google Scholar] [CrossRef]
- Kawakami, T.; Suzuki, H. Direct thioalkylation of nitroquinolines with alkanethiolate anions via nucleophilic displacement of a ring hydrogen atom. J. Chem. Soc., Perkin Trans. 1 2000, 21, 3640–3644. [Google Scholar] [CrossRef]
- Kuz’mina, N.V.; Lipina, E.S.; Kropotova, T.Y.; Berkova, G.A.; Pavlova, Z.F. 1-Nitro-2-thio(sulfonyl)alkenes in reactions with thiols. Russ. J. Org. Chem. 2001, 37, 1259–1265. [Google Scholar] [CrossRef]
- Miroshnichenko, A.V.; Demchuk, D.V.; Nikishin, G.I. Exchange reactions of 1,3-bis(nitroarylthio)propanes with propane-1,3-dithiols. Russ. Chem. Bull. 2006, 55, 168–171. [Google Scholar] [CrossRef]
- Fétizon, M.; Goulaouic, P.; Hanna, I.; Prangé, T. An unexpected synthesis of the 1,4-dithiaepino-furan-2-one system. Synth. Commun. 1989, 19, 973–977. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).