Abstract
The coordination compound [NiCl2L(2-PrOH)]n (where L is 2-(4-bromophenoxy)acetohydrazide; 2-PrOH is isopropanol) was obtained for the first time. The complex was characterized by means of elemental analyses, molar conductance, thermogravimetric analysis, IR spectroscopy, and single crystal X-ray diffraction analysis. It was determined that the coordination compound exhibits a polymeric structure. The complexing agent is six-coordinated, and its distorted octahedron forms due to the bidentate coordination of 2-(4-bromophenoxy)acetohydrazide through the carbonyl oxygen atom and the amine nitrogen. The oxygen of the molecule of isopropanol, the chlorine atom, and two chlorine atoms serve as bridges between two metal atoms.
1. Introduction
Drugs in the hydrazide functional group are an important category within antituberculosis, antibacterial, antifungal, and antimicrobial pharmaceuticals [1]. Hydrazides are considered key intermediates and valuable starting materials for synthesizing novel biologically active derivatives [1,2,3]. For example, hydrazide derivatives containing an aromatic fragment exhibit antibacterial and antifungal activities, the extent of which depends on the nature of the substituent attached to the benzene ring [2]. Benzotriazole-N-substituted acetohydrazide derivatives exhibit potent antiviral activity, characterized by their pronounced binding affinity and effective inhibition of glycoprotein B associated with Herpes Simplex Virus-I (HSV-I) [3].
The coordination of hydrazides to biometal ions often leads to an increase in the biological activity of compounds, as well as an expansion of its spectrum. The results of antibacterial and antifungal bio-efficacy tests showed that the complexes of Cu(II), Ni(II), and Fe(III) with dihydrazide of adipic acid exhibited greater effectiveness compared to the ligand alone [4]. This is attributed to the high mobility of metal complexes, facilitating their ability to cross the cell membrane. The chelation effect in complexes reduces the polarity of metal ions that increase the lipophilicity of metal complexes and, ultimately, facilitate them to cross the cell membranes. Synthesized metal complexes comprising Co(II), Ni(II), Cu(II), and Cd(II) chlorides, as well as CuBr2, Cu(CH3COO)2, and Cu(ClO4)2, in conjunction with the 2-(4-bromophenylamino)acetohydrazide ligand, were demonstrated to possess significant potential for therapeutic chemoprevention against cancerous cells, exhibiting efficacy comparable to that of doxorubicin [5]. Both the anticancer activity (tested on the human colorectal cancer HT29 cell line) and antibacterial effect (against both gram-positive and gram-negative bacteria) were demonstrated for cobalt(II) and zinc(II) complexes with thiophene-appended nicotinic acid hydrazide ligands [6]. Metal complexes of tin, antimony, and iron were synthesized from the hydrazide derivative of ursolic acid. Antibacterial and antioxidant activities were conducted for these complexes, revealing that they exhibit higher potency compared to their parent compounds [7]. The presence of electron-donating groups and transition metals was observed to enhance the biological activities, as demonstrated by the formation of complexes of malonic acid-based hydrazide derivatives with copper(II) and zinc(II) metals. These compounds were evaluated for their antioxidant, antibacterial, antifungal, chymotrypsin, and tyrosinase inhibition activities, exhibiting more effective inhibitory effects compared to standard drugs [8]. The synthesized Ni(II) chloride complex with the 2-(p-tolylamino)acetohydrazide ligand was investigated, demonstrating efficacy in vitro for the treatment of Eobania vermiculata [9].
Thus, the study of complexation of new hydrazides with transition metals opens the way to the synthesis of more effective drug substances with a wide spectrum of pharmacological action. Synthesis and investigation of diverse coordination polymers involving 3d-metals are currently attracting significant attention, primarily attributed to their distinctive structures and application in biosensing and biomedicine [10]. For example, coordination polymers, such as complexes of nickel and other metals with pyridine-2,6-dicarboxylic acid, have demonstrated antimicrobial properties [11,12].
The objective of the present work is the synthesis of 2-(4-bromophenoxy)acetohydrazide and its complex with nickel(II) chloride, to determine their composition, and the thermal stability and structure of the coordination compound. This is a continuation of the authors’ ongoing research into the synthesis of hydrazides, their derivatives, and the coordination compounds of metals with potential biological activity [13,14].
2. Results and Discussion
2-(4-Bromophenoxy)acetohydrazide (L) was obtained from the corresponding ester by reaction with hydrazine hydrate in refluxed methanol (Scheme 1). An ester precursor was synthesized from p-bromophenol by a reaction with methyl chloroacetate.
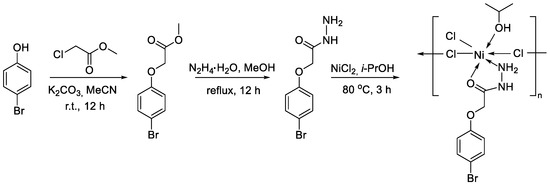
Scheme 1.
Synthesis of coordination compound [NiCl2L(2-PrOH)]n.
The synthesized metal complex is stable, nonhygroscopic, well soluble in dimethylformamide (DMF) and acetonitrile, and moderately soluble in ethanol. The results of the molar conductance of a 1 × 10−3 mol/L solution of the complex showed that it is a nonelectrolyte in DMF, where λ = 6.2 Ω−1 cm2·mol−1 [15,16], so the dissociation of the complex does not occur in this solvent. According to the results of the elemental analysis, the complex has a molar ratio of Ni:hydrazide = 1:1.
Using the method of thermogravimetric analysis (TGA), it was established that thermolysis of the complex begins in the range of 80–210 °C (Figure 1). In this temperature range (peak 180 °C, weight losses Δm = 13.5%) of the complex, one molecule of solvated 2-propanol is removed from the gas phase (calc. Δm = 13.8%). At a higher temperature, the compound undergoes oxidative thermal decomposition of the organic part of its molecule, which is accompanied by several exothermic peaks and a significant loss of mass. The final product of thermolysis is nickel(II) oxide.
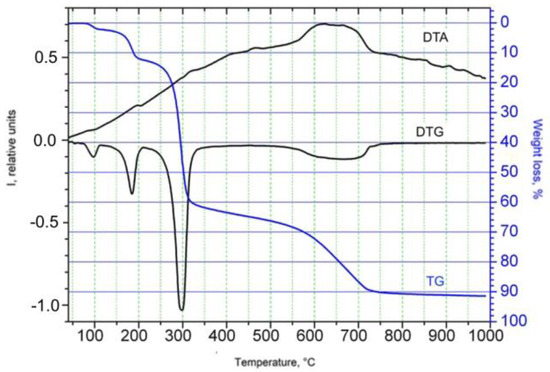
Figure 1.
The thermal decomposition of the compound.
The bands in the IR spectra for the ligand and complex (Figure S1) were matched in compliance with the literature data for hydrazides and their coordination compounds with 3d-metals [4,5,17,18].
The IR spectra of the ligand contain three characteristic absorption bands at 3420, 3322, and 3205 cm−1, assignable to νas(NH2), νs(NH2), and ν(NH), respectively. Moreover, the IR spectra have the band δ(NH2) located at 1592 cm−1 and three bands at 1698, 708, and 606 cm−1, ascribed to ν(C=O), δ(C=O), and γ(C=O), respectively.
The spectrum of the complex shows that the ligand carbonyl stretching band is relocated to a lower frequency by 45 cm−1: ν(C=O) = 1653 cm−1. In addition, δ(C=O) and γ(C=O) ligand bands undergo shifts by 7 and 5 cm−1, respectively. There are changes in the position of the bands of valence vibrations νas(NH2), νs(NH2), and ν(NH) and in their intensity after coordination with Ni(II).
The specified changes in the IR spectrum of the complex indicate the binding of hydrazone due to the coordination of C=O and NH2 groups. This is confirmed by the appearance of the spectrum of the complex new bands 504 and 424 cm−1, assigned to ν(Ni–O) and ν(Ni–N), respectively.
According to the X-ray diffraction study, the coordination compound of Ni(II) with 2-(4-bromophenoxy)acetohydrazide has a polymer structure. The polyhedron of the Ni1 atom in the complex is a distorted octahedron. Two Cl1 atoms, located in opposite directions, act as bridged ones between the metal atoms, leading to the formation of a polymer chain. The Cl2 atom and the N1 atom of the hydrazide group are located in equatorial positions, while the carbonyl oxygen atom of the organic ligand and the oxygen atom of the isopropanol molecule occupy axial positions (Figure 2). The Ni–Cl bond lengths vary within 2.374(2)–2.463(2) Å, while the Ni1–O3 and Ni1–O1 bonds are 2.036(5) Å and 2.065(4) Å, respectively (Table S1). The values of the O–Ni–Cl, O–Ni–N, Cl–Ni–N, and Cl–Ni–Cl bond angles differ within 79.6(2)–96.24(18)° (Table S1). The molecular structure of the ligand is in good agreement with the previously described compound of 2-(4-bromophenoxy)propanohydrazide [19].
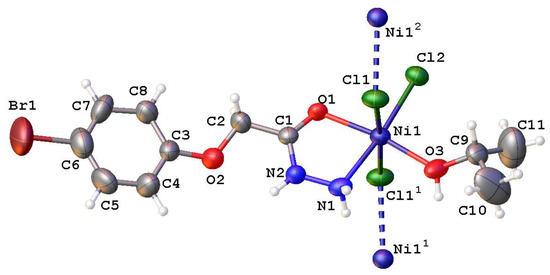
Figure 2.
Molecular structure of the [NiCl2L(2-PrOH)] complex according to the X-ray diffraction study. Thermal ellipsoids are shown at a 50% probability level.
In the crystal phase, the complex under study forms a polymeric chain in the [001] crystallographic direction, in which the Cl1 anion acts as a bridge between nickel cations (Figure 3). Ligands within a chain are bound additionally by the O3–H3…Cl2′ (the symmetry operation is x, 1.5 − y, 0.5 + z; the H…Cl distance is 2.26 Å, and the O–H…Cl angle is 158°) and N1–H1A…Cl2′ (the symmetry operation is x, 1.5 − y, 0.5 + z; the H…Cl distance is 2.73 Å, and the N–H…Cl angle is 138°) hydrogen bonds. The N2–H2...Cl2′ hydrogen bonds (the symmetry operation is 1 − x, −1/2 + y, 1.5 − z; the H…Cl distance is 2.49 Å; the N–H…Cl angle is 149°) are found between neighboring chains forming layers parallel to the (100) crystallographic plane (Figure 4).
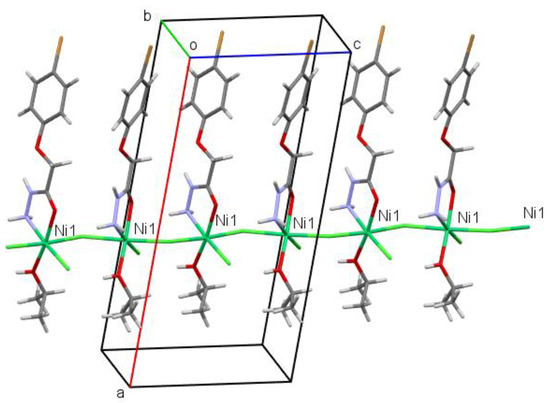
Figure 3.
Polymeric chain in the [001] crystallographic direction in the crystal of the [NiCl2L(2-PrOH)] complex.
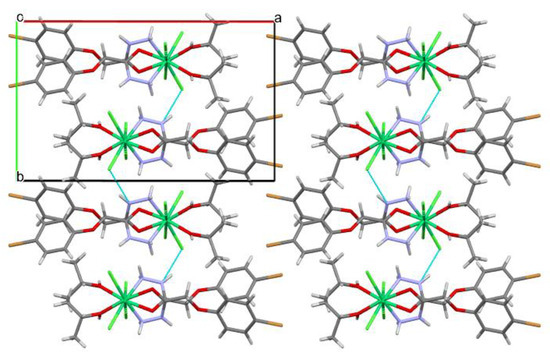
Figure 4.
Layers of polymeric chains in the crystal of [NiCl2L(2-PrOH)].
3. Materials and Methods
3.1. General Information
Commercially available chemicals were used without further purification: p-bromophenol (99%), methyl chloroacetate (99%), nickel(II) chloride hexahydrate (NiCl2·6H2O, 99.9%), and 2-propanol (2-PrOH, 99.5%). Elemental analysis for C, H, and N were performed in Elemental Analyzer CE-440 (Exeter Analytical., Inc., Chelmsford, MA, USA). The nickel content was determined using inductively coupled plasma atomic emission spectroscopy with an Optima 8000 instrument (PerkinElmer, Inc., Hopkinton, MA, USA). Molar conductance values of the complex was measured in dimethylformamide (DMF) at room temperature using a digital conductometer. Thermogravimetric analysis were carried out using a Q-1500D with a heating rate of 10 °C/min in air in the temperature range 20–1000 °С. The IR absorption spectra of the ligand and the complex were collected from KBr pellets on FT-IR-spectrophotometers 8400S (Shimadzu Corporation, Kyoto, Japan) and on Frontier FT-IR spectrometer (Perkin-Elmer, Hopkinton, MA, USA) in the 400–4000 cm−1 range.
Crystal data for complex were measured on an <Xcalibur-3> diffractometer (graphite monochromated MoKα radiation, CCD detector, ω-scanning). The structure was solved using the SHELXT [20] and refined using the SHELXL [21] programs implemented with Olex2 software [22]. Full-matrix least-squares refinement against F2 in anisotropic approximation was used for the non-hydrogen atoms. Positions of the hydrogen atoms were located from electron density difference maps and refined by the “riding” model with Uiso = nUeq of the carrier atom (n = 1.5 for methyl groups and n = 1.2 for other hydrogen atoms). The hydrogen atom of the hydroxyl group was refined using isotropic approximation.
Crystal Data: C11H17BrCl2N2NiO3, a = 18.0033(10) Å, b = 10.9202(7) Å, c = 8.6309(4) Å, β = 100.774(5)°, V = 1666.93(16) Å3, monoclinic, space group P21/c, Mr = 434.78, T = 294 K, Z = 4, µ(Mo Kα) = 3.887 mm−1. In the range up to 2θ = 50°, 13,167 reflections were measured (2941 unique, Rint = 0.1467). The final wR2 = 0.1833 was obtained using all reflections, and R1 = 0.0719 was calculated using 1887 reflections with I ≥ 2 σ(I), S = 1.016.
Final atomic coordinates, geometrical parameters, and crystallographic data have been deposited to the Cambridge Crystallographic Data Centre, 11 Union Road, Cambridge, CB2 1EZ, UK (https://www.ccdc.cam.ac.uk/structures/) and are available on request quoting the deposition number CCDC 2314158.
3.2. Synthesis of 2-(4-Bromophenoxy)acetohydrazide (L)
2-(4-Bromophenoxy)acetohydrazide (L) was synthesized following the procedure described in [23]. Briefly, p-bromophenol (1 eq.) was dissolved in acetonitrile (30 mL), and K2CO3 (1.2 eq.) was added to the solution. The resulting mixture was stirred at r.t. for 12 h, followed by the gradual addition of excess methyl chloroacetate through a dropping funnel. The resulting mixture was refluxed for 10 h. The reaction was quenched with a saturated NaCl solution, then extracted with CH2Cl2, washed with brine, dried over MgSO4, and concentrated using a rotary evaporator. This afforded methyl 2-(4-bromophenoxy)acetate with an 89% yield, which was utilized for the subsequent step without further purification. The crude ester (1 eq.) and N2H4·H2O (5 eq.) were dissolved in MeOH and refluxed for 12 h. The solvent was then removed under reduced pressure, and the product was extracted with CH2Cl2. The combined organic layers were washed with a saturated Na2CO3 aqueous solution, dried over Na2SO4, and concentrated under reduced pressure to yield the desired hydrazide (83%). The physicochemical properties of the compound align with those reported in the literature [24].
3.3. Synthesis of [NiCl2L(2-PrOH)]n
The coordination compound [NiCl2L(2-PrOH)]n was synthesized as follows: 0.305 g (1.25 mmol) L and 0.3 g NiCl2·6H2O were added to 35 mL of heated 2-propanol. The solution was evaporated at 70 °C on a magnetic stirrer for 3 h to 10 mL. After 24 h, the resulting light green precipitate was washed with 2-propanol, separated on a Schott glass filter, and dried in a vacuum desiccator. Yield: 62%. Single crystals suitable for X-ray diffraction were collected from the reaction medium. The calculated elemental composition for the complex (based on single-crystal data for C11H17BrCl2N2NiO3, M = 434.76; in %): C 30.36, H 3.91, N 6.44, Ni 13.57. Found (in %): C 30.07, H 3.79, N 6.36, Ni 13.50. Selected IR data complex (in cm−1): 3392 νas(NH2), 3210 νs(NH2), 3165 ν(NH), 1653 ν(C=O), 1592 δ(NH2), 715 δ(C=O), 589 γ(C=O), 504 ν(Ni-O), 424 ν(Ni-N).
4. Conclusions
A novel Ni(II) complex with 2-(4-bromophenoxy)acetohydrazide was obtained and fully characterized for the first time. It was established that the coordination compound has a polymer structure in which chlorine atoms that are coordinated to nickel act as bridges between two metal atoms. This compound is distinguished from previously obtained monomeric complexes of nickel(II) with different hydrazides [5,9]. The 2-(4-bromophenoxy)acetohydrazide demonstrates itself as a bidentate ligand, coordinating through the carbonyl oxygen atom and amine nitrogen. The coordination number of Ni(II) was supplemented to 6 by a chlorine atom and a molecule of isopropanol. According to the wide range of pharmacological actions of 3d-metal complexes with hydrazides, this work will contribute to the development and synthesis of new compounds with biological activity.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1: IR-spectra of ligand (a) and complex (b); Table S1: Selected geometric parameters (bond lengths /Å, bond angles/ deg) for [NiCl2L(2-PrOH)]n (Symmetry code: (i) x, −y + 3/2, z + 1/2).
Author Contributions
Conceptualization, project administration, methodology, and writing—review and editing: O.M. and I.K.; investigation and writing—original draft preparation: M.N. (synthesis of ligand), O.F. (synthesis of complex), K.T. (elemental analysis, molar conductance, TGA, IR), V.D. and S.S. (X-ray). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Final atomic coordinates, geometrical parameters, and crystallographic data have been deposited to the Cambridge Crystallographic Data Centre, 11 Union Road, Cambridge, CB2 1EZ, UK (https://www.ccdc.cam.ac.uk/structures/) and are available on request quoting the deposition number CCDC 2314158.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abidov, M. Pharmacological aspects of hydrazides and hydrazide derivatives. Health Promot. Phys. Act. 2017, 2, 9–21. [Google Scholar] [CrossRef]
- Meshcheryakova, S.; Shumadalova, A.; Beylerli, O.; Gareev, I. Synthesis and biological activity of 2-[6-methyl-4-(thietan-3-yloxy)pyrimidin-2-ylthio]acetohydrazide derivatives. ADMET DMPK 2021, 9, 167–176. [Google Scholar] [CrossRef]
- Mali, D.; Amrutkar, S. Virtual Screening, ADMET Analysis, and Synthesis of 2-(1H-benzotriazol-1-yl) N-substituted Acetohydrazide that binds to the Glycoprotein B of Herpes Simplex Virus-I (HSV-I). Anti-Infect. Agents 2023, 21, e170723218776. [Google Scholar] [CrossRef]
- Mangal, M.; Chander, S.; Goel, R.; Sharma, A. Synthesis, spectral and antimicrobial activities of metal complexes with phenyl thiourea and acid hydrazide. J. Cardiovasc. Dis. Res. 2021, 12, 605–615. [Google Scholar]
- Emara, E.; El-Sayed, W.; Khalaf-Allah, A.; Alminderej, F.; Abdel-Monem, Y.; Abd-Rabou, A. Spectral studies, thermal investigations and anticancer activity of some divalent metal complexes derived from 2-(4-bromophenylamino)acetohydrazide ligand. Appl. Organomet. Chem. 2022, 36, e6657. [Google Scholar] [CrossRef]
- Ashma, A.; Yahya, S.; Subramani, A.; Tamilarasan, R.; Sasikumar, G.; Ali, S.J.A.; Al-Lohedan, H.A.; Karnan, M. Synthesis of new nicotinic acid hydrazide metal complexes: Potential anti-cancer drug, supramolecular architecture, antibacterial studies and catalytic properties. J. Mol. Struct. 2022, 1250, 131860. [Google Scholar] [CrossRef]
- Jabeen, M.; Ahmad, S.; Shahid, K.; Sadiq, A.; Rashid, U. Ursolic acid hydrazide based organometallic complexes: Synthesis, characterization, antibacterial, antioxidant, and docking studies. Front. Chem. 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Naseem, H.A.; Ahmad, K.; Shah, H.-U.-R.; Tariq, A.; Muhammad, A.; Rauf, A. Design, synthesis and spectroscopic characterizations of medicinal hydrazide derivatives and metal complexes of malonic ester. Curr. Bioact. Compd. 2023, 19, 31–46. [Google Scholar] [CrossRef]
- Emara, E.M.; Khalaf-Allah, A.S.A.; El-Sawaf, M.A. Efficacy of 2-(p-tolylamino)acetohydrazide and its Co(II), Ni(II) complexes on the shell of Eobania vermiculata under laboratory conditions. Egypt. J. Agric. Res. 2023, 101, 1019–1026. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, J.; Ahmad, F.; Xiao, Y.; Guan, J.; Shu, T.; Zhang, X. Bimetallic Coordination Polymers: Synthesis and Applications in Biosensing and Biomedicine. Biosensors 2024, 14, 117. [Google Scholar] [CrossRef]
- Siddiqi, Z.; Khalid, M.; Kumar, S.; Shahid, M.; Noor, S. Antimicrobial and SOD activities of novel transition metal complexes of pyridine-2,6-dicarboxylic acid containing 4-picoline as auxiliary ligand. Eur. J. Med. Chem. 2010, 45, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Suku, S.; Ravindran, R. Synthesis, characterization and antimicrobial studies of 1D hetero-bimetallic coordination polymers of pyridine-2,6-dicarboxylic acid with iron and alkaline earth metals. J. Mol. Struct. 2022, 1252, 132083. [Google Scholar] [CrossRef]
- Nesterkina, M.; Barbalat, D.A.; Kravchenko, I. Design, synthesis and pharmacological profile of (−)-verbenone hydrazones. Open Chem. 2020, 18, 943–950. [Google Scholar] [CrossRef]
- Seifullina, I.; Martsinko, E.; Chebanenko, E.; Dyakonenko, V.; Shishkina, S.; Pirozhok, O. Structure of the {[Cu2Ge(μ-Cit)2(μ-INH)2]·4H2O}n Coordination Polymer, where H4Cit is Citric Acid, INH is Isonicotinic Acid Hydrazide. J. Struct. Chem. 2018, 59, 154–159. [Google Scholar] [CrossRef]
- Geary, W.J. The Use of Conductivity Measurements in Organic Solvents For The Characterisation Of Coordination Compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Khedr, M.; Saad, F.A. Synthesis, structural characterization and antimicrobial efficiency of sulfadiazine azo-azomethine dyes and their bi-homonuclear uranyl complexes for chemotherapeutic use. Turk. J. Chem. 2015, 39, 267–280. [Google Scholar] [CrossRef]
- Fouad Ibrahim, R.; Shaaban, I.; Ali, T.; Assiri, M.; Shenouda, S. Co(II), Ni(II), Cu(II) and Cd(II)-thiocarbonohydrazone complexes: Spectroscopic, DFT, thermal, and electrical conductivity studies. RSC Adv. 2021, 11, 37726–37743. [Google Scholar] [CrossRef]
- Genc, Z.; Tekin, S.; Sandal, S.; Şekerci, M.; Genc, M. Synthesis and DFT studies of structural and some spectral parameters of nickel(II) complex with 2-(2-hydroxybenzoyl)-N-(1-adamantyl) hydrazine carbothioamide. Res. Chem. Intermed. 2014, 41, 4477–4488. [Google Scholar] [CrossRef]
- Akhtar, T.; Khawar Rauf, M.; Ebihara, M.; Hameed, S. 2-(4-Bromophenoxy)propanohydrazide. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o441. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Maingot, L.; Elbakali, J.; Dumont, J.; Bosc, D.; Cousaert, N.; Urban, A.; Deglane, G.; Villoutreix, B.; Nagase, H.; Sperandio, O.; et al. Aggrecanase-2 inhibitors based on the acylthiosemicarbazide zinc-binding group. Eur. J. Med. Chem. 2013, 69, 244–261. [Google Scholar] [CrossRef] [PubMed]
- Al-Ostoot, F.H.; Khamees, H.A.; Prasad, N.; Zameer, F.; Khanum, S.A. In-silico docking, synthesis, structure analysis, DFT calculations, energy frameworks, and pharmacological intervention of [1,3,4]-thiadiazoles analogous as XO inhibitor and on multiple molecular inflammatory targets COX and LOX. J. Mol. Struct. 2022, 1270, 133963. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).