2,2’-[(1E,1’E)-{[Hexa-2,4-diyne-1,6-diylbis(oxy)]bis(2,1-phenylene)}bis(ethene-2,1-diyl)]bis(4H-chromen-4-one)
Abstract
1. Introduction
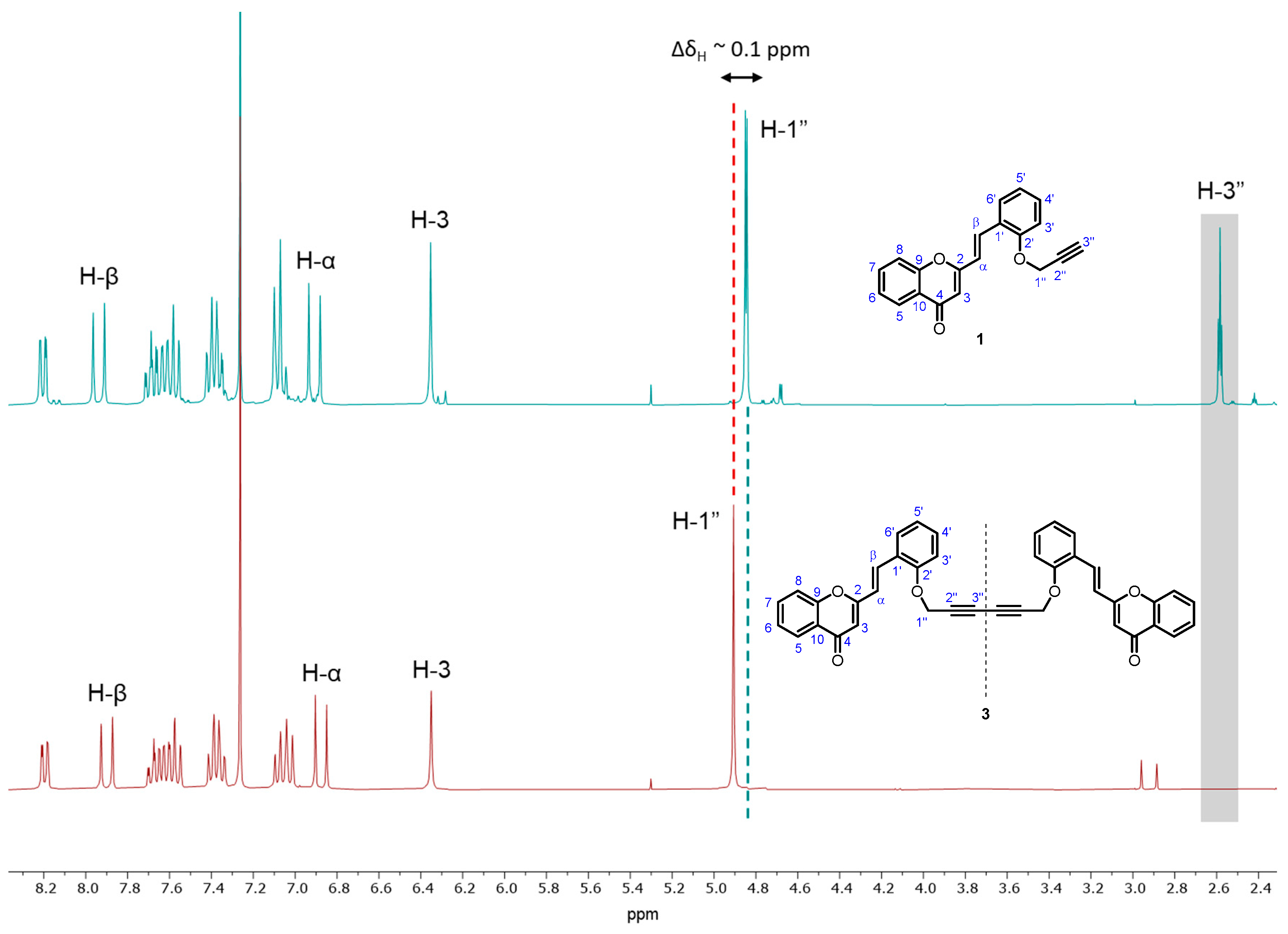
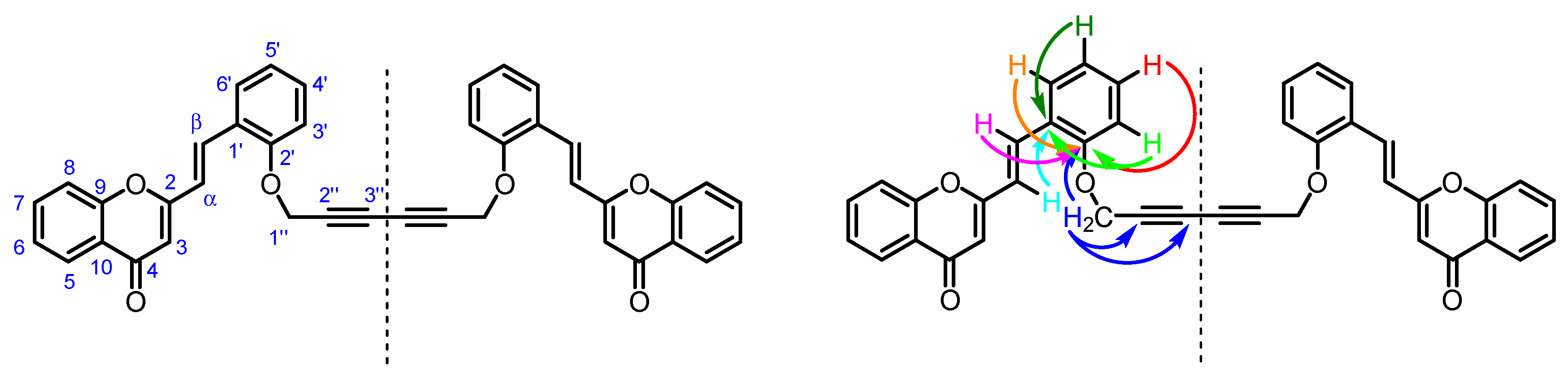
2. Results and Discussion
- (i)
- C-2″ and C-3″ (δC 71.5 and 74.5 ppm) of the 1,3-diyne moiety due to its connectivities with H-1″.
- (ii)
- C-1′ (δC 124.8 ppm) due to its connectivities with H-α and H-3′,5′.
- (iii)
- C-2′ (δC 155.7 ppm) due to its connectivities with H-1″, H-β and H-3′,4′,6′.
3. Materials and Methods
3.1. General Remarks
3.2. General Procedure for the Synthesis of 2,2’-[(1E,1’E)-{[Hexa-2,4-diyne-1,6-diylbis(oxy)]bis(2,1-phenylene)}bis(ethene-2,1-diyl)]bis(4H-chromen-4-one) (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santos, C.M.M.; Silva, A.M.S. An Overview of 2-Styrylchromones: Natural Occurrence, Synthesis, Reactivity and Biological Properties. Eur. J. Org. Chem. 2017, 2017, 3115–3133. [Google Scholar] [CrossRef]
- Gomes, A.; Freitas, M.; Fernandes, E.; Lima, J.L. Biological activities of 2-styrylchromones. Mini-Rev. Med. Chem. 2010, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, H.M.T.; Santos, C.M.M.; Cavaleiro, J.A.S.; Silva, A.M.S. First intramolecular Diels–Alder reactions using chromone derivatives: Synthesis of chromeno[3,4-b]xanthones and 2-(benzo[c]chromenyl)chromones. New J. Chem. 2018, 42, 4251–4260. [Google Scholar] [CrossRef]
- Malafaia, D.; Oliveira, A.; Fernandes, P.A.; Ramos, M.J.; Albuquerque, H.M.T.; Silva, A.M.S. Chromeno[3,4-b]xanthones as First-in-Class AChE and Aβ Aggregation Dual-Inhibitors. Int. J. Mol. Sci. 2021, 22, 4145. [Google Scholar] [CrossRef] [PubMed]
- Shi Shun, A.L.K.; Tykwinski, R.R. Synthesis of Naturally Occurring Polyynes. Angew. Chem. Int. Ed. 2006, 45, 1034–1057. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Yus, M. Heterogeneous Catalytic Homocoupling of Terminal Alkynes. ACS Catal. 2012, 2, 1441–1451. [Google Scholar] [CrossRef]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Acetylenic Polymers: Syntheses, Structures, and Functions. Chem. Rev. 2009, 109, 5799–5867. [Google Scholar] [CrossRef] [PubMed]
- Siemsen, P.; Livingston, R.C.; Diederich, F. Acetylenic Coupling: A Powerful Tool in Molecular Construction. Angew. Chem. Int. Ed. 2000, 39, 2632–2657. [Google Scholar] [CrossRef]
- Stefani, H.A.; Guarezemini, A.S.; Cella, R. Homocoupling reactions of alkynes, alkenes and alkyl compounds. Tetrahedron 2010, 66, 7871–7918. [Google Scholar] [CrossRef]
- Yerrabelly, J.R.; Porala, S.; Kasireddy, V.R.; Ghojala, V.R.; Rebelli, P. CuI/Cu(OSO2CF3)2 catalysed convenient approach to dichromenopyridines and triazole-thiazole appended chromone derivatives. Synth. Commun. 2021, 51, 3781–3790. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malafaia, D.; Sousa, J.L.C.; Silva, A.M.S.; Albuquerque, H.M.T. 2,2’-[(1E,1’E)-{[Hexa-2,4-diyne-1,6-diylbis(oxy)]bis(2,1-phenylene)}bis(ethene-2,1-diyl)]bis(4H-chromen-4-one). Molbank 2023, 2023, M1621. https://doi.org/10.3390/M1621
Malafaia D, Sousa JLC, Silva AMS, Albuquerque HMT. 2,2’-[(1E,1’E)-{[Hexa-2,4-diyne-1,6-diylbis(oxy)]bis(2,1-phenylene)}bis(ethene-2,1-diyl)]bis(4H-chromen-4-one). Molbank. 2023; 2023(2):M1621. https://doi.org/10.3390/M1621
Chicago/Turabian StyleMalafaia, Daniela, Joana L. C. Sousa, Artur M. S. Silva, and Hélio M. T. Albuquerque. 2023. "2,2’-[(1E,1’E)-{[Hexa-2,4-diyne-1,6-diylbis(oxy)]bis(2,1-phenylene)}bis(ethene-2,1-diyl)]bis(4H-chromen-4-one)" Molbank 2023, no. 2: M1621. https://doi.org/10.3390/M1621
APA StyleMalafaia, D., Sousa, J. L. C., Silva, A. M. S., & Albuquerque, H. M. T. (2023). 2,2’-[(1E,1’E)-{[Hexa-2,4-diyne-1,6-diylbis(oxy)]bis(2,1-phenylene)}bis(ethene-2,1-diyl)]bis(4H-chromen-4-one). Molbank, 2023(2), M1621. https://doi.org/10.3390/M1621








