3a-(4-Chlorophenyl)-1-methyl-3a,4-dihydroimidazo[1,5-a]quinazolin-5(3H)-one: Synthesis and In Silico Evaluation as a Ligand in the µ-Opioid Receptor
Abstract
1. Introduction
2. Results and Discussion
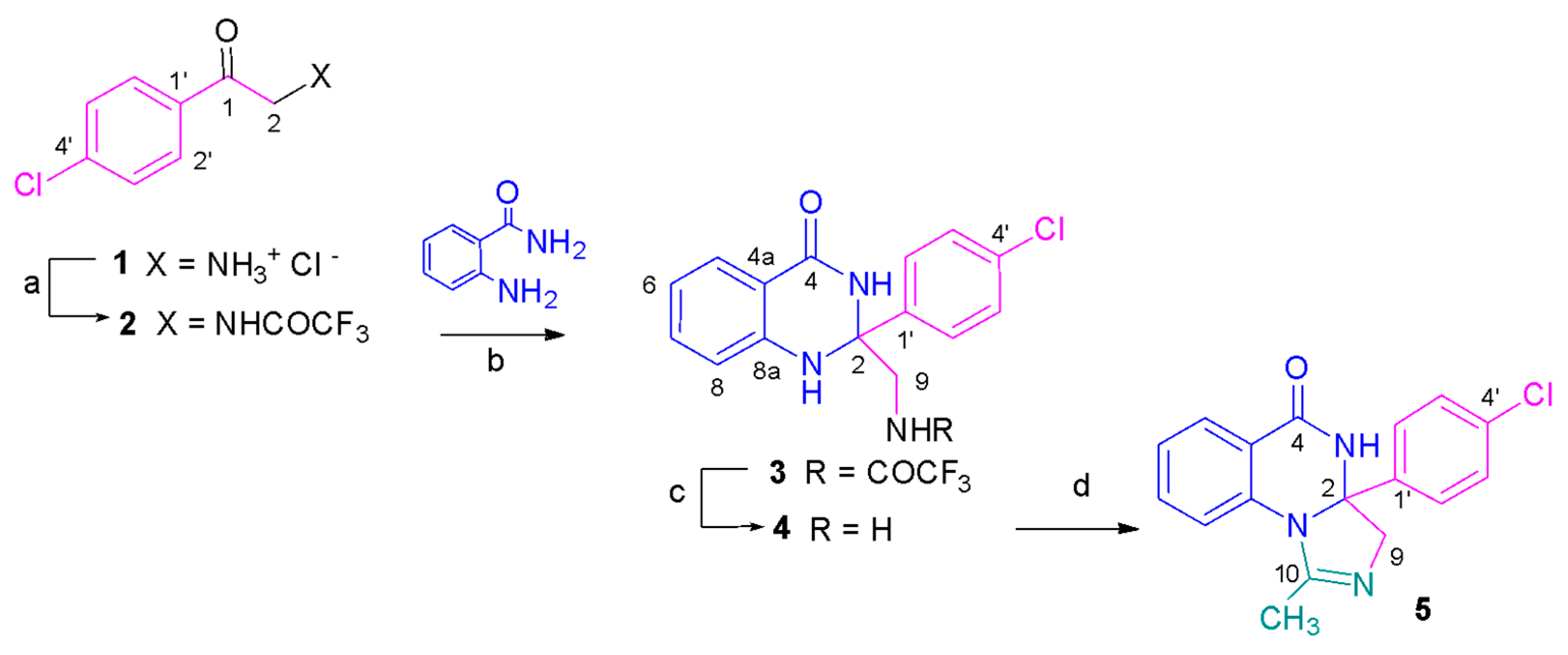
2.1. Chemistry
2.2. In Silico Analysis
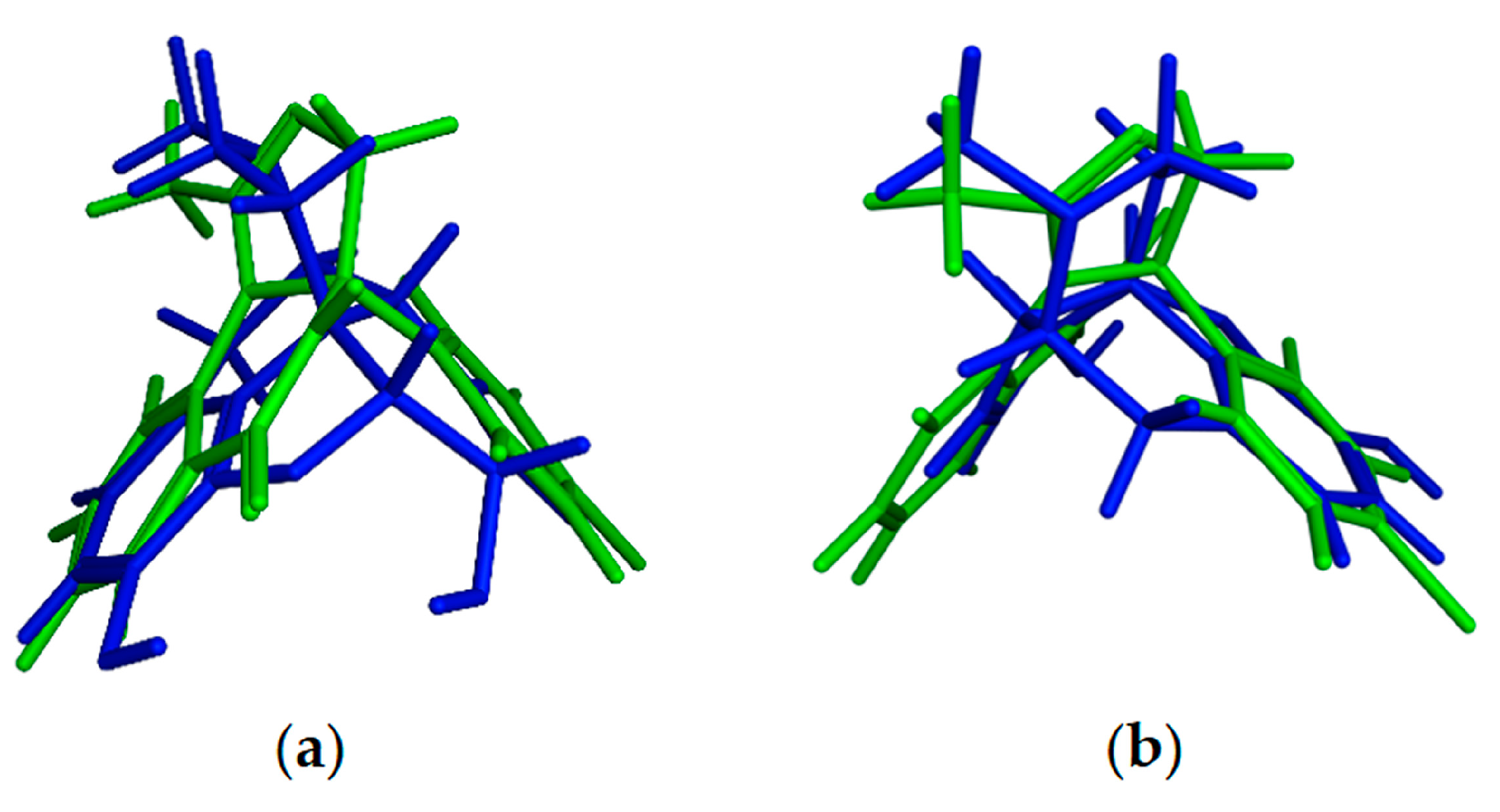
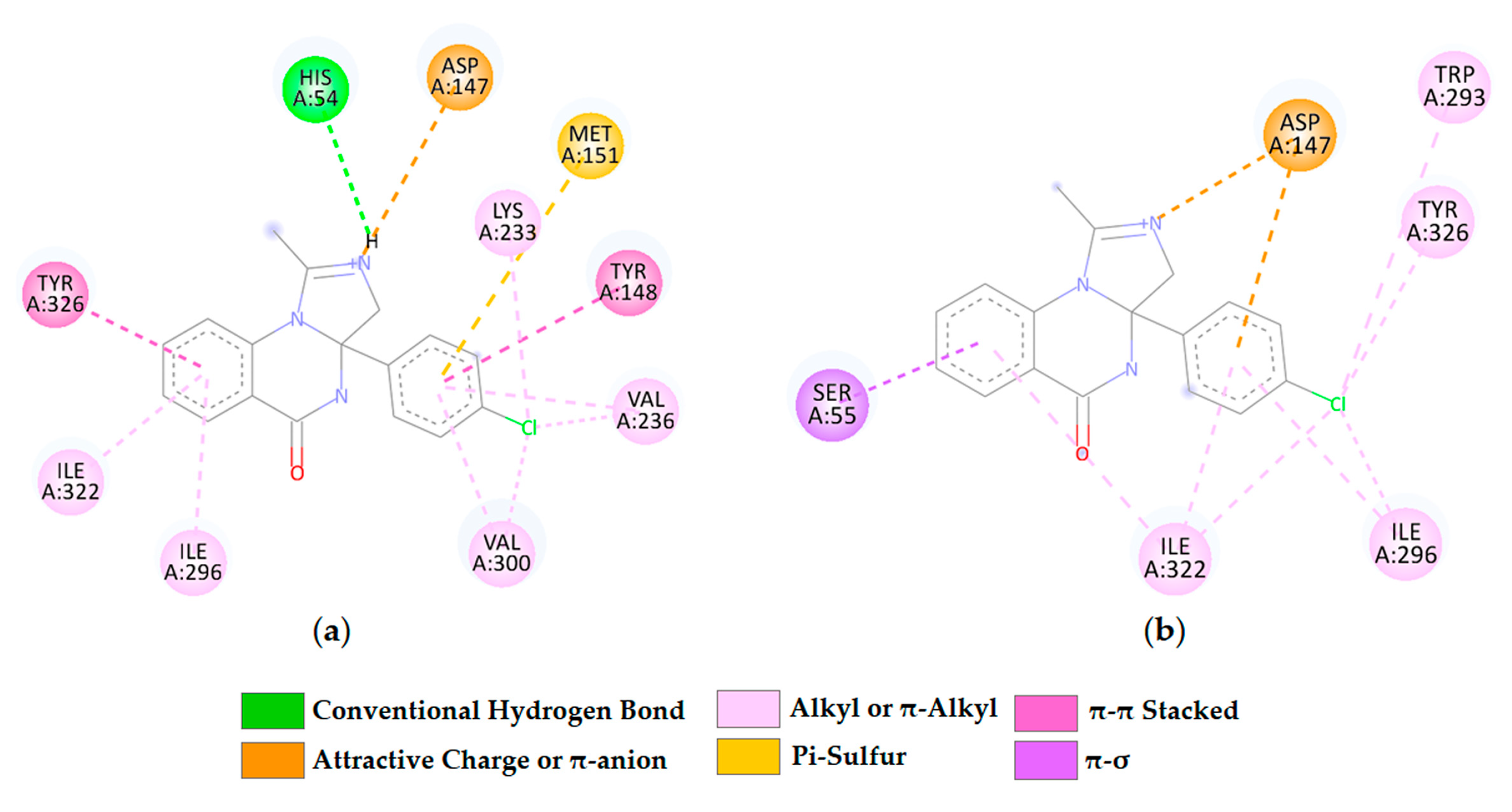
2.2.1. Docking Calculation
2.2.2. Pharmacokinetic Study
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. Synthetic Procedure
N-[2-(4-Chlorophenyl)-2-oxoethyl]-2,2,2-trifluoroacetamide (2)
N-{[2-(4-Chlorophenyl)-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl]methyl}-2,2,2-trifluoroacetamide (3)
2-(Aminomethyl)-2-(4-chlorophenyl)-2,3-dihydroquinazolin-4(1H)-one (4)
3a-(4-Chlorophenyl)-1-methyl-3a,4-dihydroimidazo[1,5-a]quinazolin-5(3H)-one (5)
3.2. Docking Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammadi, A.A.; Ahdenov, R.; Sooki, A.A. Design, synthesis and antibacterial evaluation of 2-alkyl- and 2-aryl-3-(phenylamino)quinazolin-4(3H)-one derivatives. Heterocycl. Commun. 2017, 23, 105–108. [Google Scholar] [CrossRef]
- Takaya, Y.; Tasaka, H.; Chiba, T.; Uwai, K.; Tanitsu, M.A.; Kim, H.S.; Wataya, Y.; Miura, M.; Takeshita, M.; Oshima, Y. New type of febrifugine analogues, bearing an auinolizidine moiety, Show potent antimalarial activity against plasmodium malaria parasite. J. Med. Chem. 1999, 42, 3163–3166. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Pathak, U.S. Synthesis and antihypertensive activity of novel 3-benzyl-2-substituted-3H-[1,2,4]triazolo[5,1-b]quinazolin-9-ones. Bioorg. Med. Chem. 2007, 15, 3457–3462. [Google Scholar] [CrossRef]
- Alagarsamy, V.; Solomon, V.R.; Dhanabal, K. Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted3H-quinazolin-4-one as analgesic, anti-inflammatory agents. Bioorg. Med. Chem. 2007, 15, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Wang, W.; Sun, Q.; Yang, S.; Jin, J.; Zhang, X.; Ren, X.; Zhang, J.; Zhou, J. Microwave-assisted synthesis of quinazolin-4(3H)-ones catalyzed by SbCl3. Heterocycl. Commun. 2018, 24, 293–296. [Google Scholar] [CrossRef]
- Wang, M.; Dou, G.; Shi, D. Efficient and Convenient Synthesis of Pyrrolo[1,2-a]quinazoline De-rivatives with the Aid of Tin(II) Chloride. J. Comb. Chem. 2010, 12, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Reza Safei, H.; Mohsen, S.; Shima, K.; Rahmanian, V.; Safaei, M. Diversity-oriented synthesis of quinazoline derivatives using zirconium tetrakis(dodecylsulfate) [Zr(DS)] as a reusable Lewis acid-surfactant-combined catalyst in tap water. J. Ind. Eng. Chem. 2014, 20, 3019–3024. [Google Scholar] [CrossRef]
- Bunce, R.A.; Nammalwar, B. New conditions for synthesis of (±)-2-monosubstituted and (±)-2,2-disubstituted 2,3-dihydro-4(1H)-quinazolinones from 2-nitro- and 2-aminobenzamide. J. Heterocycl. Chem. 2011, 48, 991–997. [Google Scholar] [CrossRef]
- Lu, L.; Yang, K.; Zhang, M.M.; Wanga, X.S. An efficient synthesis of pyrrolo[1,2-a]quinazoline derivatives in ionic liquid catalyzed by iodine. J. Heterocycl. Chem. 2014, 51, 841–845. [Google Scholar] [CrossRef]
- Mehedia, M.S.A.; Tepe, J.J. Recent Advances in the Synthesis of Imidazolines (2009–2020). Adv. Synth. Catal. 2020, 362, 4189–4225. [Google Scholar] [CrossRef]
- Ninković, J.; Roy, S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids 2013, 45, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Contet, C.; Kieffer, B.L.; Befort, K. Mu opioid receptor: A gateway to drug addiction. Curr. Opin. Neurobiol. 2004, 14, 370–378. [Google Scholar] [CrossRef]
- Huang, W.; Manglik, A.; Venkatakrishnan, A.J.; Laeremans, T.; Feinberg, E.N.; Sanborn, A.L.; Kato, H.E.; Livingston, K.E.; Thorsen, T.S.; Kling, R.C.; et al. Structural insights into µ-opioid receptor activation. Nature 2015, 524, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Gatta, F.; Landi Vittory, R. Reaction of Anthranilamides. II. Synthesis of 1,2-trimethylene-2-methyl-1,2,3,4-tetrahydroquinazoline derivatives. Gazz. Chim. Ital. 1969, 99, 715–722. [Google Scholar]
- Makowski, M.; Rzeszotarska, B.; Smelka, L.; Kubica, Z. Debenzyloxycarbonylation and Detrifluoroacetylation of Dehydroalanine and Dehydrophenylalanine Peptides. Liebigs Ann. Chem. 1985, 1985, 1451–1464. [Google Scholar] [CrossRef]
- Levesque, G.; Gressier, J.-C.; Proust, M. 4,5-Dihydroimidazoles from dithiocarboxylic esters, thiocarboxamides, or nitriles. Synthesis 1981, 1981, 963–965. [Google Scholar] [CrossRef]
- PASS Online. Available online: http://way2drug.com/PassOnline/index.php (accessed on 26 February 2023).
- Swiss ADME. Available online: http://www.swissadme.ch/ (accessed on 4 March 2023).
- Molinspiration. Available online: https://www.molinspiration.com/ (accessed on 4 March 2023).
- Molsft L.L.C. Available online: https://www.molsoft.com/ (accessed on 4 March 2023).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T., Jr.; Kudin, K.N.; Burant, J.C.; et al. Gaussian, Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Schmider, H.L.; Becke, A.D. Optimized density functionals from the extended G2 test set. J. Chem. Phys. 1998, 108, 9624–9631. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
| Structure |  | 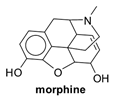 | 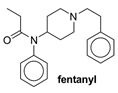 | 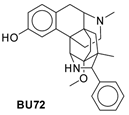 |
|---|---|---|---|---|
| Pa (a) score | 0.754 | 0.961 | 0.788 | 0.695 |
| Pi (a) score | 0.006 | 0.004 | 0.005 | 0.010 |
| Web Tool | Properties | Compound 5 | Morphine | Fentanyl | BU72 |
|---|---|---|---|---|---|
| SwissADME | TPSA (Å2) | 44.70 | 52.93 | 23.55 | 44.73 |
| WLogP | 1.92 | 0.82 | 3.76 | 3.09 | |
| Consensus Log P | 2.72 | 1.47 | 3.78 | 3.68 | |
| BBB permeant | Yes | Yes | Yes | Yes | |
| Molinspiration | TPSA (Å2) | 44.70 | 52.92 | 23.55 | 44.73 |
| miLog P | 3.52 | 1.10 | 3.79 | 4.09 | |
| BBB | - | - | - | - | |
| Molsoft L.L.C. | MolPSA (Å2) | 36.49 | 43.21 | 18.21 | 39.69 |
| MolLog P | 3.37 | 0.79 | 3.89 | 4.32 | |
| BBB score (a) | 5.12 | 4.67 | 5.61 | 4.90 | |
| Drug-likeness model score | 1.33 | 0.73 | 0.99 | 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Defant, A.; Innocenti, N.; Mancini, I. 3a-(4-Chlorophenyl)-1-methyl-3a,4-dihydroimidazo[1,5-a]quinazolin-5(3H)-one: Synthesis and In Silico Evaluation as a Ligand in the µ-Opioid Receptor. Molbank 2023, 2023, M1622. https://doi.org/10.3390/M1622
Defant A, Innocenti N, Mancini I. 3a-(4-Chlorophenyl)-1-methyl-3a,4-dihydroimidazo[1,5-a]quinazolin-5(3H)-one: Synthesis and In Silico Evaluation as a Ligand in the µ-Opioid Receptor. Molbank. 2023; 2023(2):M1622. https://doi.org/10.3390/M1622
Chicago/Turabian StyleDefant, Andrea, Nicole Innocenti, and Ines Mancini. 2023. "3a-(4-Chlorophenyl)-1-methyl-3a,4-dihydroimidazo[1,5-a]quinazolin-5(3H)-one: Synthesis and In Silico Evaluation as a Ligand in the µ-Opioid Receptor" Molbank 2023, no. 2: M1622. https://doi.org/10.3390/M1622
APA StyleDefant, A., Innocenti, N., & Mancini, I. (2023). 3a-(4-Chlorophenyl)-1-methyl-3a,4-dihydroimidazo[1,5-a]quinazolin-5(3H)-one: Synthesis and In Silico Evaluation as a Ligand in the µ-Opioid Receptor. Molbank, 2023(2), M1622. https://doi.org/10.3390/M1622







