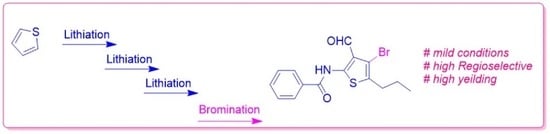
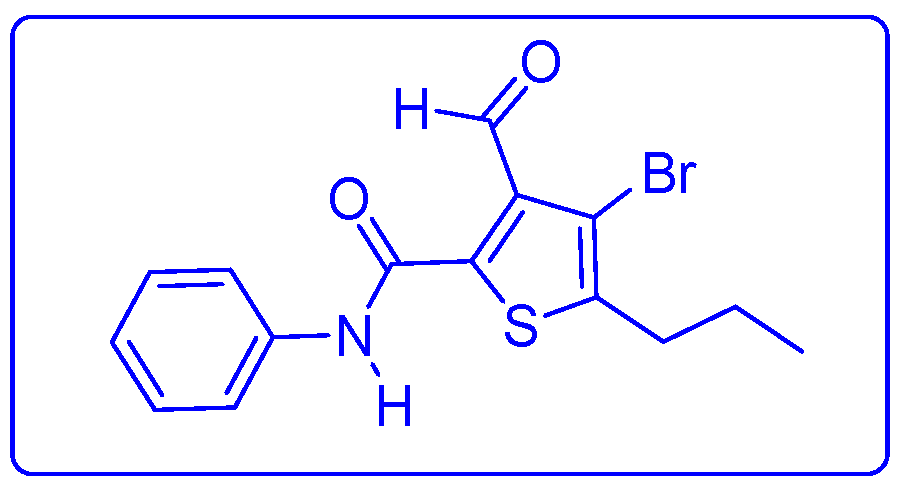
Regioselective Synthesis of 4-Bromo-3-formyl-N-phenyl-5-propylthiophene-2-carboxamide
Abstract
:1. Introduction
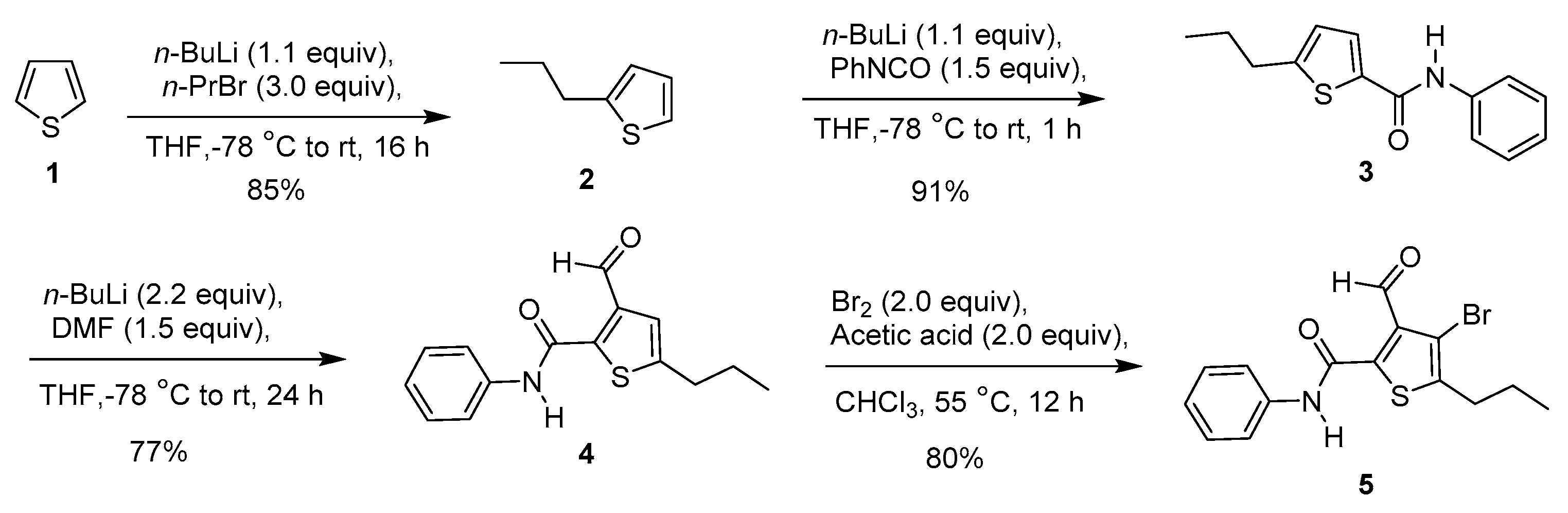
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 2-Propylthiophene 2
3.2. Synthesis of N-Phenyl-5-propylthiophene-2-carboxamide 3
3.3. Synthesis of 3-Formyl-N-phenyl-5-propylthiophene-2-carboxamide 4
3.4. Synthesis of 4-Bromo-3-formyl-N-phenyl-5-propylthiophene-2-carboxamide 5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perepichka, I.F.; Perepichka, D.F.; Meng, H.; Wudl, F. Light-Emitting Polythiophenes. Adv. Mater. 2005, 17, 2281–2305. [Google Scholar] [CrossRef]
- Barbarella, G.; Melucci, M.; Sotgiu, G. The Versatile Thiophene: An Overview of Recent Research on Thiophene-Based Materials. Adv. Mater. 2005, 17, 1581–1593. [Google Scholar] [CrossRef]
- Chan, H.S.O.; Ng, S.C. Synthesis, characterization and applications of thiophene-based functional polymers. Prog. Polym. Sci. 1998, 23, 1167–1231. [Google Scholar] [CrossRef]
- Barbarella, G.; Zangoli, M.; Di Maria, F. Synthesis and Applications of Thiophene Derivatives as Organic Materials. Adv. Heterocycl. Chem. 2017, 123, 105–167. [Google Scholar]
- Thayumanavan, S.; Mendez, J.; Marder, S.R. Synthesis of functionalized organic second-order nonlinear optical chromophores for electro-optic applications. J. Org. Chem. 1999, 64, 4289–4297. [Google Scholar] [CrossRef]
- Shah, R.; Verma, P.K. Therapeutic Importance of Synthetic Thiophene. Chem. Cent. J. 2018, 12, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, R.; Tomar, I.; Singhal, S.; Jha, K.K. Synthesis, Properties and Biological Activity of Thiophene: A Review. Der Pharma Chem. 2011, 3, 38–54. [Google Scholar]
- Perepichka, I.F.; Perepichka, D.F. (Eds.) Handbook of Thiophene-Based Materials; John Wiley & Sons, Ltd.: Chichester, UK, 2009. [Google Scholar]
- Martin-Smith, M.; Reid, S.T. Biological Activity in Compounds Possessing Thiophen Rings. J. Med. Chem. 1958, 1, 507–564. [Google Scholar] [CrossRef] [PubMed]
- Bar, S.; Hajra, S. Catalytic enantioselective synthesis of A-86929, a dopamine D1 agonist. Chem. Commun. 2011, 47, 3981–3982. [Google Scholar]
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Mancuso, R.; Gabriele, B. Recent Advances in the Synthesis of Thiophene Derivatives by Cyclization of Functionalized Alkynes. Molecules 2014, 19, 15687–15719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar, S. Regioselective Synthesis of 5-Propyl-2-((trityloxy) methyl) thiophene-3-carbaldehyde. Molbank 2021, 2021, M1289. [Google Scholar] [CrossRef]
- Fuller, L.S.; Iddon, B.; Smith, K.A. Thienothiophenes. Part 2.1 Synthesis, metallation and bromine→lithium exchange reactions of thieno[3,2-b]thiophene and its polybromo derivatives. J. Chem. Soc. Perkin Trans. 1997, 1, 3465–3470. [Google Scholar] [CrossRef]
- Shilai, M.; Kondo, Y.; Sakamoto, T. Selective metallation of thiophene and thiazole rings with magnesium amide base. J. Chem. Soc. Perkin Trans. 2001, 1, 442–444. [Google Scholar] [CrossRef]
- Okamoto, T.; Kudoh, K.; Wakamiya, A.; Yamaguchi, S. General Synthesis of Extended Fused Oligothiophenes Consisting of an Even Number of Thiophene Rings. Chem. Eur. J. 2007, 13, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Schipper, D.J.; Fagnou, K. Direct Arylation as a Synthetic Tool for the Synthesis of Thiophene-Based Organic Electronic Materials. Chem. Mater. 2011, 23, 1594–1600. [Google Scholar] [CrossRef]
- Tamon, O.; Nobuyuki, H.; Jitsuo, K. Formylation of aryl halides with carbon monoxide and sodium formate in the presence of palladium catalyst. Bull. Chem. Soc. Jpn. 1994, 67, 2329–2332. [Google Scholar]
- Sergeev, A.G.; Neumann, H.; Spannenberg, A.; Beller, M. Synthesis and Catalytic Applications of Stable Palladium Dioxygen Complexes. Organometallics 2010, 29, 3368–3373. [Google Scholar] [CrossRef]
- Ohta, A.; Akita, Y.; Ohkuwa, T.; Chiba, M.; Fukunaga, R.; Miyafuji, A.; Nakata, T.; Tani, N.; Aoyagi, Y. Palladium-catalyze Arylation of Furan, Thiophene, Benzo[b]furan and Benzo[b]thiophen. Heterocycles 1990, 31, 1951–1958. [Google Scholar] [CrossRef]
- Zheng, C.; Pu, S.; Xu, J.; Luo, M.; Huang, D.; Shen, L. Synthesis and the effect of alkyl chain length on optoelectronic properties of diarylethene derivatives. Tetrahedron 2007, 63, 5437–5449. [Google Scholar] [CrossRef]
- Khan, S.R.; Luu, H.L. Liquid Crystal Compositions Containing a Five-Membered Heterocyclic Ring, Polymer-Dispersed Liquid Crystal Compositions, and Reverse-Mode Polymer-Dispersed Liquid Crystal Elements and Associated Selectively Dimmable Devices. PCT/US2019/014992, 24 January 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bar, S.; Martin, M.I. Regioselective Synthesis of 4-Bromo-3-formyl-N-phenyl-5-propylthiophene-2-carboxamide. Molbank 2021, 2021, M1296. https://doi.org/10.3390/M1296
Bar S, Martin MI. Regioselective Synthesis of 4-Bromo-3-formyl-N-phenyl-5-propylthiophene-2-carboxamide. Molbank. 2021; 2021(4):M1296. https://doi.org/10.3390/M1296
Chicago/Turabian StyleBar, Sukanta, and Maxwell Israel Martin. 2021. "Regioselective Synthesis of 4-Bromo-3-formyl-N-phenyl-5-propylthiophene-2-carboxamide" Molbank 2021, no. 4: M1296. https://doi.org/10.3390/M1296
APA StyleBar, S., & Martin, M. I. (2021). Regioselective Synthesis of 4-Bromo-3-formyl-N-phenyl-5-propylthiophene-2-carboxamide. Molbank, 2021(4), M1296. https://doi.org/10.3390/M1296