Abstract
The synthesis of 1H-benzo[f]isoindole derivatives was achieved by the cascade radical cyclization–cyclization reaction of the active methine substrate having an allyl group and phenyl group as different two radical acceptors. This oxidative transformation proceeded by using iron(III) chloride FeCl3 as a mild oxidant via the intramolecular radical addition to the allyl group followed by the second radical addition to the phenyl group.
1. Introduction
Hexahydro-1H-benzo[f]isoindol-1-one and dihydro-1H-benzo[f]isoindol-1-one are the core structures in some natural products and the biologically active agents (Figure 1) [1,2,3,4,5,6]. Therefore, we felt attracted to the possibility of a new synthetic method based on the oxidative radical cyclization leading to γ-lactams, which was recently developed by our group [7].
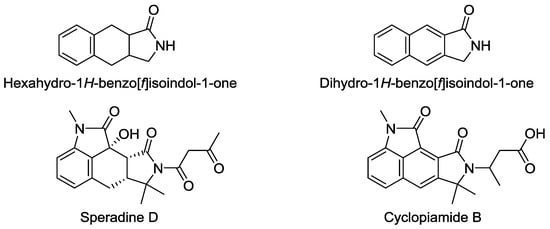
Figure 1.
1H-Benzo[f]isoindole derivatives.
The oxidative radical reactions have made great advances in synthetic chemistry mainly by using manganese (III) acetate Mn(OAc)3 and cerium (IV) ammonium nitrate (CAN) [8,9]. However, less is known about the cascade oxidative radical cyclization–cyclization reactions of the active methylenes or methines having two radical acceptors [10,11,12,13,14,15]. Furthermore, these reported transformations are dependent on a toxic strong oxidant such as Mn(OAc)3; thus, the replacement of heavy metal oxidant into the less toxic and mild electron transfer reagents is also a challenging task. We are interested in the cascade radical cyclization–cyclization reaction as a new strategy for constructing the 1H-benzo[f]isoindole structure. In this paper, we report the synthesis of 1H-benzo[f]isoindole derivatives trans-4 and cis-4 by the cascade transformation using iron(III) chloride FeCl3 as a mild and environmentally benign oxidant [16,17,18,19,20].
2. Results
At first, we prepared two substrates 3a [21] and 3b having an allyl group and a phenyl group as two different radical acceptors (Scheme 1). In the presence of N,N-dimethylaminopyridine (DMAP), treatment of ethyl benzoylacetate 1 with diallylamine 2 in toluene under reflux conditions afforded the desired methylene substrate 3a in almost quantitative yield. Another methine substrate 3b was obtained in 53% yield by α-methylation of substrate 3a using NaH and MeI.

Scheme 1.
Preparation of substrates 3a and 3b.
For the oxidative radical reactions, 2.0 equivalents of FeCl3 were initially employed as a mild oxidant in CH2ClCH2Cl under reflux conditions (Scheme 2). Although treatment of active methylene 3a with FeCl3 did not afford the desired product effectively, the FeCl3-promoted cascade radical cyclization–cyclization reaction of α-methylated methine 3b took place to afford the 1H-benzo[f]isoindole derivatives trans-4 and cis-4 in 47% combined yield and 36:11 trans/cis-selectivity, accompanied with a small amount of the mono-cyclized product trans-5 (19% yield). In marked contrast to FeCl3-promoted transformation, the use of FeBr3 did not lead to the formation of 1H-benzo[f]isoindole derivatives. The mono-cyclized product trans-6 was only obtained in 60% yield with high trans-selectivity. The excellent trans-selectivity of the mono-cyclized products trans-5 and trans-6 would be attributable to the steric effect in an initial radical intermediate. The relative stereochemistry of trans-4 and cis-4 was determined by NOESY experiments [22] and the stereochemistry of trans-5 and trans-6 was assumed on the basis of NOESY experiments and similarly based on our relative reaction [7].

Scheme 2.
Oxidative radical cyclization of 3b.
3. Discussion
In order to understand this transformation, the thermodynamic data were obtained by density functional calculation (Figure 2) [22]. These data indicate that the conversion of methine substrate 3b into trans-4 and H2 is exothermic (∆H = −17 kJ/mol at 298.15 K). Totally, the change in Gibbs energy suggests that the transformation of 3b into trans-4 is favorable to proceed in view of thermodynamics (∆G < 0 kJ/mol at 298.15 K). Furthermore, the calculation data show that cis-4 is more stable than trans-4.

Figure 2.
Calculation study.
This FeCl3-promoted transformation starts from the single electron-transfer (SET) in the enolate-Fe(III) complex A to afford Radical B (Figure 3). The product trans-4 would be formed via the 5-exo-trig cyclization of Radical B, the second cyclization of Radical C onto phenyl group, and the subsequent oxidation of Radical D with FeCl3 affording the cation intermediate E. It should be noted that the trans/cis-selectivity of 4 is determined by the stereoselectivity of the first cyclization of Radical B. We presume that the reversibility in cyclization of the resonance-stabilized Radical B affording the unstable primary Radical C leads to the erosion of trans/cis-selectivity, because 1H-benzo[f]isoindole derivative trans-4 is unstable as compared with cis-4 (Figure 2).
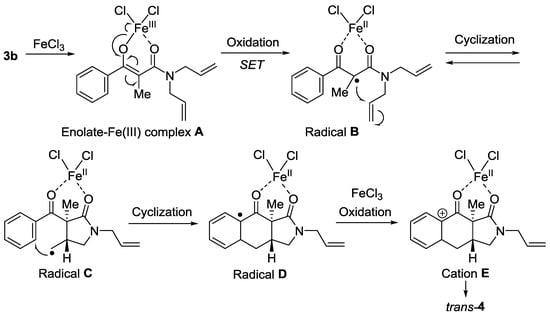
Figure 3.
Possible reaction pathway.
In the case of FeBr3-promoted transformation, the reversibility in cyclization of Radical B would be suppressed by the rapid trapping of Radical B with FeBr3 leading to the predominant formation of the mono-cyclized product trans-6. We think that high trans-selectivity in FeBr3-promoted mono-cyclization supports this hypothesis. Moreover, this result indicates that the second cyclization is not a Friedel–Crafts reaction. Consequently, the use of FeCl3 as an oxidant is essential for the second radical cyclization of Radical B.
In conclusion, we have developed the FeCl3-promoted oxidative radical cyclization–cyclization reaction for constructing the 1H-benzo[f]isoindole structure.
4. Experimental Section
4.1. General Information
Melting points are uncorrected. 1H-NMR spectra were measured in CDCl3 with TMS as an internal standard (0.00 ppm). 13C-NMR spectra were measured in CDCl3 as an internal standard (77.0 ppm). For silica gel column chromatography, SiliCycle Inc. SiliaFlash F60 was used. Preparative TLC separations were carried out on precoated silica gel plates (E. Merck 60F254, manufacturer of pharmaceutical, Kenilworth NJ, USA). The substrate 3a is the known compound [21].
4.2. N,N-Diallyl-3-oxo-3-phenylpropanamide (3a)
To a solution of diallylamine 2 (2.46 mL, 20 mmol) and N,N-dimethylaminopyridine (1.22 g, 10 mmol) in toluene (20 mL), ethyl benzoylacetate 1 (3.46 mL, 20 mmol) was added under argon atmosphere at room temperature. The stirred reaction mixture was heated at reflux for 15 h. The reaction mixture was diluted with water and then extracted with AcOEt. The organic phase was dried over Na2SO4 and then concentrated at reduced pressure. The purification of the residue by flash silica gel column chromatography (AcOEt/hexanes = 1:10 to 1:4) afforded the product 3a [21] (4.87 g, quant.).
Colorless oil. IR (KBr) 3083, 2982, 1689, 1628, 1576, 1482 cm−1. The presence of keto and enol isomers precluded a comprehensive assignment of all proton and carbon resonances. 1H-NMR (CDCl3) δ 8.01 (6/5H, m), 7.75 (4/5H, m), 7.59 (3/5H, m), 7.50–7.38 (12/5H, m), 5.88–5.74 (10/5H, m), 5.73 (2/5H, s; enol form), 5.25-5.16 (20/5H, m), 4.10 (6/5H, s; keto form), 4.09–3.92 (22/5H, m). 13C-NMR (CDCl3) δ 194.1, 172.3, 171.6, 167.1, 136.3, 134.9, 133.6, 133.1, 132.8, 132.6, 130.7, 128.7 (2C), 128.4, 125.9, 117.4, 117.1, 117.0, 85.1, 49.9, 49.3, 48.0, 45.6. HRMS (ESI+) calcd for C15H17NO2Na (M + Na+): 266.1152, Found: 266.1175.
4.3. N,N-Diallyl-2-methyl-3-oxo-3-phenylpropanamide (3b)
After NaH (60% oil suspension, 181 mg, 4.5 mmol) was washed with hexanes twice under argon atmosphere at room temperature, DMF (10.3 mL) was added. To this stirring suspension, a solution of 3a (1.00 g, 4.1 mmol) in DMF (10.3 mL) was added at 0 °C. After being stirred at the same temperature for 1 h, methyl iodide (0.28 mL, 4.5 mmol) was added to the reaction mixture. The reaction mixture was stirred at room temperature for 15 h. The reaction mixture was diluted with saturated NaHCO3 and then extracted with AcOEt. The organic phase was dried over Na2SO4 and then concentrated at reduced pressure. The purification of the residue by flash silica gel column chromatography (AcOEt/hexanes = 1:2) afforded the product 3b (558 mg, 53%).
Colorless crystals. Mp 51.5–52.5 °C (hexanes). IR (KBr) 2984, 2937, 1695, 1637, 1449, 1414 cm−1. 1H-NMR (CDCl3) δ 7.93 (2H, m), 7.56 (1H, m), 7.45 (2H, m), 5.79–5.66 (2H, m), 5.24-5.09 (4H, m), 4.44 (1H, q, J = 7.1 Hz), 4.15 (1H, dd, J = 15.1, 5.5 Hz), 3.85–3.79 (3H, m), 1.49 (3H, d, J = 7.1 Hz). 13C-NMR (CDCl3) δ 197.0, 170.5, 135.6, 133.3, 132.6, 132.5, 128.7, 128.3, 117.4, 117.3, 49.2, 47.9, 46.7, 14.7. HRMS (ESI+) calcd for C16H20NO2 (M + H+): 258.1489, Found: 258.1488.
4.4. 2-Allyl-9a-methyl-2,3,3a,9a-tetrahydro-1H-benzo[f]isoindole-1,9(4H)-dione (4) and trans-1-Allyl-3-benzoyl-4-(chloromethyl)-3-methylpyrrolidin-2-one (trans-5)
The stirred suspension of FeCl3 (127 mg, 1.0 mmol) and substrate 3b (129 mg, 0.50 mmol) in CH2ClCH2Cl (5.0 mL) was heated at reflux under argon atmosphere for 20 h. The reaction mixture was diluted with saturated NaHCO3 and then extracted with AcOEt. The organic phase was dried over Na2SO4 and then concentrated at reduced pressure. Rough purification of the residue by flash silica gel column chromatography (AcOEt/hexanes = 1:2) afforded the products as a mixture of two isomers. Second purification by preparative TLC (AcOEt/benzene = 1:4) afforded 4 as the isolated trans-4 (46 mg, 36%), cis-4 (14 mg, 11%), and trans-5 (28 mg, 19%).
trans-4: Colorless oil. IR (KBr) 2930, 2872, 1716, 1677, 1600, 1489, 1415 cm−1. 1H-NMR (CDCl3) δ 8.08 (1H, br dd, J = 7.6, 1.4 Hz), 7.47 (1H, br td, J = 7.6, 1.4 Hz), 7.34 (1H, br t, J = 7.6 Hz), 7.25 (1H, br d, J = 7.6 Hz), 5.71 (1H, m), 5.22–5.17 (2H, m), 3.97 (1H, ddt, J = 15.1, 6.4, 1.4 Hz), 3.88 (1H, ddt, J = 15.1, 6.4, 1.4 Hz), 3.36 (1H, dd, J = 9.6, 6.9 Hz), 3.25 (1H, dd, J = 10.6, 9.6 Hz), 3.08 (1H, dd, J = 16.5, 12.4 Hz), 3.01 (1H, dd, J = 16.5, 4.6 Hz), 2.79 (1H, m), 1.28 (3H, s). 13C-NMR (CDCl3) δ 194.3, 172.3, 141.0, 133.1, 132.3, 132.0, 129.0, 128.8, 127.2, 118.3, 50.1, 46.2, 45.1, 39.8, 28.1, 12.9. HRMS (ESI+) calcd for C16H18NO2 [M + H+]: 256.1332, Found: 256.1326.
cis-4: Colorless oil. IR (KBr) 2929, 1704, 1600, 1489, 1451, 1441, 1420 cm−1. 1H-NMR (CDCl3) δ 7.99 (1H, br dd, J = 7.6, 1.4 Hz), 7.52 (1H, br td, J = 7.6, 1.4 Hz), 7.34 (1H, br t, J = 7.6 Hz), 7.24 (1H, br d, J = 7.6 Hz), 5.62 (1H, m), 5.11 (1H, dd, J = 10.1, 1.4 Hz), 5.05 (1H, dd, J = 17.4 , 1.4 Hz), 3.95 (1H, ddt, J = 15.6, 6.0, 1.4 Hz), 3.78 (1H, ddt, J = 15.6, 6.4, 1.4 Hz), 3.37 (1H, dd, J = 9.6, 7.8 Hz), 3.26 (1H, dd, J = 16.5, 5.0 Hz), 2.96 (1H, dd, J = 9.6, 8.5 Hz), 2.88 (1H, dd, J = 16.5, 3.7 Hz), 2.80 (1H, m), 1.54 (3H, s). 13C-NMR (CDCl3) δ 193.4, 171.4, 140.3, 134.1, 132.9, 132.1, 129.1, 128.2, 127.3, 118.2, 55.1, 47.9, 45.7, 38.9, 27.8, 20.5. HRMS (ESI+) calcd for C16H18NO2 [M + H+]: 256.1332, Found: 256.1343.
trans-5: Colorless oil. IR (KBr) 2980, 2933, 1697, 1645, 1490, 1444 cm−1. 1H-NMR (CDCl3) δ 7.86 (2H, br d, J = 7.4 Hz), 7.52 (1H, m), 7.42 (2H, br t, J = 7.6 Hz), 5.78 (1H, m), 5.33–5.22 (2H, m), 4.06 (1H, br dd, J = 14.6, 6.4 Hz), 3.95 (1H, br dd, J = 14.6, 6.4 Hz), 3.70 (1H, dd, J = 10.1, 7.8 Hz), 3.57 (1H, dd, J = 11.0, 6.0 Hz), 3.47 (1H, dd, J = 11.0, 8.7 Hz), 3.35 (1H, m), 3.18 (1H, dd, J = 10.1, 8.3 Hz), 1.45 (3H, s). 13C-NMR (CDCl3) δ 198.6, 173.9, 135.7, 132.4, 131.5, 128.9, 128.5, 119.2, 59.1, 48.4, 45.6, 42.7, 42.4, 14.8. HRMS (ESI+) calcd for C16H1835ClNO2Na [M + Na+]: 314.0918, Found: 314.0924; calcd for C16H1837ClNO2Na (M + 2 + Na+): 316.0894, Found: 316.0902.
4.5. trans-1-Allyl-3-benzoyl-4-(bromomethyl)-3-methylpyrrolidin-2-one (trans-6)
The stirred suspension of FeBr3 (296 mg, 1.0 mmol) and substrate 3b (129 mg, 0.50 mmol) in CH2ClCH2Cl (5.0 mL) was heated at reflux under argon atmosphere for 20 h. The reaction mixture was diluted with saturated NaHCO3 and then extracted with AcOEt. The organic phase was dried over Na2SO4 and then concentrated at reduced pressure. Purification of the residue by preparative TLC (AcOEt/hexanes = 1:2) afforded trans-6 (100 mg, 60%).
Colorless crystals. Mp 71.5–72 °C (AcOEt–hexane). IR (KBr) 2925, 2853, 1733, 1697, 1445 cm−1. 1H-NMR (CDCl3) δ 7.86 (2H, br dd, J = 8.2, 1.4 Hz), 7.52 (1H, m), 7.41 (2H, br t, J = 7.8 Hz), 5.79 (1H, m), 5.31–5.26 (2H, m), 4.05 (1H, br dd, J = 14.6, 6.4 Hz), 3.95 (1H, br dd, J = 14.6, 6.4 Hz), 3.72 (1H, dd, J = 10.1, 7.3 Hz), 3.44–3.36 (2H, m), 3.29 (1H, m), 3.16 (1H, dd, J = 10.1, 7.8 Hz), 1.43 (3H, s). 13C-NMR (CDCl3) δ 198.4, 174.0, 135.6, 132.5, 131.4, 129.0, 128.4, 119.2, 59.7, 49.5, 45.6, 42.4, 30.3, 14.7. HRMS (ESI+) calcd for C16H1879BrNO2Na [M + Na+]: 358.0413, Found: 358.0423; calcd for C16H1881BrNO2Na (M + Na+): 360.0393, Found: 360.0407.
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-8599/2017/1/M929. Details of the calculation study: Table S1: Gibbs free energy, enthalpy, and entropy at 298.15 K; Table S2: Change in Gibbs free energy, enthalpy, and entropy, HMQC, HMBC, and NOESY experiments of products trans-4, cis-4, trans-5 and trans-6, and 1H and 13C-NMR spectra of Compounds 3a, 3b, trans-4, cis-4, trans-5, and trans-6.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
This work was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (C) Grant Number 16K08188 (to H.M.).
Author Contributions
E. Yoshioka performed experiments and analyzed the data. H. Miyabe contributed to design of the study and manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Ma, X.; Peng, J.; Wu, G.; Zhu, T.; Li, G.; Gu, Q.; Li, D. Speradines B-D, oxygenated cyclopiazonic acid alkaloids from the sponge-derived fungus Aspergillus flavus MXH-X104. Tetrahedron 2015, 71, 3522–3527. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Nong, X.; Wei, X.; Qi, S. Oxindole alkaloids from the fungus Penicillium commune DFFSCS026 isolated from deep-sea-derived sediments. Tetrahedron 2015, 71, 610–615. [Google Scholar] [CrossRef]
- Hu, X.; Xia, Q.-W.; Zhao, Y.-Y.; Zheng, Q.-H.; Liu, Q.-Y.; Chen, L.; Zhang, Q.-Q. Speradines B-E, four novel tetracyclic oxindole alkaloids from the marine-derived fungus Aspergillus oryzae. Heterocycles 2014, 89, 1662–1669. [Google Scholar]
- Burch, J.D.; Farand, J.; Colucci, J.; Sturino, C.; Ducharme, Y.; Friesen, R.W.; Lévesque, J.-F.; Gagné, S.; Wrona, M.; Therien, A.G.; et al. Naphthalene/quinoline amides and sulfonylureas as potent and selective antagonists of the EP4 receptor. Bioorg. Med. Chem. Lett. 2011, 21, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hui, J.; Wang, D.; Zhu, L.; Fang, J.-H.; Zhao, X.-D. Synthesis, cytotoxicity and pro-apoptosis of novel benzoisoindolin hydrazones as anticancer agent. Chem. Pharm. Bull. 2010, 58, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Nishida, Y.; Masuoka, C.; Li, J.-C.; Okawa, M.; Ikeda, T.; Nohara, T. Lignan derivatives and a norditerpene from the seeds of Vitex negundo. J. Nat. Prod. 2004, 67, 2073–2075. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Imoto, Y.; Yoshikawa, T.; Kohtani, S.; Miyabe, H. FeCl3–promoted oxidative radical cyclization for the synthesis of lactams having a quaternary carbon. Synlett 2017, 28. in press. [Google Scholar] [CrossRef]
- Snider, B.B. Manganese(III)-based oxidative free-radical cyclizations. Chem. Rev. 1996, 96, 339–363. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Deepthi, A. Recent advances in CAN mediated reactions in organic synthesis. Tetrahedron 2009, 65, 10745–10755. [Google Scholar] [CrossRef]
- Mohan, R.M.; Kates, S.A.; Dombroski, M.A.; Snider, B.B. Manganese (III) based oxidative free-radical cyclizations. 3. Polycyclization reactions proceeding through secondary radicals. Tetrahedron Lett. 1987, 28, 845–848. [Google Scholar] [CrossRef]
- Curran, D.P.; Morgan, T.M.; Schwartz, C.E.; Snider, B.B.; Dombroski, M.A. Cyclizations of unsaturated ·CR(COX)2 radicals. Manganese(III) acetate oxidative cyclizations of unsaturated acetoacetates and atom-transfer cyclizations of unsaturated haloacetoacetates give the same radicals. J. Am. Chem. Soc. 1991, 113, 6607–6617. [Google Scholar] [CrossRef]
- González, M.A.; Molina-Navarro, S. Attempted synthesis of spongidines by a radical cascade terminating onto a pyridine ring. J. Org. Chem. 2007, 72, 7462–7465. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Suda, N.; Hayashi, Y.; Hirama, M. Stereoselective synthesis of zoanthenol ABC-ring by radical strategy. Tetrahedron Lett. 2013, 54, 1389–1391. [Google Scholar] [CrossRef]
- Wong, Y.-C.; Kao, T.-T.; Huang, J.-K.; Jhang, Y.-W.; Chou, M.-C.; Shia, K.-S. Manganese(III)-catalyzed oxidative cyclization of aryl 1-cyanoalk-5-ynyl ketone systems: A convenient and general approach to cyclopenta[b]naphthalene derivatives. Adv. Synth. Catal. 2014, 356, 3025–3038. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Tan, X.; Lu, J.; Cormier, K.W.; Ma, Z.; Chen, C. A biomimetic route for construction of the [4+2] and [3+2] core skeletons of dimeric pyrrole-imidazole alkaloids and asymmetric synthesis of ageliferins. J. Am. Chem. Soc. 2012, 134, 18834–18842. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, A.A.O.; Bolm, C. Iron(III) chloride in oxidative C–C coupling reactions. Chem. Soc. Rev. 2009, 38, 2730–2744. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-R.; Cheng, L.; Kong, D.-L.; Huang, H.-Y.; Gu, C.-L.; Liu, L.; Wang, D.; Li, C.-J. FeCl3‑mediated radical tandem reactions of 3‑benzyl-2-oxindoles with styrene derivatives for the stereoselective synthesis of spirocyclohexene oxindoles. Org. Lett. 2016, 18, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Ishibashi, H. Iron-mediated radical nitro-cyclization reaction of 1,6-dienes. Org. Lett. 2010, 12, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.F.; Velu, S.S.; Weber, J.-F.F.; Lee, K.C.; Hadi, A.H.A.; Richomme, P.; Rondeau, D.; Noorbatcha, I.; Awang, K. A tandem highly stereoselective FeCl3-promoted synthesis of a bisindoline: synthetic utility of radical cations in heterocyclic construction. Tetrahedron 2004, 60, 11733–11742. [Google Scholar] [CrossRef]
- Booker-Milburn, K.I.; Barker, A.; Brailsford, W.; Cox, B.; Mansley, T.E. Fe(III) mediated oxidative radical cyclisation of cyclopropanone acetals. Tetrahedron 1998, 54, 15321–15344. [Google Scholar] [CrossRef]
- Cossy, J.; Belotti, D.; Cuong, N.K.; Chassagnard, C. Photoreductive cyclization of N,N-dialkyl-β-oxoamides: synthesis of piperidines and δ-lactams. Tetrahedron 1993, 49, 7691–7700. [Google Scholar] [CrossRef]
- See: Supplementary Material.
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).