Ethyl (1R*,10S*,12R*,15S*)-4-Hydroxy-2-oxo-15- (2-oxo-1-pyrrolidinyl)-9-oxatetracyclo[10.2.2.01,10.03,8]hexadeca-3,5,7,13-tetraene-13-carboxylate
Abstract
:1. Introduction
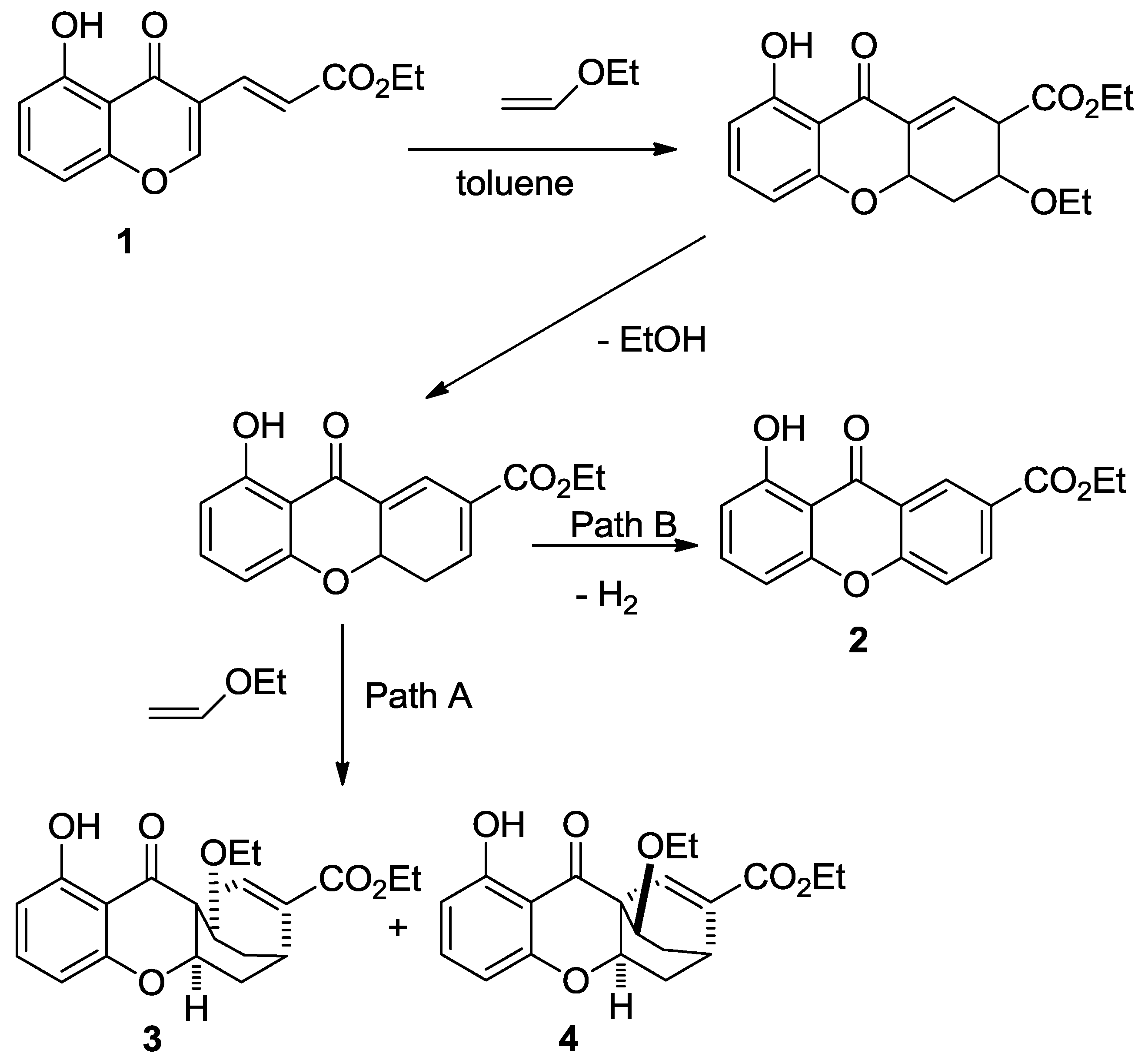
2. Results
3. Discussion
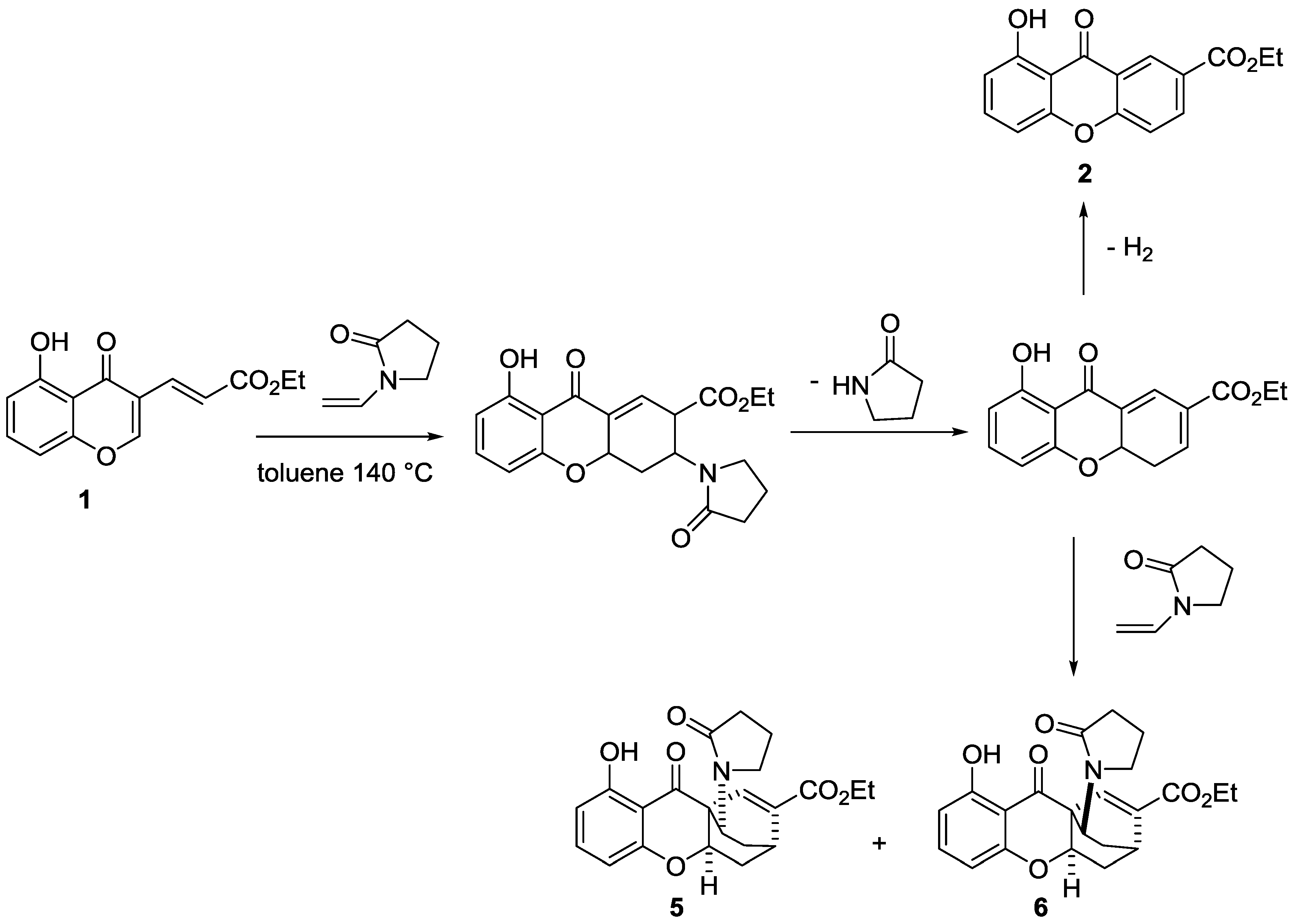
4. Materials and Methods
4.1. Domino Reaction
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Broja, T.; Fuchs, P.J.W.; Zeitler, K. Domino reactions, more than just a game. Nat. Chem. 2015, 7, 950–951. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F. Domino reactions in organic synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.W.; Combs, A.P.; Tempest, P.A.; Brown, S.D.; Keating, S.A. Multiple-component condensation strategies for combinatorial library synthesis. Acc. Chem. Res. 1996, 29, 123–131. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Rackelmann, N. The domino-Knoevenagel-hetero Diels-Alder reaction and related transformations. In Multicomponent reactions; Zhu, J., Bienaymé, H., Eds.; Wiley-VCH: Weinheim, Germany, 2005; Chapter 5; pp. 121–168. [Google Scholar]
- Tietze, L.F.; Steinmetz, A. Stereoselective solid-phase synthesis of cyclopentane and cyclohexane derivatives by two-component domino reactions: Generation of combinatorial libraries. Angew. Chem. Int. Ed. 1996, 35, 651–652. [Google Scholar] [CrossRef]
- Tietze, L.F.; Rischer, M. The tandem Sakurai–carbonyl–Ene reaction: A new and highly stereoselective sequential transformation and its use for the synthesis of steroid derivatives. Angew. Chem. Int. Ed. 1992, 31, 1221–1222. [Google Scholar] [CrossRef]
- Marko, I.E.; Dumeunier, R.; Leclercq, C.; Leroy, B.; Plancher, J.M.; Mekhalfia, A.; Bayston, D. Tandem Ene-Reaction/Intramolecular Sakurai Cyclisation (IMSC): A Novel Access to Polysubstituted Tetrahydropyrans and γ-Butyrolactones Using a Unique Allylation Strategy. Synthesis 2002, 7, 958–972. [Google Scholar] [CrossRef]
- Marko, I.E.; Leroy, B. Concise and stereocontrolled synthesis of polysubstituted tetrahydropyrans. Tetrahedron Lett. 2000, 41, 7225–7230. [Google Scholar] [CrossRef]
- Marko, I.E.; Plancher, J.M. Novel tandem “ene-ISMS” methodology. Efficient and versatile assembly of a pseudomonic acid C analogue. Tetrahedron Lett. 1999, 40, 5259–5262. [Google Scholar] [CrossRef]
- Bodwell, G.J.; Hawco, K.M.; da Silva, R.P. Electron deficient dienes 3: Rapid access to 2-hydroxybenzophenones via inverse electron demand Diels-Alder-driven domino reactions of a chromone-fused electron deficient diene with enamines. Synlett 2003, 179–182. [Google Scholar] [CrossRef]
- Bodwell, G.J.; Pi, Z. Electron deficient dienes I. Normal and inverse electron demand Diels-Alder reaction of the same carbon skeleton. Tetrahedron Lett. 1997, 38, 309–312. [Google Scholar] [CrossRef]
- Conyers, R.C.; Mazzone, J.R.; Siegler, M.A.; Posner, G.H. Highly regiocontrolled and stereocontrolled syntheses of polysubstituted aminocyclohexanes: mild inverse-electron-demand Diels–Alder cycloadditions of electrophilic 2-pyridones. Tetrahedron Lett. 2016, 57, 3344–3348. [Google Scholar] [CrossRef]
- Dang, A.T.; Miller, D.O.; Dawe, L.N.; Bodwell, G.J. Electron-deficient dienes. 5. An inverse-electron-demand Diels-Alder approach to 2-substituted 4-methoxyxanthones and 3,4-dimethoxyxanthones. Org. Lett. 2008, 10, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Moya, J.; Krohn, K.; Flörke, U.; Pessoa-Mahana, H.; Weiss-López, B.; Estévez-Braun, A.; Araya-Maturana, R. Domino inverse electron demand diels-alder reactions of chromones with ethyl vinyl ether. Heterocycles 2007, 71, 1327–1345. [Google Scholar] [CrossRef]
- Araya-Maturana, R.; Gavín-Sazatornil, J.A.; Heredia-Moya, J.; Pessoa-Mahana, H.; Weiss-López, B. Long-range correlations (nJC,H n > 3) in the HMBC spectra of 3-(4-oxo-4H-chromen-3-yl)-acrylic acid ethyl esters. J. Braz. Chem. Soc. 2005, 16, 657–661. [Google Scholar] [CrossRef]
- Boger, D.L.; Mullican, M.D. Inverse electron demand Diels-Alder reactions of 3-carbomethoxy-2-pyrones. Controlled introduction of oxygenated aromatics: Benzene, phenol, catechol, resorcinol, pyrogallol annulation. Tetrahedron Lett. 1983, 24, 4939–4942. [Google Scholar] [CrossRef]
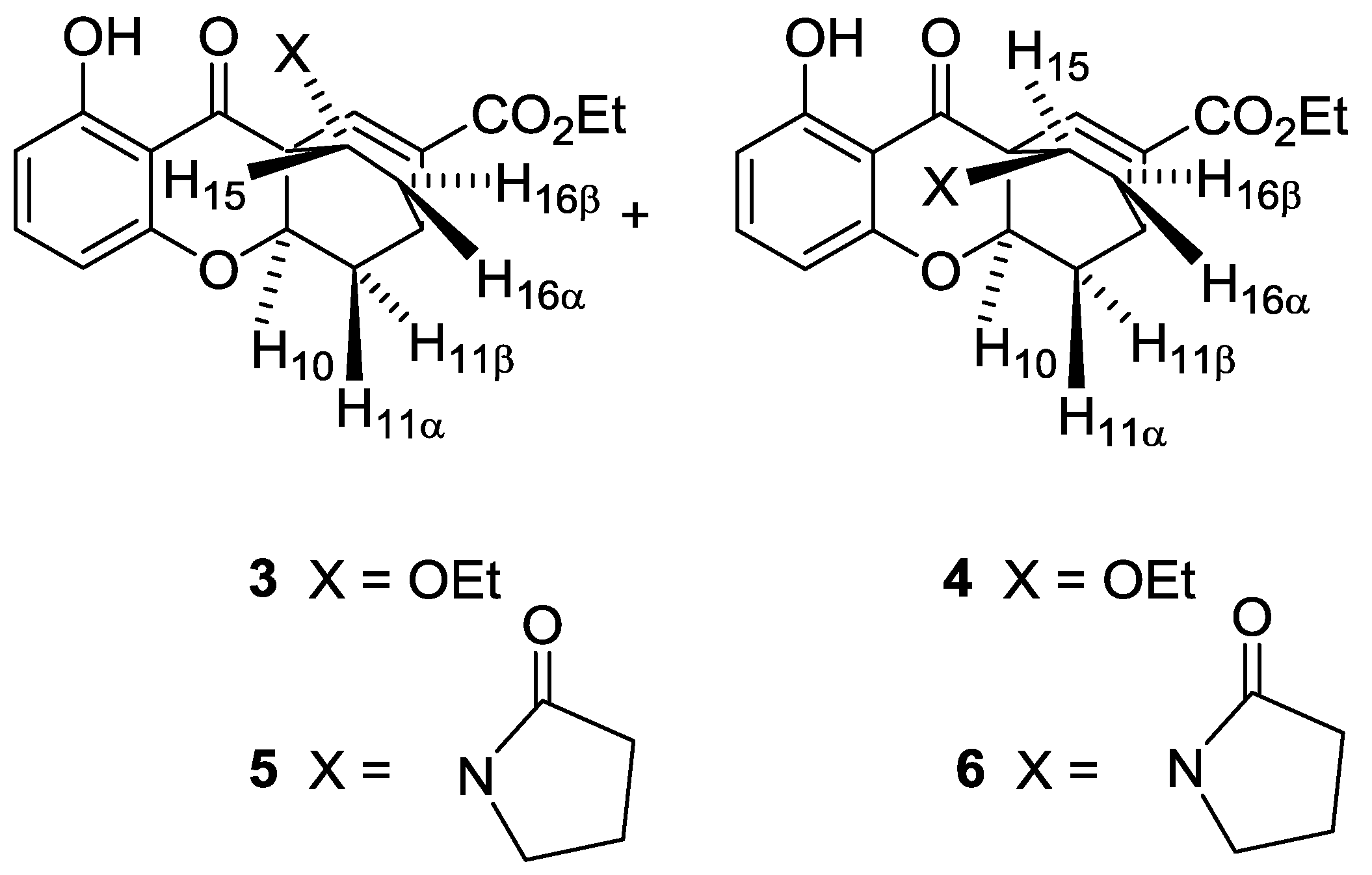
| JH,H | 3 | 4 | 5 | 6 |
|---|---|---|---|---|
| 10-11β | 10 | 8.6 | 10.3 | 8.3 |
| 10-11α | 3.4 | 2.8 | 3.8 | 2.4 |
| 11α-11β | 13.8 | 13.7 | 13.9 | 14.2 |
| 11β-16β | 3.1 | --- | 2.9 | --- |
| 11α-16α | --- | 3.7 | --- | --- |
| 15-16β | 3.1 | 7.8 | 5.6 | 9.6 |
| 15-16α | 8.1 | 2.4 | 9.8 | 5.6 |
| 16α-16β | 13 | 13.3 | 13.3 | 13.5 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia-Moya, J.; Araya-Maturana, R. Ethyl (1R*,10S*,12R*,15S*)-4-Hydroxy-2-oxo-15- (2-oxo-1-pyrrolidinyl)-9-oxatetracyclo[10.2.2.01,10.03,8]hexadeca-3,5,7,13-tetraene-13-carboxylate. Molbank 2017, 2017, M928. https://doi.org/10.3390/M928
Heredia-Moya J, Araya-Maturana R. Ethyl (1R*,10S*,12R*,15S*)-4-Hydroxy-2-oxo-15- (2-oxo-1-pyrrolidinyl)-9-oxatetracyclo[10.2.2.01,10.03,8]hexadeca-3,5,7,13-tetraene-13-carboxylate. Molbank. 2017; 2017(1):M928. https://doi.org/10.3390/M928
Chicago/Turabian StyleHeredia-Moya, Jorge, and Ramiro Araya-Maturana. 2017. "Ethyl (1R*,10S*,12R*,15S*)-4-Hydroxy-2-oxo-15- (2-oxo-1-pyrrolidinyl)-9-oxatetracyclo[10.2.2.01,10.03,8]hexadeca-3,5,7,13-tetraene-13-carboxylate" Molbank 2017, no. 1: M928. https://doi.org/10.3390/M928
APA StyleHeredia-Moya, J., & Araya-Maturana, R. (2017). Ethyl (1R*,10S*,12R*,15S*)-4-Hydroxy-2-oxo-15- (2-oxo-1-pyrrolidinyl)-9-oxatetracyclo[10.2.2.01,10.03,8]hexadeca-3,5,7,13-tetraene-13-carboxylate. Molbank, 2017(1), M928. https://doi.org/10.3390/M928






