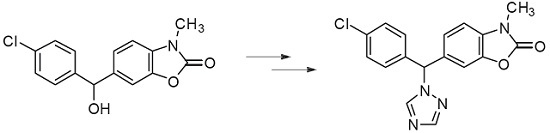
6-[(4-Chlorophenyl)(1H-1,2,4-triazol-1-yl)methyl]-3-methyl-2(3H)-benzoxazolone
Abstract
:1. Introduction
2. Results
3. Experimental Section
3.1. General Information
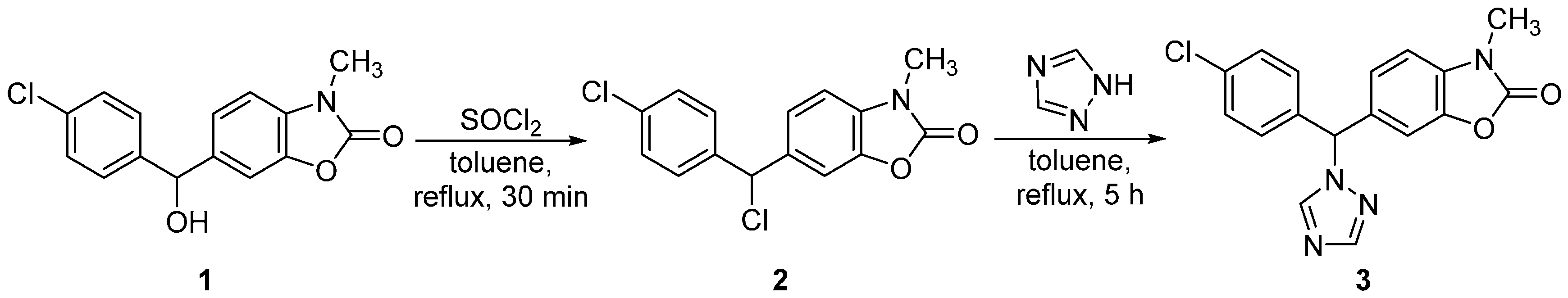
3.2. Synthesis of 6-[(4-Chlorophenyl)(1H-1,2,4-triazol-1-yl)methyl]-3-methyl-2(3H)-benzoxazolone (3)
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, S.S.; Kavitha, H.P. Synthesis and biological applications of triazole derivatives—A review. Mini-Rev. Org. Chem. 2013, 10, 40–65. [Google Scholar] [CrossRef]
- Shalini, K.; Kumar, N.; Drabu, S.; Sharma, P.K. Advances in synthetic approach to and antifungal activity of triazoles. Beilstein J. Org. Chem. 2011, 7, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Fromtling, R.A. Overview of medically important antifungal azole derivatives. Clin. Microbiol. Rev. 1988, 1, 187–217. [Google Scholar] [PubMed]
- Chen, S.C.A.; Sorrell, T.C. New Drugs, Old Drugs. Antifungal agents. Med. J. Aust. 2007, 187, 404–409. [Google Scholar] [PubMed]
- Hamilton, A.; Piccart, M. The third–generation non-steroidal aromatase inhibitors: a review of their clinical benefits in the second-line hormonal treatment of advanced breast cancer. Ann. Oncol. 1999, 10, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Goss, P. Anti-aromatase agents in the treatment and prevention of breast cancer. Cancer Control. 2002, 9, 2–8. [Google Scholar] [PubMed]
- Marchand, P.; Le Borgne, M.; Palzer, M.; Le Baut, G.; Hartmann, R.W. Preparation and pharmacological profile of 7-(α-azolylbenzyl)-1H-indoles and indolines as new aromatase inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 1553–1555. [Google Scholar] [CrossRef]
- Nativelle-Serpentini, C.; Moslemi, S.; Yous, S.; Park, C.H.; Lesieur, D.; Sourdaine, P.; Séralini, G.-E. Synthesis and evaluation of benzoxazolinonic imidazoles and derivatives as non-steroidal aromatase inhibitors. J. Enz. Inh. Med. Chem. 2004, 19, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yous, S.; Nativelle-Serpentini, C.; Seralini, G.-E.; Chang, S.-J.; Lesieur, D. Use of Benzothiazinones and Related Compounds as Aromatase Inhibitors in Treatment of Psoriasis and Neoplasm, and Their Preparation. FR 2860235, 1 April 2005. Chem. Abstr. 2005, 142, 355274. [Google Scholar]
- Botta, M.; Corelli, F.; Manetti, F.; Mugnaini, C.; Tafi, A. Rational design and synthesis of homochiral azole antifungal agents. Pure Appl. Chem. 2001, 73, 1477–1485. [Google Scholar] [CrossRef]
- Petrov, O.; Gerova, M.; Petrova, K.; Ivanova, Y. New imidazole derivatives of 2(3H)-benzazolones as potential antifungal agents. J. Heterocycl. Chem. 2009, 46, 44–48. [Google Scholar] [CrossRef]
- Rani, N.; Sharma, A.; Gupta, G.K.; Singh, R. Imidazoles as potential antifungal agents: A review. Mini-Rev. Med. Chem. 2013, 13, 1626–1655. [Google Scholar] [CrossRef] [PubMed]
- Danel, C.; Foulon, C.; Park, C.; Yous, S.; Bonte, J.-P.; Vaccher, C. Enantiomeric resolution of new aromatase inhibitors by liquid chromatography on cellulose chiral stationary phases. J. Sep. Sci. 2005, 28, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Danel, C.; Foulon, C.; Guelzim, A.; Park, C.H.; Bonte, J.-P.; Vaccher, C. Preparative Enantiomeric separation of new aromatase inhibitors by HPLC on polysaccharide-based chiral stationary phases: Determination of enantiomeric purity and assignment of absolute stereochemistry by X-ray structure snalysis. Chirality 2005, 17, 600–607. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerova, M.S.; Ivanova, Y.B.; Chanev, C.D.; Petrov, O.I. 6-[(4-Chlorophenyl)(1H-1,2,4-triazol-1-yl)methyl]-3-methyl-2(3H)-benzoxazolone. Molbank 2016, 2016, M901. https://doi.org/10.3390/M901
Gerova MS, Ivanova YB, Chanev CD, Petrov OI. 6-[(4-Chlorophenyl)(1H-1,2,4-triazol-1-yl)methyl]-3-methyl-2(3H)-benzoxazolone. Molbank. 2016; 2016(2):M901. https://doi.org/10.3390/M901
Chicago/Turabian StyleGerova, Mariana S., Yordanka B. Ivanova, Christo D. Chanev, and Ognyan I. Petrov. 2016. "6-[(4-Chlorophenyl)(1H-1,2,4-triazol-1-yl)methyl]-3-methyl-2(3H)-benzoxazolone" Molbank 2016, no. 2: M901. https://doi.org/10.3390/M901
APA StyleGerova, M. S., Ivanova, Y. B., Chanev, C. D., & Petrov, O. I. (2016). 6-[(4-Chlorophenyl)(1H-1,2,4-triazol-1-yl)methyl]-3-methyl-2(3H)-benzoxazolone. Molbank, 2016(2), M901. https://doi.org/10.3390/M901