Abstract
2-Styrylchromones (2-SCs) are interesting compounds for their biological properties as well as versatility as starting materials for further transformations. Herein, we disclose a new 2-SC derivative—2,2’-[(1E,1’E)-{[hexa-2,4-diyne-1,6-diylbis(oxy)]bis(2,1-phenylene)}bis(ethene-2,1-diyl)]bis(4H-chromen-4-one)—which is a dimeric compound formed by two units of 2-SC linked through a 1,3-diyne moiety. It was obtained in excellent yield (96%) through the copper-catalyzed homocoupling of two molecules of O-propargyl-2-SC. Its structure was unveiled by 1D (1H and 13C) and 2D (HSQC and HMBC) NMR techniques together with HRMS.
1. Introduction
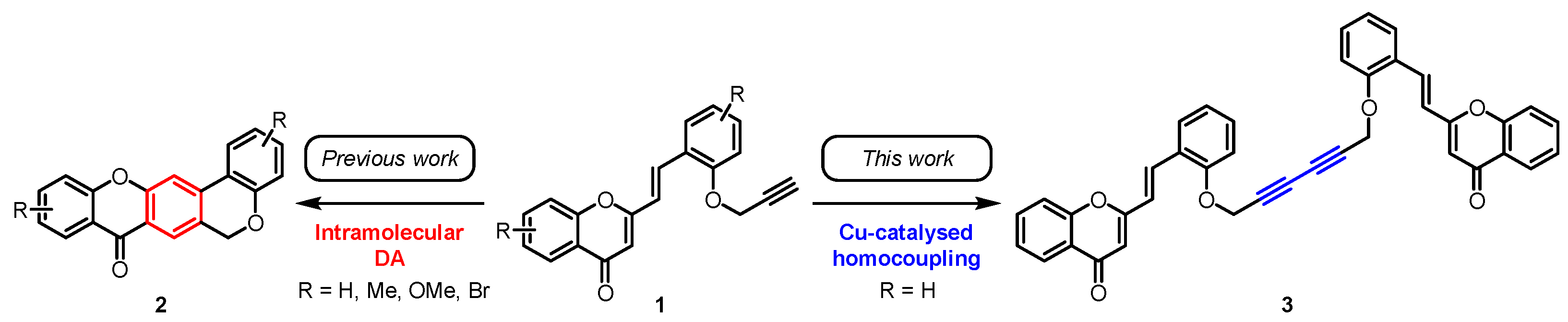
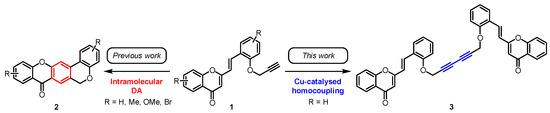
2-Styrylchromones (2-SCs) are a small class of naturally occurring compounds, which are characterized by the presence of a 2-styryl moiety at the chromone (4H-chromen-4-one) scaffold. Up to now, just nine natural 2-SCs have been reported in the literature [1]. On the other hand, there is a vast panoply of their synthetic derivatives due to their interesting biological properties such as antiallergic, anti-inflammatory, antioxidant, antitumor and antiviral activities, among others [1,2]. Our research group has a long history in the area of 2-SC chemistry, not only with respect to their great potential as bioactive compounds but also as starting materials for further transformations. In this context, in 2018, we reported for the first time the use of O-propargyl-2-SC derivatives 1 as substrates in intramolecular Diels–Alder (DA) reactions [3], allowing the synthesis of chromeno[3,4-b]xanthones 2 (Scheme 1), which showed promising results as acetylcholinesterase (AChE) and β-amyloid (Aβ) aggregation dual-targeting inhibitors [4].
Scheme 1.
O-Propargylated 2-SCs 1 as starting materials for the synthesis of chromeno[3,4-b]xanthones 2 (previous work) and of the title compound 3 (this work).
Herein, we report the synthesis of a dimeric compound 3 formed through the copper-catalyzed homocoupling of two molecules of the O-propargyl-2-SC 1 (Scheme 1). The synthesized compound 3 possesses in its structure two units of 2-SC linked through a 1,3-diyne moiety.
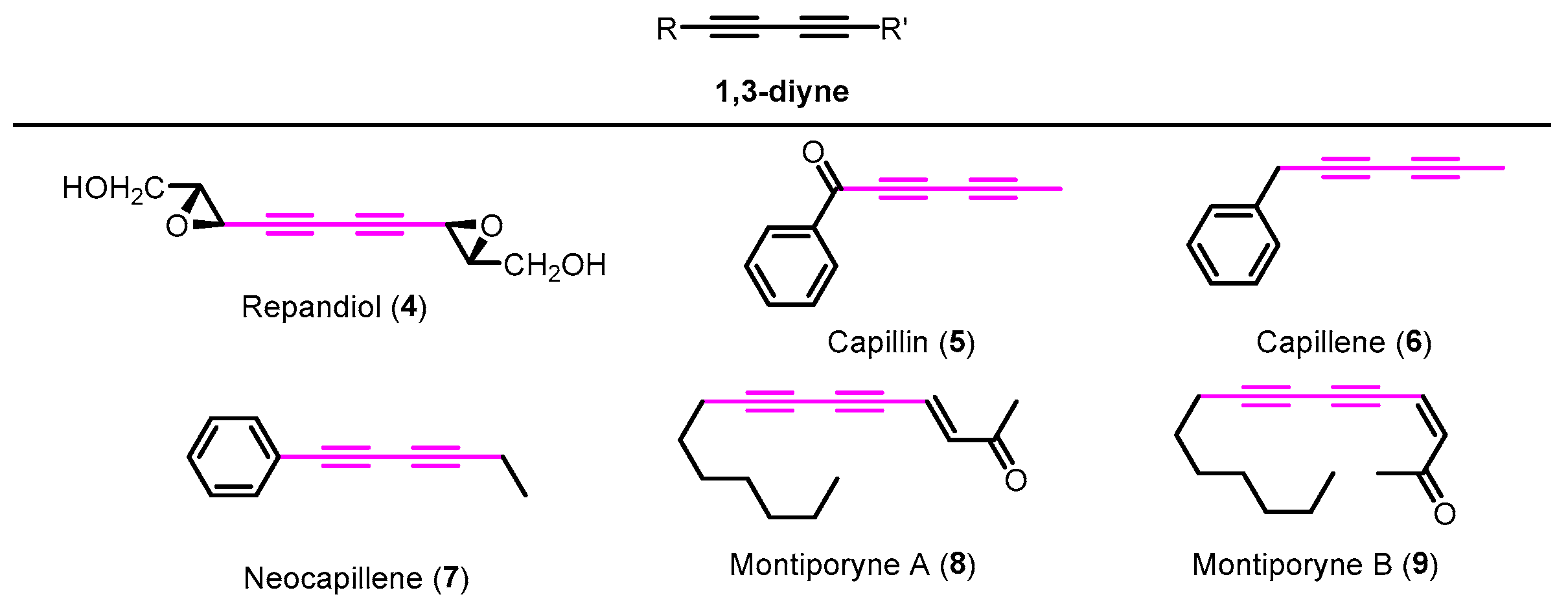
Many examples of naturally occurring 1,3-diynes displaying prominent biological activities can be found in the literature [5]. For instance, repandiol (4) is a symmetrical 1,3-diyne isolated from the mushrooms Hydnum repandum and H. repandum var. album; capillin (5) and isomeric analogues capillene (6) and neocapillene (7) were isolated from Artemisia capillaris; montiporynes A (8) and B (9) were isolated from a velvet coral of genus Montipora (Figure 1). In addition, these examples exhibit cytotoxic activity against several types of tumor cells [5]. The 1,3-diynes are also important moieties in supramolecular chemistry, namely in the construction of molecular boxes as high-efficiency hosts [6]. Moreover, this structural motif is crucial for the design of advanced materials such as conjugated polymers [7], liquid crystals, molecular wires, and nonlinear optic materials, among others [6]. This type of compound is straightforwardly obtained through metal-catalyzed homocoupling of terminal alkynes [6,8,9].
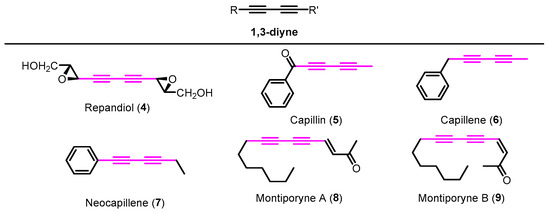
Figure 1.
Examples of naturally occurring 1,3-diynes 4–9 with cytotoxic activity.
2. Results and Discussion
The initial aim of this work was to learn the reactivity of O-propargyl-2-SCs in intramolecular DA reactions promoted by CuI/Cu(OTf)2 as a Lewis acid catalyst [10]. However, no DA reaction took place and interestingly, we isolated the 2-SC dimer linked by a 1,3-diyne 3 instead of the expected chromeno[3,4-b]xanthone 2 (Scheme 1).
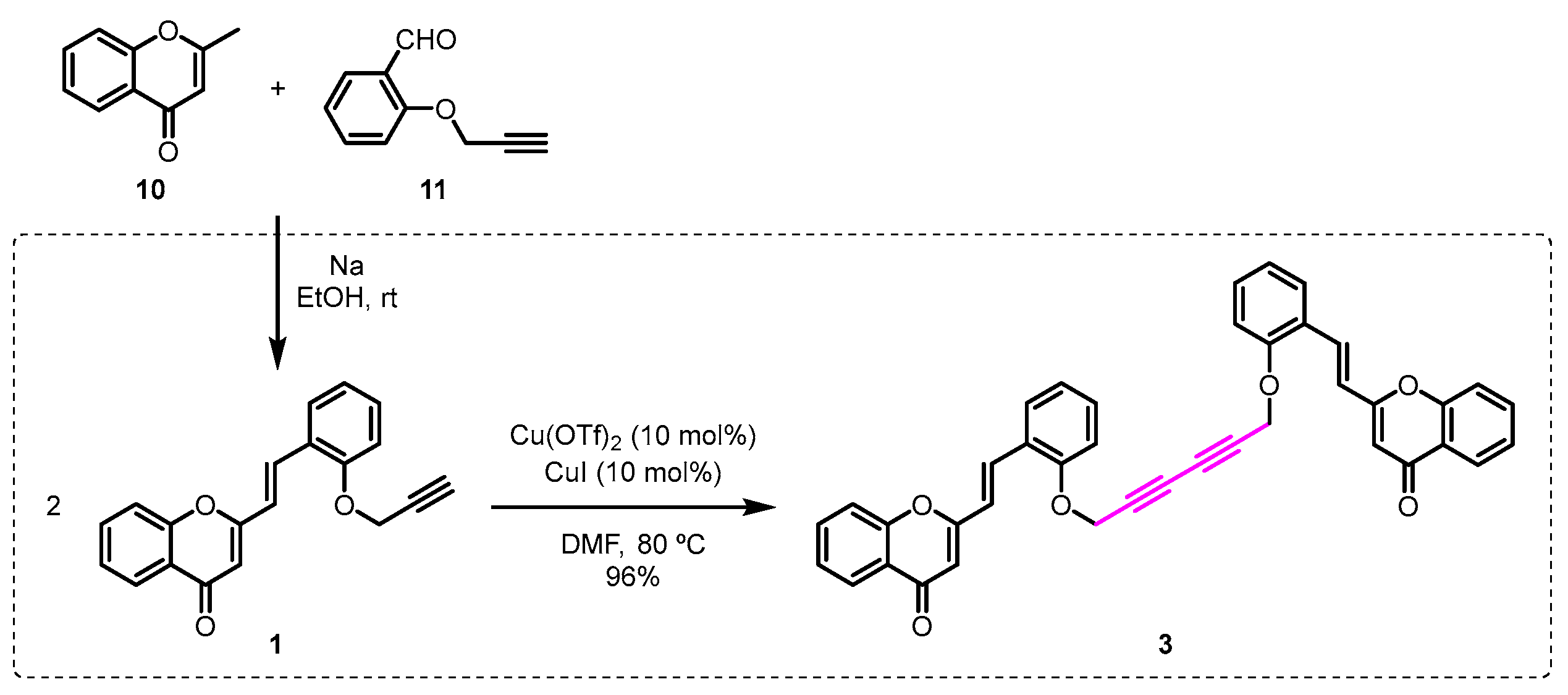
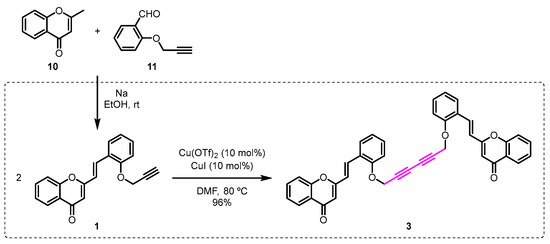
Firstly, the required O-propargyl-2-SC 1 was prepared by base-promoted aldol reaction of the 2-methylchromone 10 with the O-propargylsalicylaldehyde 11 (Scheme 2). Then, the O-propargyl-2-SC 1 was converted into the dimeric compound 3 in excellent yield (96%), in the presence of catalytic amounts of CuI/Cu(OTf)2, in dimethylformamide (DMF) at 80 °C (Scheme 2).
Scheme 2.
Synthesis of the 2,2’-[(1E,1’E)-{[hexa-2,4-diyne-1,6-diylbis(oxy)]bis(2,1-phenylene)}bis(ethene-2,1-diyl)]bis(4H-chromen-4-one) (3).
This transformation occurs via Cu-catalyzed homocoupling between the terminal triple bond of two units of O-propargyl-2-SC 1.
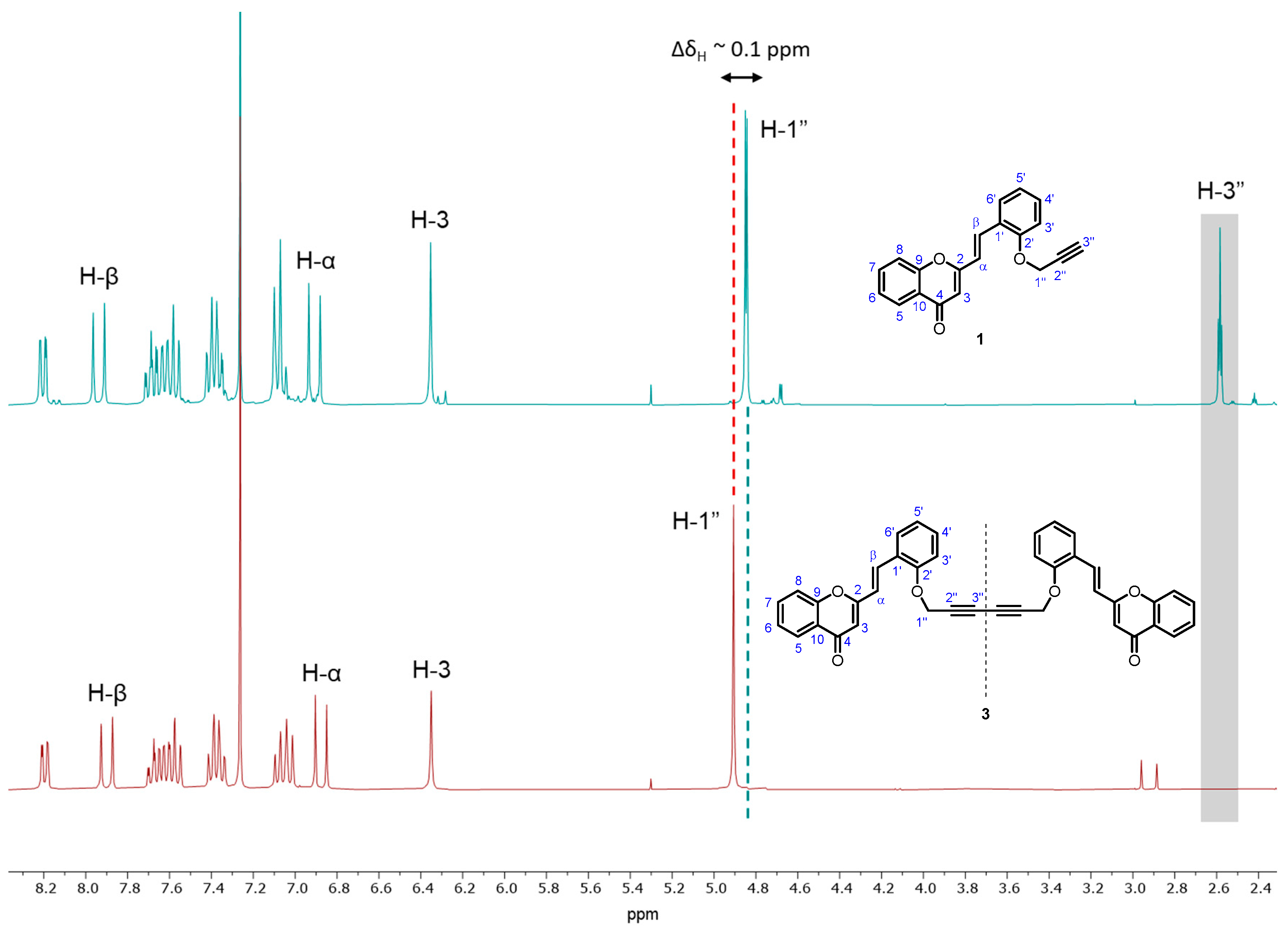
Compound 3 was thoroughly characterized by 1D (1H and 13C) (Figures S1 and S2) and 2D (HSQC and HMBC) (Figures S3 and S4) NMR techniques. Its 1H NMR spectrum is characterized by the absence of the triplet at δH~2.6 ppm corresponding to the resonance of H-3″ from the terminal triple bond (–C≡CH) of the starting O-propargyl-2-SC 1 (Figure 2). In addition, a slight deviation was observed in the chemical shift of the signal corresponding to the resonance of H-1″, which in the 1H NMR spectrum of the starting O-propargyl-2-SC 1 appears as a doublet at δH~4.8 ppm and in the spectrum of compound 3 appears as a singlet at δH ~4.9 ppm (ΔδH~0.1 ppm) (Figure 2).
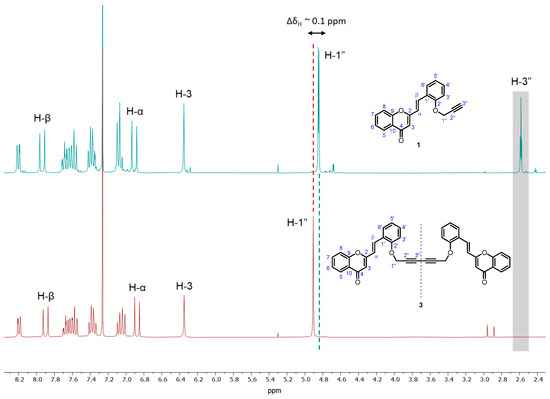
Figure 2.
Expansion of 1H NMR spectra of the O-propargyl-2-SC 1 (up) and the title compound 3 (bottom).
Furthermore, the 13C NMR spectrum of compound 3 is characterized by the deviation to lower frequency values (δC 71.5–74.5 ppm) of the signals corresponding to the resonance of C-2″ and C-3″ of the 1,3-diyne moiety in comparison with the starting O-propargyl-2-SC 1 (δC 76.1–78.2 ppm).
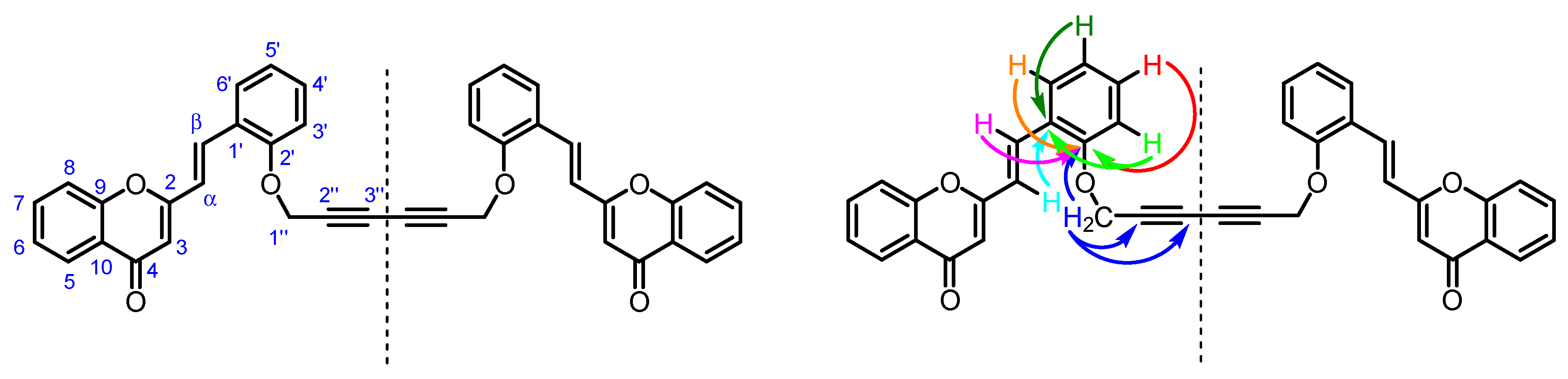
In addition, all non-protonated carbons were unequivocally assigned through the observed correlations in the HMBC spectrum of 3 (Figure 3):
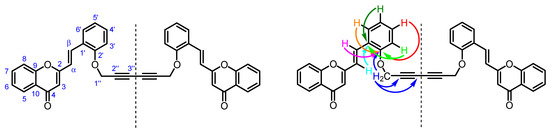
Figure 3.
Numbering system and main HMBC connectivities observed in the HMBC spectrum of compound 3.
- (i)
- C-2″ and C-3″ (δC 71.5 and 74.5 ppm) of the 1,3-diyne moiety due to its connectivities with H-1″.
- (ii)
- C-1′ (δC 124.8 ppm) due to its connectivities with H-α and H-3′,5′.
- (iii)
- C-2′ (δC 155.7 ppm) due to its connectivities with H-1″, H-β and H-3′,4′,6′.
The spectroscopic data of 4H-chromen-4-one moiety is described elsewhere [3,4].
3. Materials and Methods
3.1. General Remarks
Melting points were measured with a Büchi Melting Point B-540 apparatus and are uncorrected. NMR spectra were recorded with Bruker Avance 300 (300.13 MHz for 1H and 75.47 MHz for 13C) and 500 (500.13 MHz for 1H and 125.77 MHz for 13C) spectrometers in CDCl3 as solvent. Chemical shifts are reported in ppm and coupling constants (J) in Hz; the internal standard was tetramethylsilane (TMS). Unequivocal 13C assignments were made with the aid of 2D gHSQC and gHMBC (delays for one-bond and long-range JC/H couplings were optimized for 145 and 7 Hz, respectively) experiments. Positive ESI mass spectra were acquired with a QTOF 2 spectrometer. Preparative thin layer chromatography (TLC) was performed with Macherey–Nagel silica gel G/UV254. All chemicals and solvents were obtained from commercial sources and used as received or dried by standard procedures. O-Propargyl-2-SC 1 was prepared through a procedure described in the literature [3,4].
3.2. General Procedure for the Synthesis of 2,2’-[(1E,1’E)-{[Hexa-2,4-diyne-1,6-diylbis(oxy)]bis(2,1-phenylene)}bis(ethene-2,1-diyl)]bis(4H-chromen-4-one) (3)
Cu(OTf)2 (12 mg; 0.033 mmol) and CuI (6.3 mg; 0.033 mmol) were added to a solution of the O-propargyl-2-SC 1 (100 mg; 0.331 mmol) in DMF (1 mL). The reaction mixture was stirred at 80 °C for 24 h. After this period, it was poured onto H2O and then, the obtained precipitate was filtered off, taken into CH2Cl2, and dried over anhydrous Na2SO4. The solvent was evaporated to dryness, and compound 3 was obtained in its pure form as a yellow solid (95.4 mg, 96% yield). Mp 192–195 °C. 1H NMR (500 MHz, CDCl3): δ 4.91 (s, 4H, H-1″), 6.35 (s, 2H, H-3), 6.88 (d, J 16.2 Hz, 2H, H-α), 7.01–7.10 (m, 4H, H-3′,5′), 7.33–7.41 (m, 4H, H-6,4′), 7.56 (dd, J 1.1 and 8.6 Hz, 2H, H-8), 7.61 (dd, J 1.7 and 7.7 Hz, 2H, H-6′), 7.67 (ddd, J 1.7, 7.0 and 8.6 Hz, 2H, H-7), 7.90 (d, J 16.2 Hz, 2H, H-β), 8.20 (dd, J 1.7 and 7.9 Hz, 2H, H-5) ppm. 13C NMR (75 MHz, CDCl3): δ 56.7 (C-1″), 71.5 and 74.5 (C-2″,3″), 110.5 (C-3), 112.8 (C-3′), 118.0 (C-8), 121.4 (C-α), 122.1 (C-5′), 124.1 (C-10), 124.8 (C-1′), 124.9 (C-6), 125.6 (C-5), 128.3 (C-6′), 130.9 (C-4′), 131.8 (C-β), 133.6 (C-7), 155.7 (C-2′), 156.0 (C-9), 162.2 (C-2), 178.5 (C-4) ppm. HRMS-ESI+ m/z calcd for [C40H26O6 + H]+: 603.1808, found: 603.1771.
4. Conclusions
To conclude, we synthesized a new 2-SC-derived compound, which is characterized by its dimeric scaffold based on two units of 2-SC linked through a 1,3-dyine moiety. This compound was obtained in 96% yield with no need of further chromatography purification, through the copper-catalyzed homocoupling of two molecules of O-propargyl-2-SC. Extended libraries of the synthesized compound can be very interesting for their potential biological activities since it combines 2-SCs and a 1,3-dyine in the same molecule.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1: 1H NMR spectrum of the title compound 3; Figure S2: 13C NMR spectrum of the title compound 3; Figure S3: HSQC spectrum of the title compound 3; Figure S4: HMBC spectrum of the title compound 3; Figure S5: HRMS spectrum of the title compound 3.
Author Contributions
Conceptualization and supervision, H.M.T.A. and A.M.S.S.; methodology and investigation, D.M. and J.L.C.S.; writing—original draft preparation, J.L.C.S.; writing—review and editing, J.L.C.S., H.M.T.A. and A.M.S.S.; project administration and funding acquisition, A.M.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from PT national funds (OE) through FCT/MCTES (Fundação para a Ciência e a Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) within the projects: LAQV-REQUIMTE (UIDB/50006/2020 and UIDP/50006/2020), and MuTaTher-AD—“Multi-target theranostics for Alzheimer’s disease” (2022.06064.PTDC). Joana Sousa gratefully acknowledges LAQV-REQUIMTE for her researcher contract. Daniela Malafaia thanks FCT/MCTES (Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) and ESF (European Social Fund) through NORTE 2020 (Programa Operacional Região Norte) for her PhD grant (2021.05641.BD).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santos, C.M.M.; Silva, A.M.S. An Overview of 2-Styrylchromones: Natural Occurrence, Synthesis, Reactivity and Biological Properties. Eur. J. Org. Chem. 2017, 2017, 3115–3133. [Google Scholar] [CrossRef]
- Gomes, A.; Freitas, M.; Fernandes, E.; Lima, J.L. Biological activities of 2-styrylchromones. Mini-Rev. Med. Chem. 2010, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, H.M.T.; Santos, C.M.M.; Cavaleiro, J.A.S.; Silva, A.M.S. First intramolecular Diels–Alder reactions using chromone derivatives: Synthesis of chromeno[3,4-b]xanthones and 2-(benzo[c]chromenyl)chromones. New J. Chem. 2018, 42, 4251–4260. [Google Scholar] [CrossRef]
- Malafaia, D.; Oliveira, A.; Fernandes, P.A.; Ramos, M.J.; Albuquerque, H.M.T.; Silva, A.M.S. Chromeno[3,4-b]xanthones as First-in-Class AChE and Aβ Aggregation Dual-Inhibitors. Int. J. Mol. Sci. 2021, 22, 4145. [Google Scholar] [CrossRef] [PubMed]
- Shi Shun, A.L.K.; Tykwinski, R.R. Synthesis of Naturally Occurring Polyynes. Angew. Chem. Int. Ed. 2006, 45, 1034–1057. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Yus, M. Heterogeneous Catalytic Homocoupling of Terminal Alkynes. ACS Catal. 2012, 2, 1441–1451. [Google Scholar] [CrossRef]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Acetylenic Polymers: Syntheses, Structures, and Functions. Chem. Rev. 2009, 109, 5799–5867. [Google Scholar] [CrossRef] [PubMed]
- Siemsen, P.; Livingston, R.C.; Diederich, F. Acetylenic Coupling: A Powerful Tool in Molecular Construction. Angew. Chem. Int. Ed. 2000, 39, 2632–2657. [Google Scholar] [CrossRef]
- Stefani, H.A.; Guarezemini, A.S.; Cella, R. Homocoupling reactions of alkynes, alkenes and alkyl compounds. Tetrahedron 2010, 66, 7871–7918. [Google Scholar] [CrossRef]
- Yerrabelly, J.R.; Porala, S.; Kasireddy, V.R.; Ghojala, V.R.; Rebelli, P. CuI/Cu(OSO2CF3)2 catalysed convenient approach to dichromenopyridines and triazole-thiazole appended chromone derivatives. Synth. Commun. 2021, 51, 3781–3790. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).