3-(4-Fluorophenyl)-1-(1-(4-fluorophenyl)-3,3,3-trifluoroprop-1-en-1-yl)-5-fluoro-1H-pyrazole
Abstract
1. Introduction
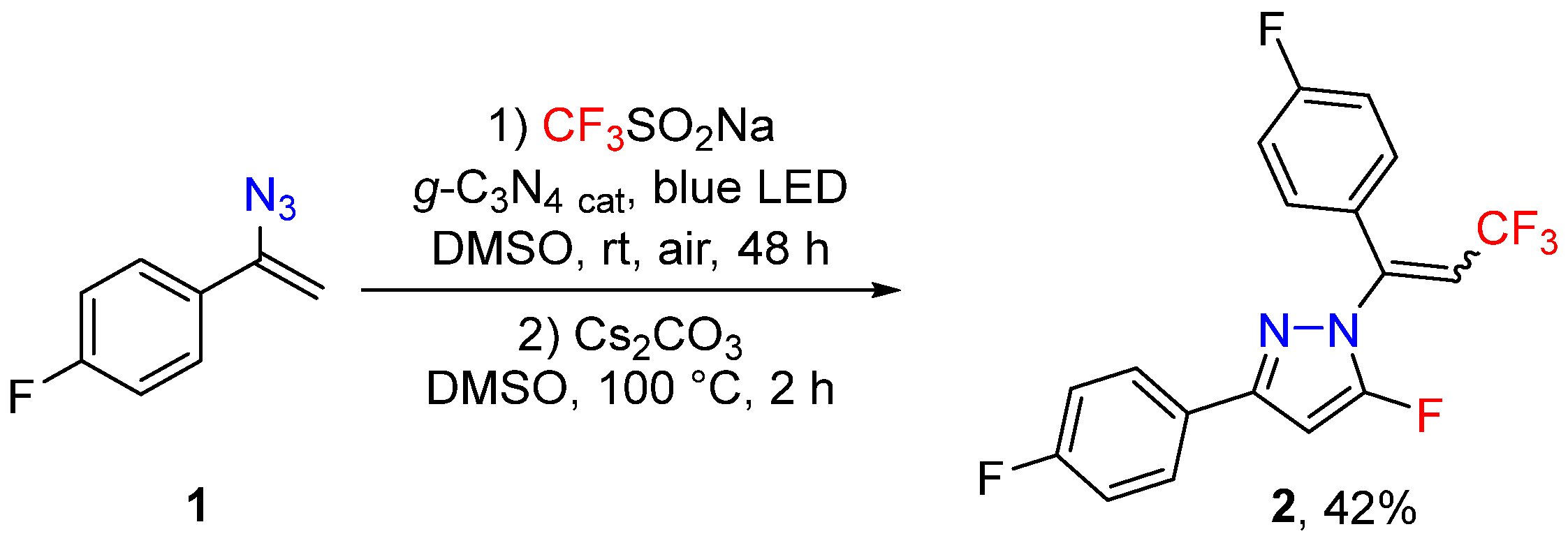
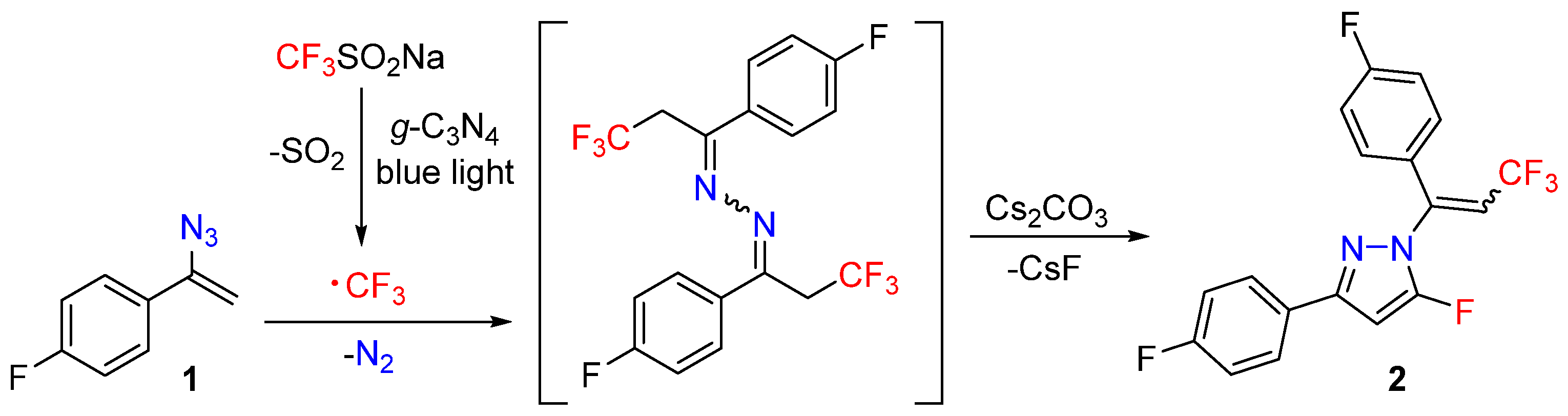
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. 3-(4-Fluorophenyl)-1-(1-(4-Fluorophenyl)-3,3,3-Trifluoroprop-1-en-1-yl)-5-Fluoro-1H-Pyrazole (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, D.M.; Román, R.; Barrio, P.; Trabanco, A.A.; Fustero, S. Biorelevant fluorine-containing N-heterocycles. In Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals; Haufe, G., Leroux, F.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 575–606. [Google Scholar]
- Zhang, B.; Wang, J. Assembly of versatile fluorine-containing structures via N-heterocyclic carbene organocatalysis. Sci. China Chem. 2022, 65, 1691–1703. [Google Scholar] [CrossRef]
- Mykhailiuk, P.K. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2021, 121, 1670–1715. [Google Scholar] [CrossRef]
- Barata-Vallejo, S.; Cooke, M.V.; Postigo, A. Radical Fluoroalkylation Reactions. ACS Catal. 2018, 8, 7287–7307. [Google Scholar] [CrossRef]
- Baguia, H.; Evano, G. Direct Perfluoroalkylation of C−H Bonds in (Hetero) arenes. Chem.—Eur. J. 2022, 28, e202200975. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef]
- Barata-Vallejo, S.; Bonesi, S.M.; Postigo, A. Photocatalytic fluoroalkylation reactions of organic compounds. Org. Biomol. Chem. 2015, 13, 11153–11183. [Google Scholar] [CrossRef]
- Koike, T. Frontiers in Radical Fluoromethylation by Visible-Light Organic Photocatalysis. Asian J. Org. Chem. 2020, 9, 529–537. [Google Scholar] [CrossRef]
- Kliś, T. Visible-Light Photoredox Catalysis for the Synthesis of Fluorinated Aromatic Compounds. Catalysts 2023, 13, 94. [Google Scholar] [CrossRef]
- Gisbertz, S.; Pieber, B. Heterogeneous Photocatalysis in Organic Synthesis. ChemPhotoChem 2020, 4, 456–475. [Google Scholar] [CrossRef]
- Ghosh, I.; Khamrai, J.; Savateev, A.; Shlapakov, N.; Antonietti, M.; König, B. Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes. Science 2019, 365, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, M.; Zha, W.; Wei, Y.; Ma, X.; Xu, C.; Lu, C.; Qin, N.; Gao, L.; Qiu, W.; et al. Mechanistic study of visible light-driven CdS or g-C3N4-catalyzed CH direct trifluoromethylation of (hetero) arenes using CF3SO2Na as the trifluoromethyl source. J. Catal. 2020, 389, 533–543. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Singh, P.P.; Srivastava, V. Recent advances in visible-light graphitic carbon nitride (g-C3N4) photocatalysts for chemical transformations. RSC Adv. 2022, 12, 18245–18265. [Google Scholar] [CrossRef]
- Qin, H.-T.; Wu, S.-W.; Liu, J.-L.; Liu, F. Photoredox-catalysed redox-neutral trifluoromethylation of vinyl azides for the synthesis of α-trifluoromethylated ketones. Chem. Commun. 2017, 53, 1696–1699. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Lonca, G.H.; Chiba, S. PhI(OAc)2-Mediated Radical Trifluoromethylation of Vinyl Azides with Me3SiCF3. Angew. Chem. Int. Ed. 2014, 53, 1067–1071. [Google Scholar] [CrossRef]
- Ikeda, I.; Kogame, Y.; Okahara, M. Synthesis of novel pyrazoles containing perfluoroalkyl groups by reactions of perfluoro-2-methylpent-2-ene and hydrazones. J. Org. Chem. 1985, 50, 3640–3642. [Google Scholar] [CrossRef]
- Paveliev, S.A.; Churakov, A.I.; Alimkhanova, L.S.; Segida, O.O.; Nikishin, G.I.; Terent’ev, A.O. Electrochemical Synthesis of O-Phthalimide Oximes from α-Azido Styrenes via Radical Sequence: Generation, Addition and Recombination of Imide-N-Oxyl and Iminyl Radicals with C−O/N−O Bonds Formation. Adv. Synth. Catal. 2020, 362, 3864–3871. [Google Scholar] [CrossRef]
- Sharma, P.; Sarngan, P.P.; Lakshmanan, A.; Sarkar, D. One-step synthesis of highly reactive g-C3N4. J. Mater. Sci. Mater. Electron. 2022, 33, 9116–9125. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paveliev, S.A.; Ustyuzhanin, A.O.; Krylov, I.B.; Terent’ev, A.O. 3-(4-Fluorophenyl)-1-(1-(4-fluorophenyl)-3,3,3-trifluoroprop-1-en-1-yl)-5-fluoro-1H-pyrazole. Molbank 2023, 2023, M1620. https://doi.org/10.3390/M1620
Paveliev SA, Ustyuzhanin AO, Krylov IB, Terent’ev AO. 3-(4-Fluorophenyl)-1-(1-(4-fluorophenyl)-3,3,3-trifluoroprop-1-en-1-yl)-5-fluoro-1H-pyrazole. Molbank. 2023; 2023(2):M1620. https://doi.org/10.3390/M1620
Chicago/Turabian StylePaveliev, Stanislav A., Alexander O. Ustyuzhanin, Igor B. Krylov, and Alexander O. Terent’ev. 2023. "3-(4-Fluorophenyl)-1-(1-(4-fluorophenyl)-3,3,3-trifluoroprop-1-en-1-yl)-5-fluoro-1H-pyrazole" Molbank 2023, no. 2: M1620. https://doi.org/10.3390/M1620
APA StylePaveliev, S. A., Ustyuzhanin, A. O., Krylov, I. B., & Terent’ev, A. O. (2023). 3-(4-Fluorophenyl)-1-(1-(4-fluorophenyl)-3,3,3-trifluoroprop-1-en-1-yl)-5-fluoro-1H-pyrazole. Molbank, 2023(2), M1620. https://doi.org/10.3390/M1620






