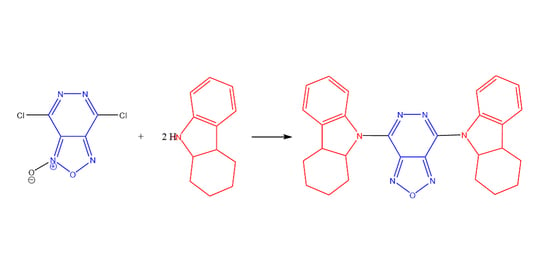
4,7-Bis(1,2,3,4,4a,9a-Hexahydro-9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Neto, B.A.D.; Lapis, A.A.M.; da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. Eur. J. Org. Chem. 2013, 2013, 228–255. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. 4,7-Dibromo-substituted 2,1,3-benzothia (selena,oxa)diazoles and [1,2,5]thia(selena)diazolo[3,4-c]pyridines as building blocks in solar cells components. Chem. Heterocycl. Comp. 2017, 53, 855–857. [Google Scholar] [CrossRef]
- Rakitin, O.A. Fused 1,2,5-thia- and 1,2,5-selenadiazoles: Synthesis and application in materials chemistry. Tetrahedron Lett. 2020, 61, 152230. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Mikhalchenko, L.V.; Golovanov, I.S.; Amelichev, S.A.; Rakitin, O.A. Synthesis of 4,7-dibromo derivative of ultrahigh electron-deficient [1,2,5]thiadiazolo[3,4-d]pyridazine heterocycle and its cross-coupling reactions. Eur. J. Org. Chem. 2018, 41, 5668–5677. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Lyssenko, K.A.; Popov, V.V.; Rakitin, O.A. Safe synthesis of 4,7-dibromo[1,2,5]thiadiazolo[3,4-d]pyridazine and its SNAr reactions. Molecules 2018, 23, 2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmovzh, T.N.; Knyazeva, E.A.; Tanaka, E.; Popov, V.V.; Mikhalchenko, L.V.; Robertson, N.; Rakitin, O.A. [1,2,5]Thiadiazolo[3,4-d]pyridazine as an internal acceptor in the D-A-π-A organic sensitizers for dye-sensitized solar cells. Molecules 2019, 24, 1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmovzh, T.N.; Rakitin, O.A. 7-Bromo-[1,2,5]selenadiazolo[3,4-d]pyridazin-4(5H)-one. Molbank 2021, 2021, M1229. [Google Scholar] [CrossRef]
- Chmovzh, T.; Knyazeva, E.; Popov, V.; Rakitin, O. 4,7-Dichloro[1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide. Molbank 2018, 2018, M982. [Google Scholar] [CrossRef] [Green Version]
- Nikonov, G.N.; Bobrov, S. 1,2,5-Oxadiazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 5, pp. 315–395. [Google Scholar] [CrossRef]
- Saito, K.; Shibata, Y.; Yamanaka, M.; Akiyama, T. Chiral Phosphoric Acid-Catalyzed Oxidative Kinetic Resolution of Indolines Based on Transfer Hydrogenation to Imines. J. Am. Chem. Soc. 2013, 135, 11740–11743. [Google Scholar] [CrossRef] [PubMed]
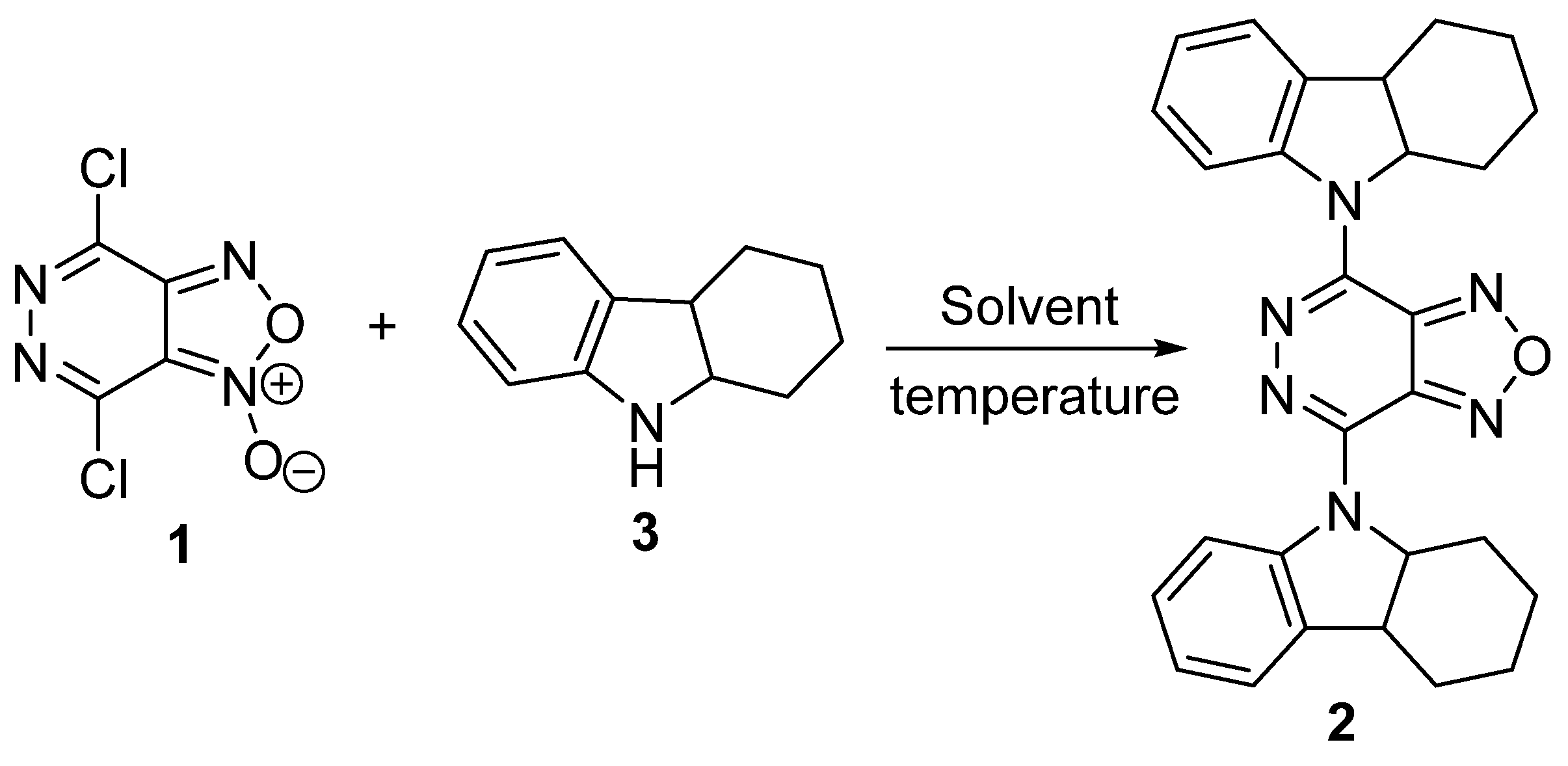
| Entry | Solvent | Temperature, °C | Time, h | Yield, of 2% |
|---|---|---|---|---|
| 1 | CH2Cl2 | 41 | 6 | 15 |
| 2 | MeCN | 81 | 6 | 65 |
| 3 | DMF | 81 | 3 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Gaisin, K.S.; Rakitin, O.A. 4,7-Bis(1,2,3,4,4a,9a-Hexahydro-9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine. Molbank 2021, 2021, M1295. https://doi.org/10.3390/M1295
Chmovzh TN, Gaisin KS, Rakitin OA. 4,7-Bis(1,2,3,4,4a,9a-Hexahydro-9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine. Molbank. 2021; 2021(4):M1295. https://doi.org/10.3390/M1295
Chicago/Turabian StyleChmovzh, Timofey N., Karim S. Gaisin, and Oleg A. Rakitin. 2021. "4,7-Bis(1,2,3,4,4a,9a-Hexahydro-9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine" Molbank 2021, no. 4: M1295. https://doi.org/10.3390/M1295
APA StyleChmovzh, T. N., Gaisin, K. S., & Rakitin, O. A. (2021). 4,7-Bis(1,2,3,4,4a,9a-Hexahydro-9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine. Molbank, 2021(4), M1295. https://doi.org/10.3390/M1295