Abstract
An interaction of water-methanol solution of sodium1,4-benzenediacetate (bda) and 4,4′-bipyridylethelene (bpee) with aqueous solution of Zn(NO3)2·6H2O at room temperature yielded colourless crystals of 1 after three weeks in a sealed glass tube. The compound with composition C22H18ZnN2O4 crystallizes in monoclinic space group P21/c, with the following cell dimensions: a = 10.4566(2), b = 13.3085(2), c = 13.7189(2) Å, β = 101.491(1)°. In the structure of 1, two Zn(II) neighbours are connected by two bda ligands adopting chelating and bidentate-bridging coordination modes to form a dimeric unit (Zn2O8N4) with the Zn–Zn distance of 4.0432(6) Å. The carboxyl-bridged dimeric units are extended along the [001] direction by bpee co-ligands and further linked by bda ligand to form a three-dimensional network structure. The IR shows the characteristic bands of the carboxylates at 1611/1507 cm−1 and 1424/1373 cm−1, respectively, for asymmetric and symmetric stretching −CO2− vibrations. The separation ∆[νasym(CO2−) − νsym(CO2−)] values of 187 and 134 cm−1 are indicative of chelating and bidentate bridging coordination modes of the carboxylate to the metal centre.
1. Introduction
In recent times, metal–organic framework (MOF) materials have received a great deal of attention not only for their unique properties, but also for their potential applications owing to their diversity in topological architectures [1,2,3,4]. Metal–organic frameworks consist of a metal ion linked by organic ligands to form one-, two-, or three-dimensional network structures [5,6]. A number of carboxylate ligands with aromatic backbones are frequently used in the design of metal–organic frameworks with desirable properties. Versatile carboxylate ligands derived from 1,4-benzenedicarboxylic acid, 1,3,5-benzenetricarboxylic acid, 1,2,4,5-benzenetetracarboxylic acid, or pyridine-2,4-dicarboxylic acid are commonly used owing to their abundant carboxylate groups possessing high affinity to metal cations [7,8,9]. Other functionalized ligands such as 1,2-bis(4-pyridyl)ethylene, 1,4-benzenediacetic acid, 4,4′-bipyridylethene, or 3-nitro-1,2-benzenedicarboxlic acid have also been extensively used under diverse synthetic conditions to prepare MOFs [10,11,12,13,14,15,16,17], which are used in several applications [18,19,20,21,22]. The interest in the use of phenylenediacetic acid as ligand in the synthesis of coordination polymers stems from the presence of the flexible −CH2 spacer that enables the −CO2− units to freely bend and rotate to meet the requirements of the coordination geometries of metal ions in the assembly process [23]. Researchers have made used of the flexible nature of phenylenediacetate along with different auxiliary ligands in preparing one-, two-, and three-dimensional structures [24,25,26,27,28]. In particular, Wang and coworkers [24,25] investigated a synthetic system containing various divalent metal acetates, 1,2-phenylenediacetate and 1,3-bis(4-pyridyl)propane co-ligand. Zhang et al. [23] reported on the structural diversities of divalent metal complexes of 1,3-phenylenediacetate with organo-nitrogen co-ligands, in which the structural characteristics of auxiliary ligands vary from chelating to bridging. However, reports on the coordination polymers of 1,4-phenylenediacetate are scant [26,27]. In this study, we were interested in using one long chain linkers 4,4-bipyridylethylene (bpee) with −CH=CH− (ethylenic) functionalities as N-donors and 1,4-benzenediacetic acid (bda) to synthesize a coordination polymer. We have been able to prepare a zinc(II)-coordination polymer, C22H18ZnN2O4, 1, with a three-dimensional structure, incorporating the two ligands, via solvent diffusion technique.
2. Results and Discussion
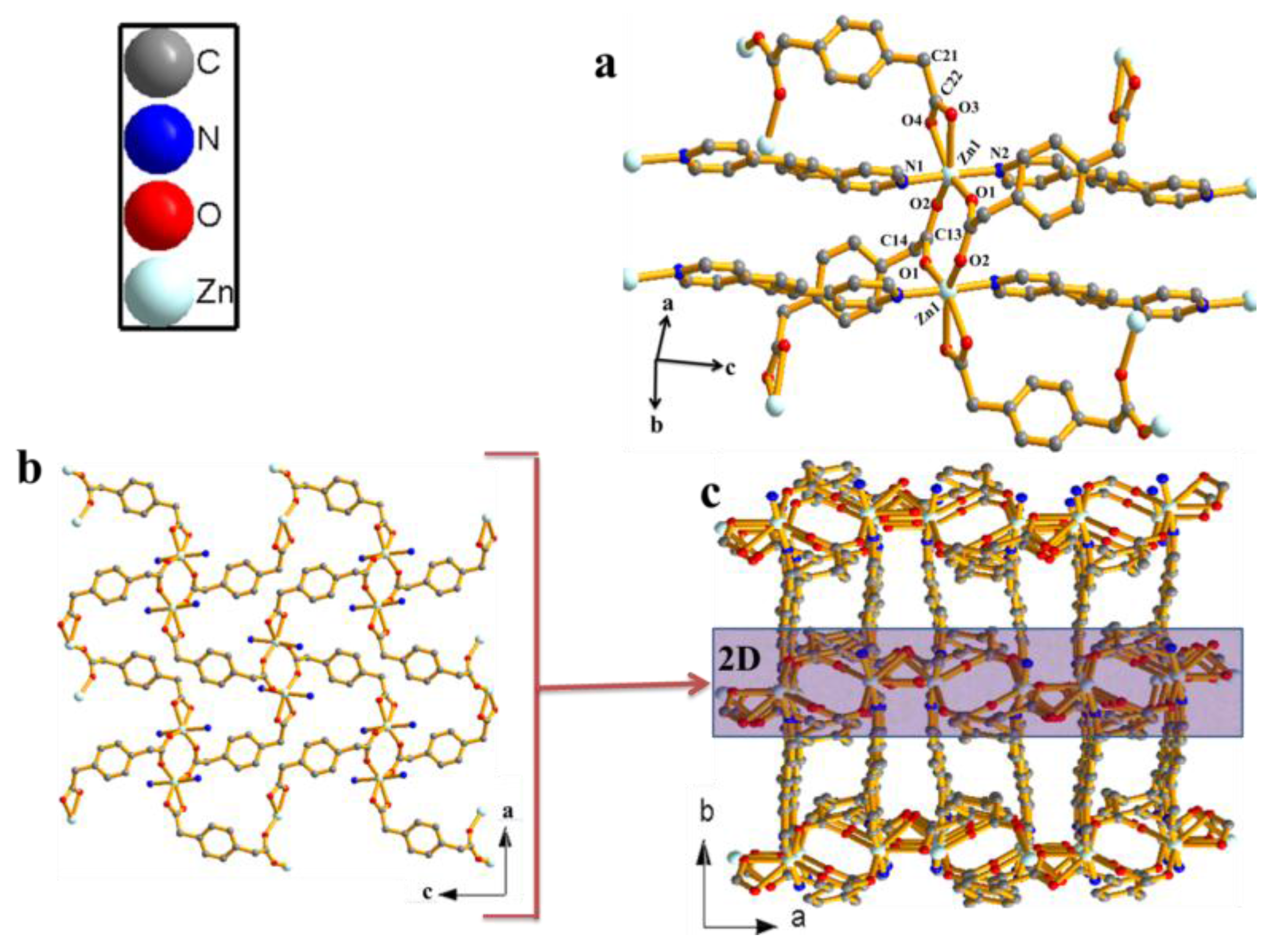
The zinc(II)-coordination polymer was prepared by layering an aquo-methanol solution of the ligand on top of aqueous solution of the metal ion in a glass tube and allowing to stand at room temperature for three weeks. Compound 1 crystallizes in monoclinic space group P21/c (Table S1). The zinc atom is six-coordinated in a distorted octahedral manner (Figure 1a). The bda ligand is tetradentate, with one carboxylate group coordinating to the zinc centre in a bidentate-bridging fashion via O1 and O2, while the other is bidentately chelating via O3 and O4. Two symmetry-related bpee ligands coordinating via N1 and N2 at the apical positions complete the sixth coordination. What is especially noteworthy about this compound is that the two Zn(II) neighbours are connected by two bda ligands through μ2-oxo bridge carboxylic oxygen atoms to form an eight-membered cyclic dimer (Zn2bda2) with the Zn–Zn distance of 4.0432(6) Å. The carboxyl-bridged dimeric units are extended along the [010] direction by bpee co-ligands to produce 1D coordination polymer with Zn-bpee-Zn separation of 13.7189(6) Å. All the Zn–O bond lengths are between 2.020(4) and 2.384(5) Å (Zn–Oav = 2.140(3)) and the Zn–N bond lengths are in the range of 2.172 (3)–2.185(3) Å (Table S2) and are in agreement with similar compounds in the literature [14,15,16,17,21,22,23,24,25,26,27,28]. The polymeric corrugated chains are connected by chelating bda into 2D layered structure as shown in Figure 1b. The second bda ligand connects the layer into a 3D network (Figure 1c). In the isomeric structure reported by Zhang et al. [23], the zinc centre is in four coordination and the 1,3-phenylenediacetate ligand shows transtrans-configuration and adopts bis(monodentate) coordination. Furthermore, in the structural diversity for a series of metal(II) complexes based on flexible 1,2-phenylenediacetate and dipyridyl-type co-ligand reported by Xin et al. [25], the phenylenediacetate anions also adopt a bis(monodentate) coordinated mode that bridges the adjacent Zn(II) atoms to yield an undulating [Zn(phda)]n chain. In the present compound, the metal centre is in six coordination and the 1,4-phenylenediacetate ligand bidentately bridges two Zn atoms and the other bidentately chelates one Zn atom. The difference between these compounds is due to the effect of ligand isomerism. The topology of the present compound is similar to [Co(phda)(dpe)]n characterized as a six-connected self-penetrated network with (48668) rob topology. It is interesting to note that although the synthesis was solvent-mediated, the solvent molecules could not be located in the lattice.
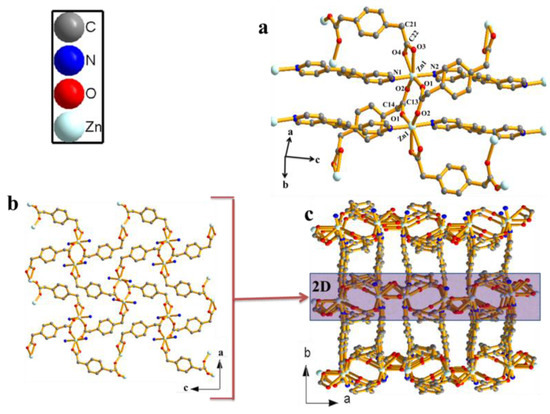
Figure 1.
(a) Zn(II) dimeric unit of compound 1. (b) 2D sheets of compound 1 observed along the b-crystallographic axis). (c) 3D nonporous networks of 1 assembled by connecting 2D layers (hydrogen are omitted for clarity).
The infrared spectrum of the as-synthesized compound (Figure S2) shows the characteristic bands of the carboxylates at 1611/1507 cm−1 and 1424/1373 cm−1, respectively, for asymmetric and symmetric stretching −CO2− vibrations. The separation ∆[νasym(CO2−) − νsym(CO2−)] values of 187 and 134 cm−1 are indicative of chelating and bidentate bridging coordination modes of the carboxylate to the metal centre [29]. The in-plane C–H bending vibrations of the aromatic ring are observed at 1197 –1062 cm−1, while the out-of-plane C–H bending vibrations appeared in the region 959–721 cm−1. Bands in the region 637–451 cm−1 are due to Zn–O vibrations.
3. Materials and Methods
3.1. Materials
The materials were obtained commercially and used without further purification. Zn(NO3)2·6H2O, 4,4′-bipyridylethelene, 1,4-benzenediacetic acid, distilled water, ethanol, methanol, and acetone were purchased from the Aldrich Chemical Co. (St. Louis, MO, USA).
3.2. Methods
3.2.1. Synthesis of C22H18ZnN2O4, 1
Zn(NO3)2·6H2O (0.50 mmol, 0.149 g) was dissolved in 50 mL of water with constant stirring. Sodium1,4-benzenediacetate (Na-bda) (0.25 mmol, 0.060 g) and 4,4′-bipyridylethelene (bpee) (0.25 mmol, 0.045 g) were dispersed in 25 mL mixture of water (H2O) and methanol (CH3OH) (7:3), respectively, and sonicated for 15 min to make a homogeneous solution. Water/methanol mixture of the ligands (Na-bda and bpee) (2 mL) was slowly and carefully layered on top of the aqueous metal ion [Zn(NO3)2·6H2O] solution (2 mL) using the 2 mL buffer solution of water and methanol (7:3) in a crystal tube, which was sealed and left uninterrupted at room temperature. Colourless block type single crystals were grown at the junction of the two different solvents after three weeks. The crystals, which were separated and washed with water and methanol, were found to be air-stable and insoluble in a wide spectrum of solvent. A bulk amount of the compound was synthesized by direct mixing of the corresponding ligands (sodium 1,4-benzenediacetate and 4,4′-bipyridylethelene) solution with a water-methanol solution of Zn(NO3)2·6H2O under stirring for 24 h. Yield: 82%. The PXRD pattern of 1 compared with the simulated pattern from single crystal analyses is presented in Figure S3. Anal. Calcd. For C22H18N2O4Zn: C, 60.08; H, 4.13; N, 6.37. Found: C, 60.21; H, 4.08; N, 6.41%.
3.2.2. Physical Measurements
The elemental analysis of the compound was carried out on a Thermo Fisher Flash 2000 Elemental Analyzer (ThermoFisher Scientific Warrington, UK). Fourier transformed IR spectroscopic investigation was carried out using a KBr pellet on Bruker IFS-66v (Bruker, Ettlingen, Germany).
3.2.3. Single-Crystal X-ray Diffraction.
A good single crystal of compound 1 was mounted on a thin glass fibber with commercially available super glue. X-ray single-crystal structural data were collected on a Bruker Smart-CCD diffractometer (Bruker AXS, Gmbh, Germany) equipped with a normal focus and a 2.4 kW sealed tube X-ray source with graphite monochromated Mo-Kα radiation (λ = 0.71073 Å) operating at 50 kV and 30 mA. The program SAINT [30] was used for the integration of diffraction profiles, and absorption correction was made with the SADABS [31] program. The structure was solved by SIR 92 [32] and refined by the full-matrix least-squares method using SHELXL [33]. All the hydrogen atoms were fixed by HFIX and placed in ideal positions. The stilbene-type C=C atoms were positionally disordered. The potential solvent accessible area or void space was calculated using the PLATON [34] multipurpose crystallographic software. All crystallographic and structure refinement data of compound 1 is summarized in Table S1. Selected bond lengths and angles for compounds 1 are given in Tables S2, supporting information. All calculations were carried out using SHELXL 97, PLATON, SHELXS 97, and WinGX system, Ver 1.80.05 [35]. Crystal Data for C22H18ZnN2O4 (M = 439.77 g/mol): monoclinic, space group P21/c (no. 14), a = 10.4566(2) Å, b = 13.3085(2) Å, c = 13.7189(2) Å, β = 101.491(1)°, V = 1870.88(5) Å3, Z = 4, T = 293(2) K, μ(Mo Kα) = 1.346 mm−1, Dcalc = 1.561 g/cm3, 27316 reflections measured (3.6° ≤ 2θ ≤ 25.9°), 3791 unique (Rint = 0.060) which were used in all calculations. The final R1 was 0.0400 (I > 2σ(I)) and wR2 was 0.1557 (all data).
4. Conclusions
We have successfully constructed a Zn(II) coordination polymer under solvent diffusion condition by employing mixed linkers system of 4,4′-bipyridylethene and 1,4-benzenediacetic acid. The compound [Zn(bpee)(bda))]n, 1, has a 3D network. We are currently investigating these mixed linkers system with first row transition elements with a view to unravel their magnetic properties; the results will be published elsewhere.
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-8599/2017/3/M952, Figure S1: Asymmetric unit of 1, Figure S2: Infrared spectrum of 1, Figure S3: Simulated and experimental XRD powder patterns for compound 1, Table S1: Crystallographic data and structural refinement parameters for compound 1, Table S2: Selected bond distances and bond angles in 1. “CCDC 1566264” contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html.
Supplementary File 1Acknowledgments
Stephen Adie Adalikwu is thankful to TWAS for the award of Research and Advanced Training Fellowship and C.N.R. Rao for giving me the opportunity to carry out research in ICMS, JNCASR-India. We are grateful to T.K. Maji and Arpan Hazra of JNCASR, Bangalore, for solving single crystal structure.
Author Contributions
S.A.A. and O.E.O. conceived and designed the experiments; S.A.A. performed the experiments; S.A.A. and A.A.A. analyzed the data; A.A.A. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1213. [Google Scholar] [CrossRef] [PubMed]
- Kinnibrugh, T.L.; Ayi, A.A.; Bakhmutov, V.I.; Zoń, J.; Clearfield, A. Probing structural changes in a phosphonate-based metal–organic framework exhibiting reversible dehydration A. Cryst. Growth Des. 2013, 13, 2973–2981. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-like metal–organic frameworks (ZMOFs): Design, synthesis, and properties. Chem. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Zhao, L.; Wang, X.-L.; Shao, K.-Z.; Su, Z.-M. Luminescent metal–organic frameworks with anthracene chromophores: Small-molecule sensing and highly selective sensing for nitro explosives. Cryst. Growth Des. 2016, 16, 4374–4382. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fanga, Q.-R.; Li, J.-R.; Makala, T.A.; Younga, M.D.; Yuana, D.; Zhaoa, D.; Zhuanga, W.; Zhoua, H.-C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O- and N-donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Li, X.; Cao, R.; Sun, D.; Yuan, D.; Bi, W.; Li, X.; Wang, Y. A three-dimensional zinc(II) complex consisting of single metal centers and pentanuclear clusters bridged by 1,3,5-benzenetricarboxylate. J. Mol. Struct. 2004, 694, 205–210. [Google Scholar] [CrossRef]
- Shi, Z.; Li, G.; Wang, L.; Gao, L.; Chen, X.; Hua, J.; Feng, S. Two Three-dimensional metal−organic frameworks from secondary building units of Zn8(OH)4(O2C−)12 and Zn2((OH)(O2C−)3: [Zn2(OH)(btc)]2(4,4‘-bipy) and Zn2(OH)(btc)(pipe). Cryst. Growth Des. 2004, 4, 25–27. [Google Scholar] [CrossRef]
- Inah, B.E.; Ayi, A.A.; Adhikary, A. Crystal structure of a chloride-bridged copper(II) dimer: Piperazine-1,4-dium bis(di-μ-chloridobis[(4-carboxypyridine-2-carboxylato-k2N,O2)chloridocuprate(II)]. Acta Cryst. 2017, E73, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, C.M.; Haldar, R.; Maji, T.K.; Rao, C.N.R. Chiral porous metal-organic frameworks of Co(II) and Ni(II): Synthesis, structure, magnetic properties, and CO2 uptake. Cryst. Growth Des. 2012, 12, 975–981. [Google Scholar] [CrossRef]
- De Lill, D.T.; Cahill, C.L. Synthesis and characterization of a praseodymium-adipate framework templated with 1,2-bis(4-Pyridyl)ethene: Host-guest interactions and structural survey. Cryst. Growth Des. 2007, 7, 2390–2393. [Google Scholar] [CrossRef]
- Kongshaug, K.O.; Fjellvag, H. Design of novel bilayer compounds of the CPO-8 type containing 1D channels. Inorg. Chem. 2006, 45, 2424–2429. [Google Scholar] [CrossRef] [PubMed]
- Madhu, V.; Das, S.K. Neutral coordination polymers based on a metal–mono(dithiolene) complex: Synthesis, crystal structure and supramolecular chemistry of [Zn(dmit)( 4,4′-bpy)]n, [Zn(dmit)( 4,4′-bpe)]n and [Zn(dmit)(bix)]n (4,4′-bpy = 4,4′-bipyridine, 4,4′-bpe = trans-1,2-bis(4-pyridyl)ethene, bix = 1,4-bis(imidazole -1-ylmethyl)-benzene. Dalton Trans. 2011, 40, 12901–12908. [Google Scholar] [PubMed]
- Wu, Y.; Zhao, Y.; Yang, G.-P.; Guo, Y.; Wang, Y.-Y.; Shi, Q.-Z. Influence of the steric effect of flexible isomeric phenylenediacetic acids on the resultant lead(II) coordination polymers. J. Solid State Chem. 2015, 223, 131–137. [Google Scholar]
- Yang, G.-P.; Wang, Y.-Y.; Zhang, W.-H.; Fu, A.-Y.; Liu, R.-T.; Lermontova, E.K.; Shi, Q.-Z. A series of Zn(II) coordination complexes derived from isomeric phenylenediacetic acid and dipyridyl ligands: Syntheses, crystal structures, and characterizations. CrystEngComm 2010, 12, 1509–1517. [Google Scholar] [CrossRef]
- Yin, W.-D.; Li, G.-L. Crystal structure of poly[aqua(1,2-bis(4-pyridyl) ethane-k2 N:N′)(4-nitro1,2-benzenedicarboxylate-k3O,O′:O′′)cobalt(II)], C20H17CoN3O7. Z. Krist. New Cryst. Struct. 2015, 230, 11–12. [Google Scholar]
- Haldar, R.; Maji, T.K. Metal–organic frameworks (MOFs) based on mixed linker systems: Structural diversities towards functional materials. CrystEngComm 2013, 15, 9276–9295. [Google Scholar] [CrossRef]
- Han, M.-L.; Liu, J.; Wang, S.-C.; Liu, G.-Z.; Du, D.-G. Two New Cu(II) Coordination polymers based on 1,2-bi(4-pyridyl)ethene and 4-R-phthalic acid (R = −C(CH3)3 or −CH3): Syntheses, structures, and characteristics. Synth. React. Inorg. Met.-Org. Chem. 2016, 48, 338–342. [Google Scholar] [CrossRef]
- Leng, F.; Wang, W.; Zhao, X.J.; Hu, X.L.; Li, Y.F. Adsorption interaction between a metal-organic framework of chromium-benzenedicarboxylates and uranine in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 164–169. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Huang, Y.-S.; Zhao, Y.-Y.; Jia, Z.-B. Molecular tectonics of entangled metal-organic frameworks based on different conformational carboxylates mixed with a flexible N,N′-type ligand. Cryst. Growth Des. 2011, 11, 569–574. [Google Scholar] [CrossRef]
- Liu, X.-M.; Xie, L.-H.; Lin, J.-B.; Lin, R.-B.; Zhang, J.-P.; Chen, X.-M. Flexible porous coordination polymers constructed from 1,2-bis(4-pyridyl)hydrazine via solvothermal in situ reduction of 4,4′-azopyridine. Dalton Trans. 2011, 40, 8549–8554. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-F.; Li, B.; Sun, X.-Y.; Wang, L.-Y.; Fan, Y.-T. Hydrothermal syntheses and characterizations of three ZnII coordination polymers tuned by pH value and base. Z. Anorg. Allg. Chem. 2010, 636, 1606–1611. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Li, D.-S.; Wang, J.-J.; Fu, F.; Du, M.; Zoua, K.; Gaob, X.-M. Structural diversity and properties of ZnII and CdII complexes with a flexible dicarboxylate building block 1,3-phenylenediacetate and various heterocyclic co-ligands. Dalton Trans. 2009, 5355–5364. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.Y.; Liu, G.Z.; Wang, L.Y. New coordination polymers from 1D chain, 2D layer to 3D framework constructed from 1,2-phenylenediacetic acid and 1,3-bis(4-pyridyly)propane flexible ligands. J. Solid State Chem. 2011, 184, 1387–1392. [Google Scholar] [CrossRef]
- Xin, L.-Y.; Liu, G.-Z.; Li, X.-L.; Wang, L.-Y. Structural diversity for a series of metal(II) complexes based on flexible 1,2-phenylenediacetate and dipyridyl-type Co-ligand. Cryst. Growth Des. 2012, 12, 147–157. [Google Scholar] [CrossRef]
- Zhang, M.; Ren, Y.; Ma, Z.; Qiao, L. Synthesis, crystal structure, and luminescent properties of two coordination polymers based on 1,4-phenylenediacetic acid. J. Mol. Struct. 2017, 1137, 606–609. [Google Scholar] [CrossRef]
- Wang, L.-H.; Lei, L.; Wang, X. Synthesis, structural characterization and catalytic activity of A Cu(II) coordination polymer constructed from 1,4-phenylenediacetic acid and 2,2’-bipyridine. Bull. Chem. React. Eng. Catal. 2017, 12, 113–118. [Google Scholar]
- Paraschiv, C.; Cucos, A.; Shova, S.; Madalan, A.M.; Maxim, C.; Visinescu, D.; Cojocaru, B.; Parvulescu, V.I.; Andruh, M. New Zn(II) coordination polymers constructed from amino-alcohols and aromatic dicarboxylic acids: Synthesis, structure, photocatalytic properties, and solid-state conversion to ZnO. Cryst. Growth Des. 2015, 15, 799–811. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Madison, W.I. SMART (V 5.628), SAINT (V 6.45a), XPREP, and SHELXTL; Bruker AXS Inc.: Fitchburg, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. SADABS: Siemens Area Detector and Empirical Absorption Correction Program; University of Gottingen: Gottingen, Germany, 1994. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Gualaradi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97: Program for the Solution of Crystal Structure; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX-A windows program for crystal structure analysis. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).