Abstract
Functionally substituted 1,2-benzisoxazoles are very important and promising heterocycles with various pharmacological activities. Benzoxazoles containing reactive 3-chloromethyl and 5-amino groups are practically unexplored derivatives in this series. In this communication, the simple method for the synthesis of N-[3-(chloromethyl)-1,2-benzisoxazol-5-yl]acetamide which is an interesting precursor for the preparation of a series of 3,5-disubstituted benzoxazoles was described. The structure of the synthesized compound was established by elemental analysis, high-resolution mass spectrometry, 1H, 13C NMR and IR spectroscopy, and mass spectrometry.
1. Introduction
1,2-Benzisoxazole derivatives are oxygen- and nitrogen-containing heterocycles with a wide range of synthetic and pharmaceutical applications. They possess significant pharmacological and biological activities such as analgesics [1], anticonvulsant [2,3], antipscychotic [4], anticancer [5], and antimicrobial [6], and also showed affinity for serotonergic and dopaminergic receptors [7]. New functional derivatives of this class can be considered as compounds with great potential for biological activity. 5-Amino derivatives of 1,2-benzisoxazoles are actively investigated as biologically active compounds [3,8,9,10]. There is information about the synthesis of 3-chloromethyl 1,2-benzisoxazoles [11]. To expand the range of functionally substituted 1,2-benzisoxazoles, we aimed to combine in one molecule a protected amino group in the fifth position and a functionally active chloromethyl group in the third position of the heterocycle. Herein, we report the synthesis of N-[3-(chloromethyl)-1,2-benzisoxazol-5-yl]acetamide 1, which can be considered as an important intermediate for the preparation of previously inaccessible 3,5-disubstituted 1,2-benzisoxazoles.
2. Results and Discussion
There are several synthetic protocols for the synthesis of 3-substituted 1,2-benzisoxazoles [12]. We chose the method of base-catalyzed cyclization of o-hydroxyphenylketoximes [13]. It was found that the treatment of N-[4-(chloroacetyl)-3-hydroxyphenyl]acetamide 3 [14] with hydroxylamine hydrochloride and pyridine in EtOH gave oxime 2 in high yield. The reaction of oxime 2 with thionyl chloride in the presence of pyridine in anhydrous THF yielded the target N-[3-(chloromethyl)-1,2-benzisoxazol-5-yl]acetamide 1 (Scheme 1).
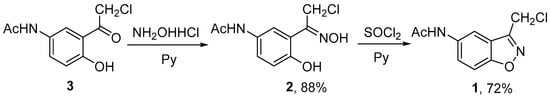
Scheme 1.
Synthesis of N-[3-(chloromethyl)-1,2-benzisoxazol-5-yl]acetamide 1.
The structure of N-[3-(chloromethyl)-1,2-benzisoxazol-5-yl]acetamide 1 was fully confirmed by elemental analysis, high-resolution mass spectrometry, 1H, 13C NMR and IR spectroscopy, and mass spectrometry. HRMS and elemental analysis confirm the brutto formula of compound 1. The mass spectrum of the compound 1 contains two molecular ion peaks (244 and 242) with intensities characteristic of compounds containing one chlorine atom. The 1H NMR spectrum of 1 showed characteristic singlets of Me (2.11 ppm), ClCH2 (5.22 ppm) groups and C-H signals of the benzene ring (7.74 and 8.34 ppm). The IR spectrum contains signals characteristic of the acetamide group: NH (3300 cm–1) and C=O (1611 cm–1).
In conclusion, we synthesized N-[3-(chloromethyl)-1,2-benzisoxazol-5-yl]acetamide 1, which is a convenient precursor of various functionally disubstituted 1,2-benzisoxazoles, compounds with useful pharmacological properties.
3. Materials and Methods
N-(3-(2-Chloroacetyl)-4-hydroxyphenyl)acetamide 3 was prepared according to the published method [14]. The solvents and reagents were purchased from commercial sources and used as received. Elemental analysis was performed on a 2400 Elemental Analyzer (Perkin Elmer Inc., Waltham, MA, USA). Melting point was determined on a Kofler hot-stage apparatus and is uncorrected. 1H and 13C NMR spectra were taken with a Bruker AM-300 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA), at frequencies of 300 and 75 MHz, in DMSO-d6 solution, with TMS as the standard. J values are given in Hz. MS spectrum (EI, 70 eV) was obtained with a Finnigan MAT INCOS 50 instrument (Hazlet, NJ, USA). IR spectrum was measured with a Bruker “Alpha-T” instrument (Santa Barbara, CA 93117, USA) in KBr pellet. High-resolution MS spectrum was measured on a Bruker micrOTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization (ESI).
Synthesis of N-(3-(2-chloro-1-(hydroxyimino)ethyl)-4-hydroxyphenyl)acetamide 2 (Supplementary Materials).
Hydroxylamine hydrochloride (29.2 g, 0.42 mol) was added to a suspension of N-[4-(chloroacetyl)-3-hydroxyphenyl]acetamide (71.5 g, 0.31 mol) in EtOH (1200 mL). After stirring for 40 min at room temperature, pyridine (26.7 mL, 0.33 mol) was added dropwise within 30 min. The reaction mixture was stirred at room temperature until consumption of the starting compound 3 (TLC) for 5 h. The solvent was distilled off in vacuo; the residue was treated with water and hydrochloric acid to pH 4-5. The precipitate formed was filtered off, washed with water and dried. Yield 66.7 g (88%), white crystals, mp. 172–173 °C. IR spectrum (KBr), ν, cm–1: 3339 and 3165 (NH, OH), 2862 (CH), 1611 (C=O), 1553, 1394, 1273, 1008, 735. 1H-NMR (DMSO-d6, ppm): 2.02 (s, 3H), 4.70 (s, 2H), 6.85 (d, J = 8.8, 1H), 7.49 (d, J = 8.8, 1H), 7.64 (s, 1H), 9.82 (s, 1H), 10.30 (s, 1H), 12.12 (s, 1H). 13C-NMR (DMSO-d6, ppm): 23.8 (CH3), 32.8 (CH2), 116.3, 118.6, 119.9, 122.0, 131.3, 152.1, 154.2, 167.8. MS (EI, 70 Ev), m/z (I, %): 244 (M + 2, 7), 242 (M+, 25), 182 (22), 147 (48), 43 (100). HRMS (ESI-TOF): calcd for C10H12ClN2O3 [M + H]- 243.0531; found m/z 243.0539. Anal. calcd for C10H11ClN2O3: C, 49.50; H, 4.57; N, 11.54; found: C, 49.75; H, 4.62; N, 11.69%.
Synthesis of N-[3-(chloromethyl)-1,2-benzisoxazol-5-yl]acetamide 1 (Supplementary Materials).
A solution of thionyl chloride (5.5 mL, 75 mmol) in dry THF (50 mL) was added dropwise to a solution of oxime 2 (19.19 g, 75 mmol) and pyridine (20 mL) in dry THF (300 mL), at such a rate that the temperature did not rise above 0 °C. The reaction mixture was stirred at room temperature for 8 h, poured to cold water (500 mL) and acidified to pH~3; the THF was distilled off and residue was extracted with EtOAc (3 × 100 mL). The combined extracts were washed with water and dried over MgSO4. The solvent was partially distilled off in vacuo; the residue was filtered off and crystallized from a mixture of ethyl acetate–tert-butyl methyl ether. Yield 13.2 g (72%), white crystals, mp. 198–199 °C. IR spectrum (KBr), ν, cm–1: 3300 (NH), 2983 (CH), 1662 (C=O), 1565, 1519, 1476, 1338, 1265, 818, 738. 1H-NMR (DMSO-d6, ppm): 2.11 (s, 3H), 5.22 (s, 2H), 7.74 (m, 2H), 8.34 (s, 1H), 10.26 (s, 1H). 13C-NMR (DMSO-d6, ppm): 23.9 (CH3), 34.9 (CH2), 110.1, 110.5, 119.8, 123.5, 135.9, 155.7, 159.3, 168.5. MS (EI, 70 Ev), m/z (I, %): 226 (M + 2, 3), 224 (M+, 10), 182 (25), 147 (38), 43 (100). HRMS (ESI-TOF): calcd for C10H10ClN2O2 [M + 1]− 225.0425; found m/z 225.0432. Anal. calcd for C10H9ClN2O2: C, 53.47; H, 4.04; N, 12.47; found: C, 53.62; H, 4.15; N, 12.62%.
Supplementary Materials
The following are available online: copies of 1H and 13C NMR, IR, HRMS and mass-spectra for the compounds 1 and 2.
Author Contributions
Conceptualization, E.N.K.; methodology, O.A.R.; software, E.N.K.; validation, O.A.R.; formal analysis, investigation, E.N.K.; resources, O.A.R.; data curation, O.A.R.; writing—original draft preparation, E.N.K.; writing—review and editing, E.N.K.; visualization, O.A.R.; supervision, O.A.R.; project administration, O.A.R.; funding acquisition, O.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1 and 2 are available from the authors.
References
- Hasegawa, H. Utilization of zonisamide inpatients with chronic pain orepilepsy refractory to othertreatments: A retrospective, open label, uncontrolled studyin a VA hospital. Curr. Med. Res. Opin. 2004, 20, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Utsui, Y.; Shiraishi, Y.; Karasawa, T.; Yoshida, K.; Shimizu, M. Relationships Between Plasma Concentrations of Diphenylhydantoin, Phenobarbital, Carbamazepine, and 3-Sulfamoylmethyl-1,2-Benzisoxazole (AD-810), a New Anticonvulsant Agent, and Their Anticonvulsant or Neurotoxic Effects in Experimental Animals. Epilepsia 1979, 20, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Uno, H.; Kurokawa, M.; Masuda, Y.; Nishimura, H. Studies on 3-substituted 1,2-benzisoxazole derivatives. 6. Syntheses of 3-(sulfamoylmethyl)-1,2-benzisoxazole derivatives and their anticonvulsant activities. J. Med. Chem. 1979, 22, 180–183. [Google Scholar] [CrossRef]
- Hrib, N.J.; Jurcak, J.G.; Burgher, K.L.; Conway, P.G.; Hartman, H.B.; Kerman, L.L.; Roehr, J.E.; Woods, A.T. Benzisoxazole- and Benzisothiazole-3-carboxamides as Potential Atypical Antipsychotic Agents. J. Med. Chem. 1994, 37, 2308–2314. [Google Scholar] [CrossRef]
- Jain, M.; Kwon, C.-H. 1,2-Benzisoxazole Phosphorodiamidates as Novel Anticancer Prodrugs Requiring Bioreductive Activation. J. Med. Chem. 2003, 46, 5428–5436. [Google Scholar] [CrossRef] [PubMed]
- Priya, B.S.; Swamy, S.N.; Rangappa, K.S. Synthesis and characterization of novel 6-fluoro-4-piperidinyl-1,2-benzisoxazole amides and 6-fluoro-chroman-2-carboxamides: Antimicrobial studies. Bioorg. Med. Chem. 2005, 13, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Nuhrich, A.; Varache-Lembège, M.; Vercauteren, J.; Dokhan, R.; Renard, P.; Devaux, G. Synthesis and binding affinities of a series of 1,2-benzisoxazole-3-carboxamides to dopamine and serotonin receptors. Eur. J. Med. Chem. 1996, 31, 957–964. [Google Scholar] [CrossRef]
- Gopalsamy, A.; Shi, M.; Golas, J.; Vogan, E.; Jacob, J.; Johnson, M.; Lee, F.; Nilakantan, R.; Petersen, R.; Svenson, K.; et al. Discovery of Benzisoxazoles as Potent Inhibitors of Chaperone Heat Shock Protein 90. J. Med. Chem. 2008, 51, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Pippione, A.C.; Carnovale, I.M.; Bonanni, D.; Sini, M.; Goyal, P.; Marini, E.; Pors, K.; Adinolfi, S.; Zonari, D.; Festuccia, C.; et al. Potent and selective aldo-keto reductase 1C3 (AKR1C3) inhibitors based on the benzoisoxazole moiety: Application of a bioisosteric scaffold hopping approach to flufenamic acid. Eur. J. Med. Chem. 2018, 150, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Maoyi, L.; Yunfu, L.; Yu, X.; Guoli, Z.; Zhijuan, Y.; Jian, L.; Shuhui, C. Tricyclic Compounds Acting on CRBN Proteins. Patent EP3848367, 14 July 2021. [Google Scholar]
- Arava, V.R.; Gorentla, L.; Siripalli, U.B.R.; Dubey, P.K. An Efficient Synthesis of 3-Chloromethyl-1,2-benzisoxazoles via Modified Boekelheide Rearrangement. Indian J. Chem. B 2011, 50, 119–125. [Google Scholar]
- Shastri, R.A. Review on Synthesis of 3-Substituted 1,2-Benzisoxazole Derivatives. Chem. Sci. Trans. 2016, 5, 8–20. [Google Scholar] [CrossRef]
- Kalkote, U.; Goswami, D. New synthesis of 1,2-benzisoxazole derivatives. Aust. J. Chem. 1977, 30, 1847–1850. [Google Scholar] [CrossRef]
- Kunckell, F. Darstellung von Oxyamido- und Oxyamidochlor-Ketonen. Ber. Dtsch. Chem. Ges. 1901, 34, 124–129. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).