Abstract
1,3-Alternate calix[4]arenes were prepared, having bulky adamantyl groups in the p-positions of all four aromatic units of the macrocycles and pairs of propargyl or 2-azidoethyl groups alternating with n-propyl groups at the phenol oxygen atoms. The step-wise syntheses were carried out through a selective distal alkylation of the parent p-adamantylcalix[4]arene with propargyl bromide or 1,2-dibromoethane, resulting in calix[4]arenes bearing pairs of propargyl or 2-bromoethyl groups at their narrow rims. The bromine atoms were replaced by azide groups, and then both calix[4]arene diethers were exhaustively alkylated at the remaining OH-groups with 1-iodopropane under stereoselective conditions to fix the macrocycles in an 1,3-alternate shape. The structures of the prepared p-adamantylcalix[4]arenes were confirmed by NMR and HRMS data, and, for the 1,3-alternate dipropargyl ether, the X-ray diffraction data were also collected. Preliminary data on the reactivity of the prepared calixarenes under the CuAAC conditions suggested a strong steric hampering created by the adamantane units nearby the reacting alkyne or azide groups that affected the outcome of the two-fold cycloaddition involving the calixarene bis(azides) or bis(alkynes) as complementary partners.
1. Introduction
The adamantane structure is well recognized as a molecular core for biomedical and pharmaceutical applications, which is due to its specific shape, size and lipophilicity, empowered by functionalization capabilities [1,2]. Since their introduction into calixarene chemistry three decades ago [3,4], adamantane-containing groups have been demonstrated to be useful building blocks capable of bringing receptor functionalities to the macrocycles or/and tuning their receptor properties. Indeed, several unsubstituted adamantyl units arranged directly at the wide rim of the calixarene skeleton, or those connected to the narrow rim of the cores through spacers, are responsible for intermolecular interactions between calixarene metal complexes in crystals [5] and may affect the cation extraction processes [6]; these groups alter the mechanical properties of calixarene-containing polymers [7] or can anchor calixarenes onto pre-functionalized surfaces through host–guest interactions [8]. The wide-rim-located adamantyl units regulate penetration of charged guests into/through the aromatic cavity of calixarene macrorings [9], or they may contain additional groups and serve as linkers to insert receptor or/and reporter functionalities into the (multi)calixarene structures [10]. Also, when attached directly to the calixarene receptor sites, adamantyl groups affect the efficiency and selectivity of their supramolecular interactions due to steric reasons [11,12,13,14].
Since its discovery [15,16] and first implementation in calixarene chemistry [17], the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) [18,19] has rapidly become a useful tool for the preparation of calixarene conjugates bearing sophisticated functional units, e.g., sugars and amino acids/peptides [20,21,22]. Even more importantly, the enrichment of calixarene chemistry with the CuAAC approach has made available macrocycles having several 1,4-disubstituted 1,2,3-triazole groups attached to a common platform, which appeared to be efficient multidentate ligands for binding and sensing transition metal cations [23,24,25,26,27]. Most of these triazolated calixarenes contain pairs of the receptor groups attached through short linkers to the distal phenol oxygen atoms of the calix[4]arene macrocycle having the cone or 1,3-alternate shape. In the latter case, the inverted calixarene aromatic units form a common pocket with the functional groups, thus drastically affecting the receptor capabilities of the whole site. Taking into account the above features of the adamantane units, their attachment to the p-positions of 1,3-alternate calix[4]arenes may provide additional shielding of the functional groups residing at the oxygen atoms of the macrocycle, which in turn must affect the accessibility of these groups for supramolecular interactions, thus improving selectivity of the triazole-containing receptor sites. In line with this, herein we report the synthesis of the 1,3-alternate-shaped p-adamantylcalix[4]arenes bearing pairs of propargyl or 2-azidoethyl groups at distal phenol oxygen atoms of the macrocycles, which can be further converted into the respective 1-R-4-triazolylmethylated or 4-R-1-triazolylethylated receptor macrocycles upon reacting with azides or alkynes under the CuAAC conditions.
2. Results and Discussion
The readily available p-adamantylcalix[4]arene 1 (in the form of a stable 1:1 complex with p-xylene) [4] was used as the starting compound. First, calixarene 1 was reacted with propargyl bromide in acetone using sodium carbonate as a base, which was crucial for alkylation of only two of four phenol OH-groups of the calixarene macrocycle (Scheme 1). The resulting dipropargyl ether 2, which was thus prepared in good yield, appeared to be substituted in distal aromatic units of the macrocycle as it followed from the signal count in the NMR spectra. Though formally flexible due to inversion of the unsubstituted calixarene aromatic units, calixarene 2 adopted a cone conformation in a CDCl3 solution because of intramolecular hydrogen bonds between the OH and OR groups, as it followed from the characteristic patterns [28] observed for the signals of the calixarene methylene groups in 1H and 13C NMR spectra (see Section 3 and Figures S1 and S2 in the Supplementary Materials).
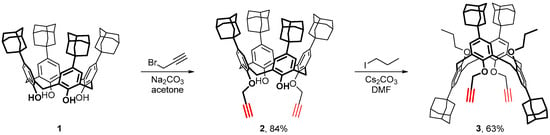
Scheme 1.
Synthesis of adamantylated calix[4]arene 3 bearing pairs of n-propyl and propargyl groups at the opposed sides of the 1,3-alternate macrocycle.
Next, the remaining phenol hydroxyl groups in calixarene 2 were alkylated with 1-iodopropane to block the inversion of the aromatic units and fix the macrocycle in an 1,3-alternate shape. In this case, Cs2CO3 was selected as a base because the template effect from the Cs+ cation was well-documented as being responsible for the formation of the 1,3-alternate calix[4]arene macrocycles during alkylation [29]. The targeted 1,3-alternate calix[4]arene 3 was expected to be not the only product of the alkylation due to competing formation of a partial cone isomer. Still, the yield of compound 3 was surprisingly high, which might be explained by steric repulsions between bulky adamantane units stabilizing the 1,3-alternate shape of the macrocycle during the reaction. For the NMR spectra of compound 3 see Section 3 and Figures S3 and S4.
Upon slow evaporation of a dichloromethane/methanol solution of calixarene 3, single crystals were collected and subjected to X-ray diffraction analysis. The obtained data confirmed unambiguously the structure of this compound having the 1,3-alternate shape and bearing pairs of the alternating n-propyl and propargyl groups attached to the adamantylated macrocycle (Figure 1).
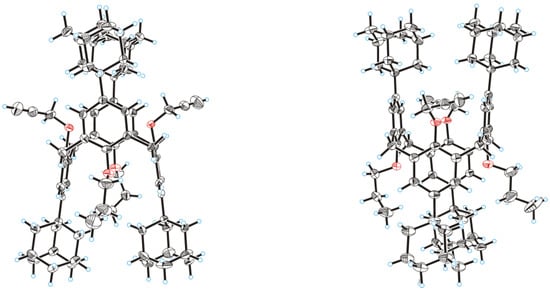
Figure 1.
Molecular structure of the calixarene 3 in two projections; thermal ellipsoids are drawn at a 50% probability level.
For the preparation of the adamantylated bis(azide) 6, the parent adamantylated calixarene 1 was first alkylated with 1,2-dibromoethane (Scheme 2). In contrast to the synthesis of the dipropargyl ether, a stronger base (K2CO3) was required to complete the process, and a huge excess of the alkylating reactant had to be used to suppress the undesired alkylation of calixarene 1 by the just-formed dibromide 4. Next, the obtained calixarene 4 (see Section 3 and Figures S5 and S6 for the NMR spectra) was reacted with sodium azide to replace the bromine atoms with the azide groups, and the resulting bis(azide) 5 (see Section 3 and Figures S7 and S8 for the NMR spectra) was subjected to alkylation with 1-iodopropane in the presence of Cs2CO3 as a base. Similar to the synthesis of compound 3, the desired 1,3-alternate calix[4]arene bearing pairs of n-propyl and 2-azidoethyl groups at the opposed sides of the macrocycle was obtained in relatively high yield, which could result from the intramolecular repulsions between bulky groups during the reaction.
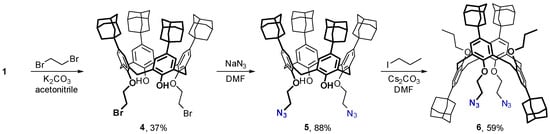
Scheme 2.
Synthesis of adamantylated calix[4]arene 6 bearing pairs of n-propyl and 2-azidoethyl groups.
The structure of bis(azide) 6 was concluded from the NMR data (see Section 3 and Figures S9 and S10). In particular, the 13C NMR spectrum of this compound contains a single resonance from the methylene groups of the calixarene macroring at ~39 ppm, which is characteristic for the 1,3-alternate shape of the molecule [28]. Also, for all the prepared compounds, HRMS data were obtained to confirm their structures.
We have recently shown that the 1,3-alternate calix[4]arene bis(alkyne) 7 and bis(azide) 8, both having no substituents in the p-positions of the calixarene macrocycles, can be readily assembled under the Cu+-catalysis into a semi-tubular biscalixarene 9 (Scheme 3). This compound, in which two triazole groups played both the linker and the receptor functions simultaneously, has been prepared in relatively high yield for such a two-fold reaction using CuI/Et3N or, better, CuSO4·5H2O/sodium ascorbate catalytic systems. Polymeric/oligomeric substances were the only by-products detected in the reaction mixtures, thus indicating a complete conversion of the starting calixarenes 7 and 8 [30].
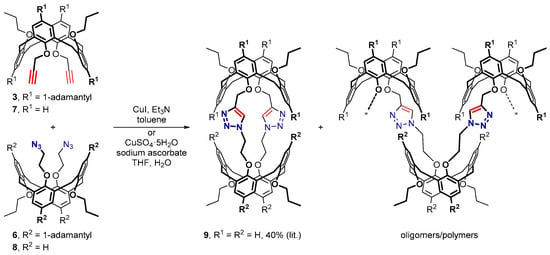
Scheme 3.
Two-fold CuAAC reactions between propargylated and 2-azidoethylated calix[4]arenes.
To assess the effect of the bulky adamantane units surrounding the propargyl or 2-azidoethyl groups in compounds 3 and 6 on their reactivity under the CuAAC conditions, similar experiments involving these compounds were carried out. It was found that neither CuI/Et3N nor CuSO4·5H2O/sodium ascorbate could efficiently catalyze the desired two-fold CuAAC between calixarenes 3 and 6, and only the signals from the starting compounds were detected in the 1H NMR spectra of the reaction mixtures after removal of the copper salts, though a conversion of a small part of the starting materials into a polymeric/oligomeric substance was detected in the CuI/Et3N-catalyzed reaction. Reasonably, the adamantane-shielded azide groups of calixarene 6 can hardly reach the dicopper-acetylide intermediate formed inside the adamantylated pocket of calixarene 3 during the CuAAC, so neither the desired semi-tube formation nor the less hindered polymerization/oligomerization took place.
In line with this, under the CuI/Et3N-catalysis (toluene, 60 °C, 24 h), bis(alkyne) 3 and bis(azide) 6 reacted completely with their less hindered CuAAC-partners 8 and 7, respectively, though only polymeric/oligomeric products were detected in the reaction mixtures. On the other hand, in the CuSO4·5H2O/sodium ascorbate-catalyzed reactions conducted in a more polar media (THF/water (5:1), 60 °C, 24 h), calixarenes 3 and 6 behaved differently. In these cases, a complete conversion of the starting materials into polymeric/oligomeric products was observed for the 6/7 bis(azide)/bis(alkyne) pair but not for the 8/3 one. Tentatively, the steric hindrance provided by the bulky adamantane units in the 1,3-alternate calix[4]arenes affects more drastically the CuAAC-reactivity of bis(alkyne) 3 rather than bis(azide) 6.
3. Materials and Methods
Column chromatography was performed on silica gel 60 (0.063–0.200 mm). Commercial reagents were used as received. Compounds 1 [4], 7 and 8 [30] were synthesized by the reported procedures.
1H and 13C (APT) NMR spectra were acquired on a Bruker Avance 400 spectrometer (Bruker, Billerica, MA, USA) at room temperature. High resolution ESI mass spectra were obtained from a Sciex TripleTOF 5600+ spectrometer (AB Sciex, Singapore). FT-IR spectra were registered on a Nicolet iS 5 (Thermo Fisher Scientific, Waltham, MA, USA) with iD3 ATR accessory (ZnSe).
Crystallographic data were collected on a Bruker D8 Venture diffractometer using graphite monochromatized Mo–Kα radiation (λ = 0.71073 Å) using an ω-scan mode. Absorption correction based on measurements of equivalent reflections was applied [31]. The structure was solved by direct methods and refined by full matrix least-squares on F2 with anisotropic thermal parameters for all non-hydrogen atoms [32,33,34]. Some components of the disordered groups were refined isotropically. The hydrogen atoms were placed in calculated positions and refined using a riding model.
CCDC 2348304 contains crystallographic data for compound 3. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures (accessed on 20 April 2024).
3.1. 5,11,17,23-Tetra(1-adamantyl)-25,27-dihydroxy-26,28-di(2-propynyloxy)calix[4]arene 2
A mixture of p-(1-adamantyl)calix[4]arene 1 (1:1 complex with p-xylene, 1.07 g, 1.00 mmol), anhydrous Na2CO3 (1.27 g, 12.0 mmol), propargyl bromide (80% solution in toluene, 1.00 mL, 9.0 mmol) and dry acetone (40 mL) was stirred at reflux for 50 h. After cooling to room temperature, the mixture was filtered, the solid was collected, washed with acetone, and the acetone filtrate was discarded. The solid was washed repeatedly with dichloromethane; the combined dichloromethane solution was concentrated under reduced pressure and methanol was added. The solid formed was collected, washed with methanol and dried. Yield 0.87 g (84%, white solid). M.p. 239–241 °C (decomp.). 1H NMR (CDCl3, 400 MHz): δ = 7.04 (s, 4H; ArH), 6.70 (s, 4H; ArH), 6.45 (s, 2H; OH), 4.73 (d, 4H, 4JHH = 2.4 Hz; OCH2), 4.35 (d, 4H, 2JHH = 13.3 Hz; ArCH2Ar), 3.33 (d, 4H, 2JHH = 13.3 Hz; ArCH2Ar), 2.52 (t, 2H, 4JHH = 2.4 Hz; CH), 2.12–2.05 (m, 6H; CHAd), 1.93–1.89 (m, 12H; CH2,Ad), 1.88–1.83 (m, 6H; CHAd), 1.82–1.70 (m, 12H; CH2,Ad), 1.67–1.59 (m, 6H; CH2,Ad), 1.54–1.47 (m, 6H; CH2,Ad), 1.46–1.41 (m, 12H; CH2,Ad) ppm. 13C NMR (CDCl3, 100 MHz): δ = 150.29, 149.22, 147.26, 141.86, 132.51, 127.93 (CAr), 125.08, 124.47 (CHAr), 78.73 (CCH), 76.25 (CCH), 63.19 (OCH2), 43.69, 42.78, 36.91, 36.63 (CH2,Ad), 35.35, 35.30 (CAd), 31.89 (ArCH2Ar), 29.09, 28.77 (CHAd) ppm. HRMS ESI-MS: m/z: 1054.6701 [M + NH4]+ for C74H88NO4 (1054.6708).
3.2. 5,11,17,23-Tetra(1-adamantyl)-25,27-di(1-propyloxy)-26,28-di(2-propynyloxy)calix[4]arene (1,3-alternate) 3
A mixture of calixarene 2 (0.414 g, 0.40 mmol) and anhydrous Cs2CO3 (0.520 g, 1.60 mmol) in dry DMF (40 mL) was stirred at room temperature for 2 h. 1-Iodopropane (0.160 mL, 1.60 mmol) was added, and the mixture was stirred at room temperature for 48 h. The solvent was removed under reduced pressure with heating below 50 °C, and the residue was parted between dichloromethane and aqueous HCl (2 M). The organic layer was separated, washed with water, dried with MgSO4 and concentrated to dryness. The product was isolated by crystallization from a dichloromethane/methanol mixture. Yield 0.28 g (63%, white solid). M.p. > 300 °C. 1H NMR (CDCl3, 400 MHz): δ = 7.08 (s, 4H; ArH), 6.94 (s, 4H; ArH), 3.86 (d, 4H, 2JHH = 15.4 Hz; ArCH2Ar), 3.73 (d, 4H, 2JHH = 15.4 Hz; ArCH2Ar), 3.71 (d, 4H, 4JHH = 2.4 Hz; OCH2CCH), 3.36–3.28 (m, 4H; OCH2CH2), 2.30 (t, 2H, 4JHH = 2.4 Hz; CH), 2.08–2.01 (m, 12H; CHAd), 1.95–1.89 (m, 12H; CH2,Ad), 1.87–1.82 (m, 12H; CH2,Ad), 1.80–1.66 (m, 24H; CH2,Ad), 1.35–1.23 (m, 4H; CH2CH3), 0.76 (t, 6H, 3JHH = 7.5 Hz; CH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 154.42, 153.34, 144.52, 144.39, 133.92, 133.32 (CAr), 126.60, 126.12 (CHAr), 81.73 (CCH), 73.94 (CCH), 72.47 (OCH2CH2), 58.24 (OCH2CCH), 43.26, 43.19 (CH2,Ad), 38.82 (ArCH2Ar), 36.98, 36.95 (CH2,Ad), 35.43, 35.41 (CAd), 29.11, 29.09 (CHAd), 23.02 (CH2CH3), 10.37 (CH3) ppm. HRMS ESI-MS: m/z: 1138.7648 [M + NH4]+ for C80H100NO4 (1138.7647). Crystal data (CCDC 2348304): temp. (K) = 150 (2); cryst. system: triclinic; space group: P-1; a (Å) = 11.6732 (5); b (Å) = 16.7232 (7); c (Å) = 17.6214 (8); α (°) = 98.9870 (10); β (°) = 96.472 (2); γ (°) = 109.4080 (10); V (Å3) = 3154.2 (2); Z = 2; θ range (deg): 1.87 < θ < 26.42; collected/unique reflections: 30026/12821; completeness to θ (%) = 99.1; data/restraints/parameters: 12821/41/801; goodness of fit on F2 = 1.057; final R indices (I > 2σ(I)): R1 = 0.0846, wR2 = 0.1933; largest diff peak/hole (e/Å3): 0.43/−0.40.
3.3. 5,11,17,23-Tetra(1-adamantyl)-25,27-di(2-bromoethyloxy)-26,28-dihydroxycalix[4]arene 4
A mixture of p-(1-adamantyl)calix[4]arene 1 (1:1 complex with p-xylene, 0.926 g, 0.87 mmol), anhydrous K2CO3 (0.264 g, 1.91 mmol) and dry acetonitrile (25 mL) was stirred at reflux for 1 h and then cooled to room temperature. 1,2-Dibromoethane (3.0 mL, 34.8 mmol) was added, and the mixture was stirred at reflux for 30 h. After cooling, the precipitate was collected, washed with acetonitrile, and the acetonitrile filtrate was discarded. The solid was washed repeatedly with dichloromethane; the combined dichloromethane solution was washed with aqueous HCl (2 M), then with water and dried with MgSO4. After removal of the solvent under reduced pressure, the product was purified by column chromatography (silica, gradient from hexane to hexane/dichloromethane, 1:3) followed by re-crystallization from a dichloromethane/methanol solvent mixture. Yield 0.375 g (37%, white solid). M.p. > 300 °C. 1H NMR (CDCl3, 400 MHz): δ = 7.05 (s, 4H; ArH), 6.82 (s, 2H; OH), 6.76 (s, 4H; ArH), 4.35–4.25 (m, 8H; CH2Br, ArCH2Ar), 3.87–3.78 (m, 4H; OCH2), 3.33 (d, 4H, 2JHH = 13.1 Hz; ArCH2Ar), 2.16–2.04 (m, 6H; CHAd), 1.95–1.86 (m, 18H; CHAd, CH2,Ad), 1.84–1.71 (m, 12H; CH2,Ad), 1.70–1.63 (m, 6H; CH2,Ad), 1.58–1.51 (m, 6H; CH2,Ad), 1.51–1.45 (m, 12H; CH2,Ad) ppm. 13C NMR (CDCl3, 100 MHz): δ = 150.44, 149.11, 147.28, 141.94, 132.28, 127.75 (CAr), 125.21, 124.61 (CHAr), 75.29 (OCH2), 43.72, 42.83, 36.93, 36.66 (CH2,Ad), 35.43, 35.35 (CAd), 31.51 (ArCH2Ar), 29.32 (CH2Br), 29.10, 28.81 (CHAd) ppm. HRMS ESI-MS: m/z: 1213.4513 [M + K]+ for C72H86Br2KO4 (1213.4504).
3.4. 5,11,17,23-Tetra(1-adamantyl)-25,27-di(2-azidoethyloxy)-26,28-dihydroxycalix[4]arene 5
A mixture of calixarene 4 (0.590 g, 0.50 mmol) and NaN3 (0.130 g, 2.0 mmol) in dry DMF (25 mL) was stirred at 60 °C for 6 h. The solvent was removed under reduced pressure, and the residue was parted between dichloromethane and aqueous HCl (2 M). The organic layer was separated, washed with water, dried with MgSO4 and then evaporated. The residue was dissolved in a minimum volume of dichloromethane; methanol was added and the solid formed was collected, washed with methanol and dried. Yield 0.485 g (88%, white solid). M.p. 279–281 °C (decomp.). 1H NMR (CDCl3, 400 MHz): δ = 7.06 (s, 4H; ArH), 6.85 (s, 2H; OH), 6.76 (s, 4H; ArH), 4.32 (d, 4H, 2JHH = 13.1 Hz; ArCH2Ar), 4.12–4.02 (m, 4H; CH2N3), 3.86–3.77 (m, 4H; OCH2), 3.33 (d, 4H, 2JHH = 13.1 Hz; ArCH2Ar), 2.17–2.07 (m, 6H; CHAd), 1.97–1.92 (m, 12H; CH2,Ad), 1.91–1.86 (m, 6H; CHAd), 1.85–1.72 (m, 12H; CH2,Ad), 1.71–1.62 (m, 6H; CH2,Ad), 1.57–1.50 (m, 6H; CH2,Ad), 1.50–1.43 (m, 12H; CH2 Ad) ppm. 13C NMR (CDCl3, 100 MHz): δ = 150.60, 149.20, 147.20, 141.80, 132.32, 127.80 (CAr), 125.17, 124.53 (CHAr), 73.99 (OCH2), 51.06 (CH2N3), 43.77, 42.86, 36.97, 36.68 (CH2,Ad), 35.43, 35.36 (CAd), 31.29 (ArCH2Ar), 29.14, 28.83 (CHAd) ppm. HRMS ESI-MS: m/z: 1137.6345 [M + K]+ for C72H86KN6O4 (1137.6342). IR (neat): υ = 2105 (N3) cm–1.
3.5. 5,11,17,23-Tetra(1-adamantyl)-25,27-di(2-azidoethyloxy)-26,28-di(1-propyloxy)calix[4]arene (1,3-alternate) 6
A mixture of calixarene 5 (0.220 g, 0.20 mmol) and anhydrous Cs2CO3 (0.260 g, 0.80 mmol) in dry DMF (20 mL) was stirred at room temperature for 2 h. 1-Iodopropane (0.080 mL, 0.80 mmol) was added, and the mixture was stirred at room temperature for 48 h. The solvent was removed under reduced pressure, and the residue was parted between dichloromethane and aqueous HCl (2 M). The organic layer was separated, washed with water, dried with MgSO4 and concentrated to dryness. The product was isolated by crystallization from a dichloromethane/methanol mixture. Yield 0.14 g (59%, white solid). M.p. > 300 °C. 1H NMR (CDCl3, 400 MHz): δ = 6.98 (s, 4H; ArH), 6.93 (s, 4H; ArH), 3.87 (d, 4H, 2JHH = 16.7 Hz; ArCH2Ar), 3.81 (d, 4H, 2JHH = 16.7 Hz; ArCH2Ar), 3.37–3.31 (m, 4H; CH2N3), 3.31–3.25 (m, 4H; OCH2), 2.61–2.53 (m, 4H; OCH2), 2.13–2.03 (m, 12H; CHAd), 1.94–1.88 (m, 12H; CH2,Ad), 1.87–1.83 (m, 12H; CH2,Ad), 1.80–1.68 (m, 24H; CH2,Ad), 0.95–0.83 (m, 4H; CH2CH3), 0.58 (t, 6H, 3JHH = 7.5 Hz; CH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 154.90, 153.43, 145.00, 144.84, 133.30, 132.85 (CAr), 125.19, 124.75 (CHAr), 71.26, 66.28 (OCH2), 48.98 (CH2N3), 43.54, 43.39 (CH2,Ad), 39.14 (ArCH2Ar), 36.88, 36.78 (CH2,Ad), 35.51, 35.44 (CAd), 29.05, 28.99 (CHAd), 22.19 (CH2CH3), 10.06 (CH3) ppm. HRMS ESI-MS: m/z: 1200.7989 [M + NH4]+ for C78H102N7O4 (1200.7988). IR (neat): υ = 2090 (N3) cm–1.
4. Conclusions
We have shown that the 1,3-alternate calix[4]arenes bearing 1-adamantyl groups in p-positions of all four aromatic units and pairs of propargyl or 2-azidoethyl groups at distal phenol oxygen atoms can be readily prepared in a multistep synthesis starting from p-(1-adamantyl)calix[4]arene. Though the bulky adamantane units seem to assist the formation of the targeted 1,3-alternate-shaped macrocycles during the synthesis of the bis(alkyne) and bis(azide), they can also hamper their participation in the CuAAC reactions. Thus, tuning of the reaction conditions may be required to achieve a complete conversion of the adamantylated calixarene bis(alkyne) and bis(azides) into the respective triazolated products using the CuAAC approach.
Supplementary Materials
Figure S1: 1H NMR spectrum of compound 2; Figure S2: 13C NMR spectrum (APT) of compound 2; Figure S3: 1H NMR spectrum of compound 3; Figure S4: 13C NMR spectrum (APT) of compound 3; Figure S5: 1H NMR spectrum of compound 4; Figure S6: 13C NMR spectrum (APT) of compound 4; Figure S7: 1H NMR spectrum of compound 5; Figure S8: 13C NMR spectrum (APT) of compound 5; Figure S9: 1H NMR spectrum of compound 6; Figure S10: 13C NMR spectrum (APT) of compound 6.
Author Contributions
Conceptualization, A.G. and I.V.; methodology, A.G. and I.V.; investigation, M.M., A.G. and I.L.; resources, S.B. and A.G.; data curation, I.L., A.G. and S.B.; validation, I.L. and A.G.; writing—original draft preparation, I.V. and A.G.; writing—review and editing, V.K., A.G. and I.L.; supervision, A.G. and V.K.; project administration, A.G.; funding acquisition, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Russian Science Foundation (Grant No. 22-73-00052).
Data Availability Statement
The data are contained within the article or Supplementary Materials.
Acknowledgments
X-ray diffraction studies were performed at the Centre of Shared Equipment of IGIC RAS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dragomanova, S.; Andonova, V. Adamantane-containing drug delivery systems. Pharmacia 2023, 70, 1057–1066. [Google Scholar] [CrossRef]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The Lipophilic Bullet Hits the Targets: Medicinal Chemistry of Adamantane Derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Neu, F.; Collins, E.M.; Deasy, M.; Ferguson, G.; Harris, S.J.; Kaitner, B.; Lough, A.J.; McKervey, M.A.; Marques, E.; Ruhl, B.L.; et al. Synthesis, X-ray crystal structures, and cation-binding properties of alkyl calixaryl esters and ketones, a new family of macrocyclic molecular receptors. J. Am. Chem. Soc. 1989, 111, 8681–8691. [Google Scholar] [CrossRef]
- Khomich, A.N.; Shokova, E.A.; Kovalev, V.V. Synthesis of p-(1-Adamantyl)- and p-(3-Substituted-1-Adamantyl)calix[4]arenes. Synlett 1994, 1994, 1027–1028. [Google Scholar] [CrossRef]
- Ovsyannikov, A.S.; Strelnikova, I.V.; Samigullina, A.I.; Islamov, D.R.; Cherosov, M.A.; Batulin, R.G.; Kiiamov, A.G.; Gubaidullin, A.T.; Dorovatovskii, P.V.; Solovieva, S.E.; et al. Influence of neutral auxiliary ligands on crystal structure and magnetic behaviour of new [Mn II2Mn III2] clusters supported by p-adamantylcalix[4]arene. New J. Chem. 2024, 48, 203–215. [Google Scholar] [CrossRef]
- Vatsouro, I.; Serebryannikova, A.; Wang, L.; Hubscher-Bruder, V.; Shokova, E.; Bolte, M.; Arnaud-Neu, F.; Böhmer, V. Narrow rim CMPO/adamantylcalix[4]arenes for the extraction of lanthanides and actinides. Tetrahedron 2011, 67, 8092–8101. [Google Scholar] [CrossRef]
- Yang, Y.; Swager, T.M. Main-Chain Calix[4]arene Elastomers by Ring-Opening Metathesis Polymerization. Macromolecules 2007, 40, 7437–7440. [Google Scholar] [CrossRef]
- Bruinink, C.M.; Nijhuis, C.A.; Péter, M.; Dordi, B.; Crespo-Biel, O.; Auletta, T.; Mulder, A.; Schönherr, H.; Vancso, G.J.; Huskens, J.; et al. Supramolecular Microcontact Printing and Dip-Pen Nanolithography on Molecular Printboards. Chem. Eur. J. 2005, 11, 3988–3996. [Google Scholar] [CrossRef]
- Iuliano, V.; Talotta, C.; Gaeta, C.; Hickey, N.; Geremia, S.; Vatsouro, I.; Kovalev, V.; Neri, P. Influence of exo-Adamantyl Groups and endo-OH Functions on the Threading of Calix[6]arene Macrocycle. J. Org. Chem. 2020, 85, 12585–12593. [Google Scholar] [CrossRef]
- Puchnin, K.; Zaikin, P.; Cheshkov, D.; Vatsouro, I.; Kovalev, V. Calix[4]tubes: An Approach to Functionalization. Chem. Eur. J. 2012, 18, 10954–10968. [Google Scholar] [CrossRef]
- Ferreira, A.S.D.; Ascenso, J.R.; Marcos, P.M.; Schurhammer, R.; Hickey, N.; Geremia, S. Calixarene-Based lead receptors: An NMR, DFT and X-Ray synergetic approach. Supramol. Chem. 2021, 33, 231–244. [Google Scholar] [CrossRef]
- Lazzarotto, M.; Los Weinert, P.; Lazzarotto, M. Electronic parameters of cation complexation by calixarene ionophores. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 313–322. [Google Scholar] [CrossRef]
- Pop, A.; Vysotsky, M.; Saadioui, M.; Böhmer, V. Self-assembled dimers with supramolecular chirality. Chem. Commun. 2003, 10, 1124–1125. [Google Scholar] [CrossRef]
- Pinkhassik, E.; Sidorov, V.; Stibor, I. Calix[4]arene-Based Receptors with Hydrogen-Bonding Groups Immersed into a Large Cavity. J. Org. Chem. 1998, 63, 9644–9651. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Ryu, E.-H.; Zhao, Y. Efficient Synthesis of Water-Soluble Calixarenes Using Click Chemistry. Org. Lett. 2005, 7, 1035–1037. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Rivera-Monroy, Z.J.; Maldonado, M. Copper(I)-Catalyzed Alkyne–Azide Cycloaddition (CuAAC) “Click” Reaction: A Powerful Tool for Functionalizing Polyhydroxylated Platforms. ACS Omega 2023, 8, 3650–3666. [Google Scholar] [CrossRef]
- Požar, J.; Cvetnić, M.; Usenik, A.; Cindro, N.; Horvat, G.; Leko, K.; Modrušan, M.; Tomišić, V. The Role of Triazole and Glucose Moieties in Alkali Metal Cation Complexation by Lower-Rim Tertiary-Amide Calix[4]arene Derivatives. Molecules 2022, 27, 470. [Google Scholar] [CrossRef]
- Schneider, J.P.; Tommasone, S.; Della Sala, P.; Gaeta, C.; Talotta, C.; Tarnus, C.; Neri, P.; Bodlenner, A.; Compain, P. Synthesis and Glycosidase Inhibition Properties of Calix[8]arene-Based Iminosugar Click Clusters. Pharmaceuticals 2020, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Kajouj, S.; Marcelis, L.; Mattiuzzi, A.; Grassin, A.; Dufour, D.; Van Antwerpen, P.; Boturyn, D.; Defrancq, E.; Surin, M.; De Winter, J.; et al. Synthesis and photophysical studies of a multivalent photoreactive RuII-calix[4]arene complex bearing RGD-containing cyclopentapeptides. Beilstein J. Org. Chem. 2018, 14, 1758–1768. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.; Kim, S.K. Calix[4]arenes bearing triazolyl anthracenes: Hg2+-selective receptors exhibiting fluorescence or dual optical responses. New J. Chem. 2021, 45, 18609–18614. [Google Scholar] [CrossRef]
- Georghiou, P.E.; Rahman, S.; Alrawashdeh, A.; Alodhayb, A.; Valluru, G.; Unikela, K.S.; Bodwell, G.J. Synthesis, supramolecular complexation and DFT studies of a bis(pyrene)-appended ‘capped’ triazole-linked calix[4]arene as Zn2+ and Cd2+ fluorescent chemosensors. Supramol. Chem. 2020, 32, 325–333. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Domehri, E.; Tajbakhsh, M.; Bekhradnia, A. New fluorescent sensor based on a calix[4]arene bearing two triazole–coumarin units for copper ions: Application for Cu2+ detection in human blood serum. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 245–252. [Google Scholar] [CrossRef]
- Gorbunov, A.; Kuznetsova, J.; Puchnin, K.; Kovalev, V.; Vatsouro, I. Triazolated calix[4]arenes from 2-azidoethylated precursors: Is there a difference in the way the triazoles are attached to narrow rims? New J. Chem. 2019, 43, 4562–4580. [Google Scholar] [CrossRef]
- Song, M.; Sun, Z.; Han, C.; Tian, D.; Li, H.; Kim, J.S. Calixarene-Based Chemosensors by Means of Click Chemistry. Chem. Asian J. 2014, 9, 2344–2357. [Google Scholar] [CrossRef]
- Jaime, C.; De Mendoza, J.; Prados, P.; Nieto, P.M.; Sanchez, C. 13C NMR chemical shifts. A single rule to determine the conformation of calix[4]arenes. J. Org. Chem. 1991, 56, 3372–3376. [Google Scholar] [CrossRef]
- Baklouti, L.; Harrowfield, J.; Pulpoka, B.; Vicens, J. 1,3-Alternate, the Smart Conformation of Calix[4]arenes. Mini-Rev. Org. Chem. 2006, 3, 355–384. [Google Scholar] [CrossRef]
- Gorbunov, A.; Ozerov, N.; Malakhova, M.; Eshtukov, A.; Cheshkov, D.; Bezzubov, S.; Kovalev, V.; Vatsouro, I. Assembling triazolated calix[4]semitubes by means of copper(I)-catalyzed azide–alkyne cycloaddition. Org. Chem. Front. 2021, 8, 3853–3866. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS. Version 2008/1; Bruker AXS Inc.: Karlsruhe, Germany, 2008. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).