Abstract
This note describes a sequence converting an oxime-substituted pyrrolidine into a trisubstituted pyrrole structure. The synthetic route is based on a double chlorination of the pyrrolidine substrate followed by the base induced formation of both an imine and a nitrile oxide functionality. The latter reacts with an immobilized thiourea to yield an isothiocyanate which upon elimination generates the final pyrrole in an unprecedented cascade of events.
1. Introduction
Pyrroles are amongst the most prevalent electron-rich heteroaromatic architectures and can be found in the structures of numerous materials, drugs, and natural products [1,2,3]. Consequently, many synthetic routes are described for their assembly [4]. Most commonly, these heterocycles arise from acyclic precursors via cyclocondensation or cycloaddition approaches. It is therefore desirable to develop new routes towards highly substituted pyrroles that harness alternative synthetic strategies, especially if these explore unprecedented transformations or reaction cascades [5].
2. Results
In a recent research project [6], we reported on a flow-based transformation of chloro-oximes (2) to isothiocyanates (6) via the intermediacy of a nitrile oxide dipole (3) that underwent a [3 + 2]-cycloaddition with a thiourea species (4). As indicated in Scheme 1, a skeletal rearrangement of the initial cycloadduct 5 subsequently rendered the desired isothiocyanate product 6.
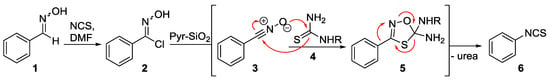
Scheme 1.
Previous synthesis of isothiocyanates via a dipolar cycloaddition approach.
During this study, we also subjected some more elaborate substrates, such as substituted pyrrolidines [7], to this sequence and were pleased to obtain the expected isothiocyanate. However, we also observed the simultaneous generation of a cyclic imine functionality, which indicated that chlorination had not only occurred on the oxime to yield a chloro-oxime, but also on the pyrrolidine nitrogen (7, Scheme 2). Upon treatment of this dichlorinated material (7) with silica-supported pyridine (Pyr-SiO2, 2.5 equiv.) both the highly reactive nitrile oxide and the more stable oxime were generated, subsequently leading to the corresponding isothiocyanate product 9. Importantly, this procedure worked equally well for diastereopure samples, as well as mixtures of diastereomers at the quaternary carbon.
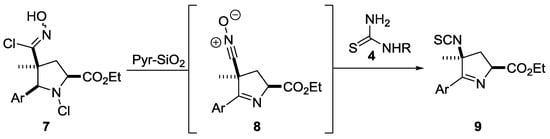
Scheme 2.
Concomitant imine formation for pyrrolidine substrates.
In a more recent extension to this work, we investigated an increase in the stoichiometric excess of base which unexpectedly led to a different product outcome. When a solution of 7 (Ar = 4-bromophenyl, 2:1 mixture of diastereomers) was heated at 60 °C for 6 h in the presence of 3.5 equivalents of base complete conversion to the new species was observed. The new product was isolated and 1H-NMR analysis revealed the disappearance of the diagnostic diastereotopic methylene protons, whilst a new resonance appeared at 6.8 ppm. Furthermore, a broad singlet was found at 9.0 ppm that was assigned as a NH proton. Interestingly, subjecting product 9 instead of 7 (Ar = 4-bromophenyl) to the analogous reaction with 1.5 equivalents of base (either Pyr-SiO2 or NEt3) gave rise to the same product as the sole isolated species. Upon purification and recrystallization of this product, material crystals suitable for single crystal X-ray diffraction experiments were obtained. The crystal structure revealed that the pyrrolidine ring had undergone aromatization to the related pyrrole heterocycle (Figure 1). The X-ray shows that the pyrrole ring is twisted out of plane with respect to the arene ring by 27° due to the steric hindrance of the methyl group.
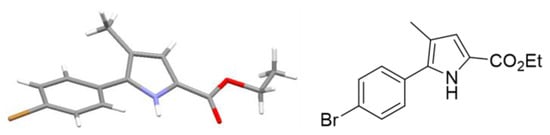
Figure 1.
Crystal structure of pyrrole 10 (CCDC 1562557) [8].
3. Discussion
To account for this process, we propose a mechanism consistent with the above-mentioned formation of imine intermediate 9, which thus requires 2 equivalents of base to remove the HCl formed. A further equivalent of base is then required to eliminate thiocyanic acid (HSCN) to furnish pyrrole product 10 (Scheme 3).
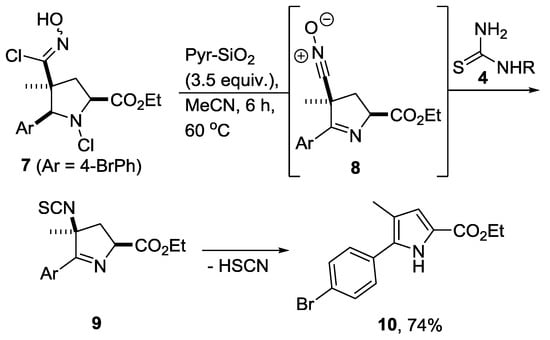
Scheme 3.
Proposed mechanism for the formation of pyrrole 10.
4. Materials and Methods
A solution of the dichloride 7 (Ar = 4-BrPh) was freshly prepared by adding N-chlorosuccinimide (NCS, 1.1 mmol, 147 mg, 2.2 equiv.) to a solution of the corresponding oxime (1.0 mmol, 175 mg, d.r. 2:1) in DMF (2 mL). After stirring this mixture for 4 h at ambient temperature, diethyl ether (10 mL) and water (10 mL) were added and the mixture was extracted into diethyl ether. The organic layer was dried over anhydrous sodium sulfate, filtered, and evaporated to dryness to yield the crude dichloride intermediate as a colorless oil. This material was redissolved in MeCN (6 mL) and combined with silica-supported pyridine (2.45 g, 3.5 equiv., [9]) and an immobilized thiourea (QP-SA, 500 mg, 2.0 mmol, 4.0 equiv., [10]). After heating the resulting mixture at 60 °C for 6 h, complete conversion of substrate was observed by TLC. Filtration and evaporation yielded a yellow oil that was further purified by silica column chromatography (10–20% EtOAc/hexanes) to furnish pyrrole 10 as a colorless solid, which was recrystallized from DCM/toluene giving the desired product as colorless crystals (74%, 114 mg). Please see SI for further information.
1H-NMR (600 MHz, CDCl3) δ/ppm 9.03 (1H, br s, NH), 7.96 (2H, d, J = 8.0 Hz), 7.35 (2H, d, J = 8.0 Hz), 6.80 (1H, d, J = 2.6 Hz), 4.32 (2H, q, J = 7.2 Hz), 2.23 (3H, s), 1.35 (3H, t, J = 7.2 Hz). 13C-NMR (150 MHz, CDCl3) δ/ppm 161.1 (C), 132.2 (C), 132.0 (2CH), 131.2 (C), 128.4 (2CH), 121.9 (C), 121.4 (C), 118.7 (C), 118.0 (CH), 60.4 (CH2), 14.5 (CH3), 12.4 (CH3). IR (neat, cm−1) ν 3305 (m), 2976 (w), 1685 (s), 1463 (m), 1281 (s), 1217 (s), 1184 (s), 1073 (m), 1032 (m), 1007 (m), 830 (m), 764 (m). HRMS (TOF MS AP+) calculated for C14H1479BrNO2 307.0208, found 307.0191 (Δ 1.7 mDa). X-ray data: CCDC 1562557; space group P-1; a = 4.8001(3) Å, b = 11.6381(6) Å, c = 11.6810(6) Å; α = 86.908(2)°, β = 86.919(2)°, γ = 89.587(2)°.
5. Conclusions
In conclusion, we describe an efficient reaction sequence converting an oxime bearing pyrrolidine into its pyrrole counterpart. This is based on dichlorination of the oxime substrate followed by treatment with excess base and an immobilized thiourea species, triggering a series of events including formation of a cyclic imine, rearrangement of the oxime into an isothiocyanate, and its subsequent elimination to render the final pyrrole product after tautomerization. The identity and connectivity of this structure was unambiguously established by single crystal X-ray diffraction experiments.
Supplementary Materials
The following are available online at www.mdpi.com/1422-8599/2017/3/M951: Copies of NMR spectra of 10.
Supplementary File 1Acknowledgments
We are grateful for support by the Royal Society (UF130576; to MB and IRB) and thank Dmitrii Yufit (Department of Chemistry, University of Durham) for solving the crystal structure of 10.
Author Contributions
M.B. conducted the research and both M.B. and I.R.B. wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References and Notes
- Rasmussen, S.C.; Evenson, S.J. Dithieno [3,2-b:2′,3′-d]pyrrole-based materials: Synthesis and application to organic electronics. Prog. Polym. Sci. 2013, 38, 1773–1804. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R.; Ley, S.V.; Nikbin, N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem. 2011, 7, 442–495. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; Thornton, P.D.; Thompson, A. Synthesis of natural products containing the pyrrolic ring. Nat. Prod. Rep. 2010, 27, 1801–1839. [Google Scholar] [CrossRef] [PubMed]
- Estevez, V.; Villacampa, M.; Menendez, J.C. Multicomponent reactions for the synthesis of pyrroles. Chem. Soc. Rev. 2010, 39, 4402–4421. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-B.; Selander, N. Divergent iron-catalyzed coupling of o-acyloximes with silyl enol ethers. Chem. Eur. J. 2017, 23, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Baxendale, I.R. The rapid generation of isothiocyanates in flow. Beilstein J. Org. Chem. 2013, 9, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- These pyrrolidines are conveniently prepared by dipolar cycloaddition reactions between azomethine ylides and methyl methacrylate, giving the endo-diastereomer in preference over the exo-diastereomer. See reference [5] for further details.
- The X-ray structure of pyrrole 10 has been deposited with the Cambridge Crystallographic Data Centre as CCDC 1562557.
- Silica-supported pyridine (Pyr-SiO2, 40–63 μm, 1.39 mmol/g) is commercially available from Silicycle.
- QuadraPure™ Thiourea resin (QP-SA, loading 4.0–5.5 mmol/g) is commercially available from Johnson-Matthey.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).