Abstract
Deoxygenation of a secondary hydroxy group on β-cyclodextrin was conducted to prepare the title compound 2II-VII, 3I-VII, 6I-VII-icosa-O-acetyl-2I-deoxy-cyclomaltoheptaose. The synthetic procedure comprised a two-step reaction—phenoxythiocarbonylation and Barton-McCombie deoxygenation. The synthesized compound was characterized by 1H-NMR, 13C-NMR, HRMS, and elemental analysis.
1. Introduction
Cyclodextrins, which have a hydrophobic core and hydrophilic exterior, can form inclusion complexes with various hydrophobic compounds and thereby improve the solubility and bioavailability of such compounds. For this reason, cyclodextrins are widely used in materials science, as well as in pharmaceutical and dietary supplement applications in which hydrophobic compounds are delivered [1,2,3,4,5]. Regioselective modification of the hydroxyl groups of cyclodextrins has been attempted by various research groups in order to enhance the functions of the original compound [6,7,8,9,10,11]. For instance, the primary hydroxy groups at the 6-position react selectively with bulky reagents such as tert-butyldimethylsilyl chloride and p-toluenesulfonyl chloride [10,12]. Secondary hydroxy groups on cyclodextrins are able to react selectively in the presence of unprotected primary hydroxy groups by using strong bases such as sodium hydroxide and sodium hydride. Furthermore, their tosylation and alkylation have been reported, even though they required enormous efforts [6,7,9].
In this report, the hydroxy group at the 2-position of 2II-VII, 3I-VII, 6I-VII-Icosa-O-acetyl-2I-deoxy-cyclomaltoheptaose 1—prepared by monobenzylation at the 2-position, followed by acetylation [13,14,15]—was substituted with hydrogen via Barton-McCombie deoxygenation. The synthesis procedure and data for the synthesized compound (3) are reported herein.
2. Results and Discussion
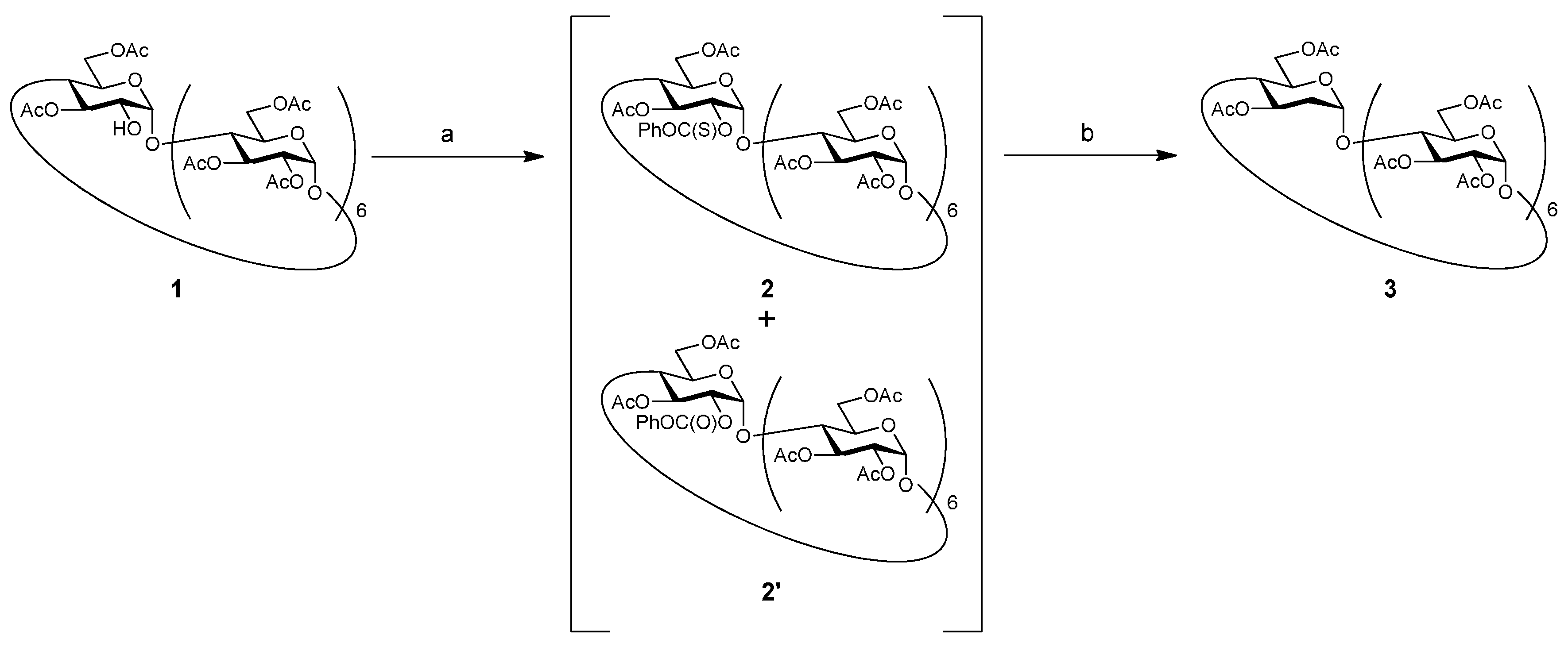
The hydroxy groups are usually substituted with a thiocarbonyl group and halogen during deoxygenation. However, the substitution of a secondary hydroxy group with a halogen by using bulky reagents is often difficult; in particular, sterically hindered hydroxy groups on cyclodextrins severely restrict activation of the reagents. Therefore, thiocarbonate was selected as the leaving group for deoxygenation (Scheme 1). Compound 1 was treated with 4-dimethylaminopyridine (DMAP) and O-phenyl chlorothionoformate to introduce the thiocarbonyl group at the 2-position. However, even after the reaction mixture was subjected to silica gel chromatography, thiocarbonyl derivative 2 was not isolated in pure state, and a mixture including byproduct 2′ was obtained. Compound 2′—an analogue of 2 wherein the S atom of the thiocarbonyl group is replaced by with O—was separated after the next reaction, and its structure was confirmed by 1H-NMR and mass spectroscopy (MS) data. A similar unexpected oxygen substitution was reported in a previous study [16]. Unfortunately, in our case, 2′ was produced in 51% yield. The mixture of 2 and 2′ was treated with n-Bu3SnH and 2,2′-azodiisobutyronitrile (AIBN) to give the title compound (deoxy compound) 3, albeit in a low yield of 25.3% (in two steps). The structure of 3 was elucidated by 1H-NMR, 13C-NMR, high-resolution mass spectroscopy (HRMS), and elemental analysis.
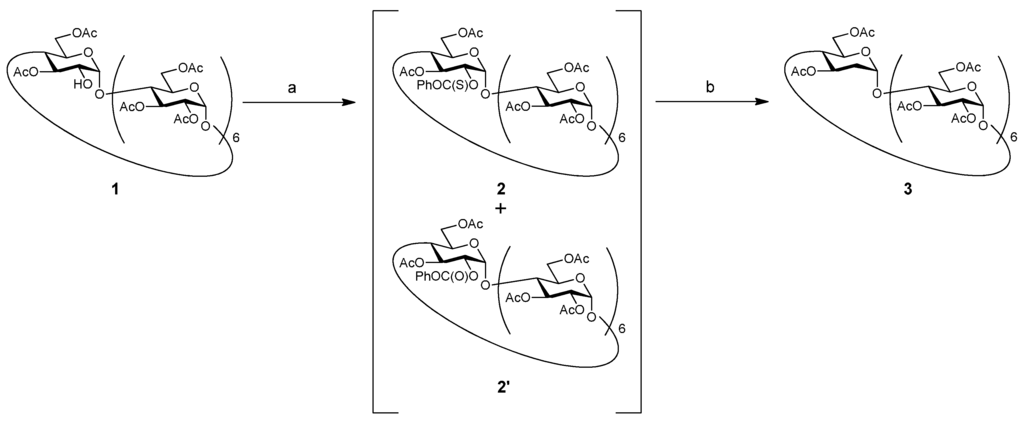
Scheme 1.
Synthesis of deoxy compound 3. Reagents and conditions: (a) PhOC(S)Cl, DMAP, pyridine, rt, 45 h; (b) n-Bu3SnH, AIBN, toluene, 90 °C, 5 h.
3. Experimental
All reagents were purchased from commercial sources and used without further purification. AIBN was recrystallized from methanol before use. The solvents employed in the reactions were dried using molecular sieves 4 Å activated in vacuo at 200 °C, and subsequently stored over molecular sieves. Thin layer chromatography (TLC) was performed on silica gel 60 F254 0.25 mm plates (Merck KGaA, Darmstadt, Germany). The compounds were detected under UV light and/or by treatment with EtOH/H2O/H2SO4/resorcinol and subsequent heating. Column chromatography was performed with silica gel 60 N, spherical neutral, particle size 40−50 μm or 63−210 μm (Kanto Chemical Co., Inc., Tokyo, Japan). The solvents for column chromatography were purchased from commercial sources and used as received. 1H and 13C-NMR spectra were recorded on a Bruker AVANCE 500US system (Bruker BioSpin GmbH, Rheinstetten, Germany) with a cryoprobe (500 MHz for 1H; 125.8 MHz for 13C). Chemical shifts (in ppm) were referenced to tetramethylsilane (δ = 0 ppm) in deuterated chloroform. 13C-NMR spectra were also recorded on the same NMR spectrometers and were calibrated with the CDCl3 peak (δ = 77.00 ppm). High-resolution mass spectra were recorded on a JEOL JMS-S3000 spectrometer (JEOL Ltd., Tokyo, Japan) using 2,5-dihydroxybenzoic acid (DHBA) as the matrix. Elemental analysis was performed using Elementar Vario EL cube (Elementar Analysensysteme GmbH, Frankfurt, Germany).
2II-VII, 3I-VII, 6I-VII-Icosa-O-acetyl-2I-deoxy-cyclomaltoheptaose (3)
Compound 1 (920 mg, 466 μmol), which was co-evaporated thrice with dry pyridine, was dissolved in dry pyridine (23 mL). To this solution were added DMAP (300 mg, 2.46 mmol) and O-phenyl chlorothionoformate (1.40 mL, 10.4 mmol) dissolved in dry dichloromethane (2.0 mL). Furthermore, the mixture was stirred at room temperature for 45 h under argon atmosphere. The resulting mixture was diluted with toluene (30 mL), filtered, and concentrated. The residue was purified by silica gel chromatography using hexane–acetone (1:1 (v/v)) as the eluent to give a mixture of 2 and 2′: Rf 0.63 (toluene–acetone = 4:3). The mixture was co-evaporated thrice with dry toluene and dissolved in dry toluene (30 mL). To the solution were added n-Bu3SnH (1.20 mL, 4.45 mmol) and AIBN (1.0 mg, 6.02 mmol). The mixture was stirred at 90 °C for 5 h under argon atmosphere, concentrated, diluted with acetonitrile, washed with hexane three times, and finally evaporated. The residue was purified by silica gel chromatography using toluene—acetone (7:3 (v/v)) to the give the desired compound 3 (229 mg, 26.8%) along with the O-substitution analogue 2′ (496 mg, 51%).
Rf 0.50 (toluene–acetone = 4:3) for 3
1H-NMR (500 MHz, CDCl3): δ 5.41−5.26 (m, 6H, H-3), 5.13−5.06 (m, 6H, H-1), 5.04−5.00 (m, 1H, H-3I), 4.96 (t, J1I,2I = 3.9 Hz, 1H, H-1I), 4.84−4.72 (m, 6H H-2), 4.59−4.49 (m, 7H, H-6a), 4.34−4.22 (m, 7H, H-6b), 4.20−4.05 (m, 7H, H-5), 3.80−3.65 (m, 6H, H-4), 3.57 (dd, J3I, 4I = 6.3 Hz, J4I,5I = 8.9 Hz, 1H, H-4I), 2.26−2.19 (m, 1H, H-2Ieq), 2.18−2.03 (m, 60H, COCH3), 1.78−1.74 (m, 1H, H-2Iax).
13C-NMR (125 MHz, CDCl3): δ 170.9, 170.7, 170.7, 170.7, 170.6, 170.5, 170.5, 170.4, 170.3, 170.3, 170.3 169.7, 169.4, 169.4, 169.3, 169.3, 169.0(C=O), 99.6 (C-1I), 97.4, 96.9, 96.7, 96.7, 96.5, 96.5 (C-1), 78.1, 77.6 (C-4), 76.4, 76.0, 71.3, 71.3, 71.0, 70.9, 70.8, 70.7, 70.5, 70.5, 70.3, 70.3, 70.0, 69.9, 69.8, 69.7, 69.6, 69.5, 69.4 (C-2, 3, 5), 63.1 (C-6I), 62.5, 62.5, 62.4 (C-6), 33.7 (C-2I), 21.1, 21.0, 20.8, 20.7, 20.6.
MALDI-TOF-HRMS (m/z) C82H110NaO54 [M + Na]+ calcd: 1981.5759, found: 1981.5693; C82H110KO54 [M + K]+ calcd: 1997.5499, found: 1997.5438.
Elemental analysis C82H110O54 calcd: C 50.26, H 5.66, found: C 49.99, H 5.58.
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4The following are available online at http://www.mdpi.com/1422-8599/2016/2/M900, Figure S1: 1H-NMR of 3, Figure S2: 13C-NMR of 3, Figure S3: MALDI-TOF-MS of 3, Figure S4: 1H-NMR of 2′, Figure S5: MALDI-TOF-MS of 2′.
Author Contributions
A.M. and H.Y. conceived and designed the experiments; K.K. and A.Y. performed the experiments; A.M. and K.K. analyzed the data; H.Y. contributed reagents/materials/analysis tools; A.M. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.J.; Schollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–192. [Google Scholar] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS Pharm.Sci.Tech. 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Saenger, W. Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. Engl. 1980, 19, 344–362. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for selective modifications of cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Dienst, E.V.V.; Snellink, B.H.; Piekartz, I.V.; Gansey, M.H.G.; Venema, F.; Feiters, M.C.; Nolte, R.J.M.; Engbersen, J.F.J.; Reinhoudt, D.N. Selective functionalization and flexible coupling of cyclodextrins at the secondary hydroxyl face. J. Org. Chem. 1995, 60, 6537–6545. [Google Scholar] [CrossRef]
- Atsumi, M.; Izumida, M.; Yuan, D.Q.; Fujita, K. Selective synthesis and structure determination of 6A, 6C, 6E-tri (O-sulfonyl)-β-cyclodextrins. Tetrahedron Lett. 2000, 41, 8117–8120. [Google Scholar] [CrossRef]
- Tian, S.; Forgo, P.; D'Souza, V.T. Selective modification at the 3-position of β-cyclodextrin. Tetrahedron Lett. 1996, 37, 8309–8312. [Google Scholar] [CrossRef]
- Takeo, K.; Mitoh, H.; Uemura, K. Selective chemical modification of cyclomalto-oligosaccharides via tert-butyldimethylsilylation. Carbohydr. Res. 1989, 187, 203–221. [Google Scholar] [CrossRef]
- Boger, J.; Corcoran, R.J.; Lehn, J.M. Cyclodextrin chemistry. Selective modification of all primary hydroxyl groups of α-and β-cyclodextrins. Helv. Chim. Acta 1978, 61, 2190–2218. [Google Scholar] [CrossRef]
- Yamamura, H.; Fujita, K. Preparation of heptakis(6-O-(p-tosyl))-β-cyclodextrin and heptakis(6-O-(p-tosyl))-2-O-(p-tosyl)-β-cyclodextrin and their conversion to heptakis(3, 6-anhydro)-β-cyclodextrin. Chem. Pharm. Bull. 1991, 39, 2505–2508. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Zhu, H.; Forgo, P.; D’Souza, V.T. Selectively monomodified cyclodextrins. Synthetic strategies. J. Org. Chem. 2000, 65, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Masurier, N.; Estour, F.; Lefèvre, B.; Brasme, B.; Masson, P.; Lafont, O. Improved access to 2-O-monobenzyl ethers of β-cyclodextrin as precursors of catalysts for organophosphoryl esters hydrolysis. Carbohydr. Res. 2006, 341, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Cousin, H.; Cardinael, P.; Oulyadi, H.; Pannecoucke, X.; Combret, J.C. Synthesis of the three isomeric mono-2-, 3-, or 6-hydroxy permethylated β-cyclodextrins and unambiguous high field NMR characterisation. Tetrahedron Asymmetry 2001, 12, 81–88. [Google Scholar] [CrossRef]
- Cromer, R.; Spohr, U.; Khare, D.P.; LePendu, J.; Lemieux, R.U. Molecular recognition XII. The binding of the H human blood group determinants and congeners by a lectin of Galactia tenuiflora. Can. J. Chem. 1992, 70, 1511–1530. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).