Abstract
An efficient and cost-effective synthesis of 6-nitro-4H-benzo[d][1,3]thiazin-2-amine based on a sequential SN2-SNAr process is reported. The synthesis is accomplished with an overall yield of 80%.
1. Introduction
The tandem SN2-SNAr reaction has proven to be useful for the synthesis of a number of aryl-fused heterocyclic systems [1,2]. The current work describes the application of this strategy to the preparation of 6-nitro-4H-benzo[d][1,3]thiazin-2-amine (1). Ring-fused 1,3-thiazin-2-amines have recently been explored as potential agents for the treatment of Alzheimer's disease and other neurological disorders as they have potent inhibitory activity against amyloid precursor protein (APP) and beta-secretase proteins (BACE1 and BACE2) [3,4,5,6,7,8,9,10,11,12,13,14]. We planned to incorporate this moiety into compounds of biological interest to our research group.
Many previous syntheses of 1,3-thiazin-2-amines have involved reactions of variously substituted enones with thiourea, and several cases proceeded in a tandem fashion [15,16,17]. Several others have utilized metal catalysts to promote the heterocyclization [18]. Our synthesis began with 2-fluoro-5-nitrobenzyl bromide (2) [1] and represents a potentially new approach to these compounds (see Scheme 1). With a reactive benzylic bromine and an activated fluorine situated ortho to each other on the aromatic ring, 2 is perfectly suited to react with two of the nucleophilic sites in thiourea (3) to generate the target heterocycle. It was hoped that this would lead to the title compound 1 by a tandem process, but in practice, the conversion was possible only by a sequential reaction. Nevertheless, it was possible to efficiently perform this transformation in a single reaction vessel in high yield.
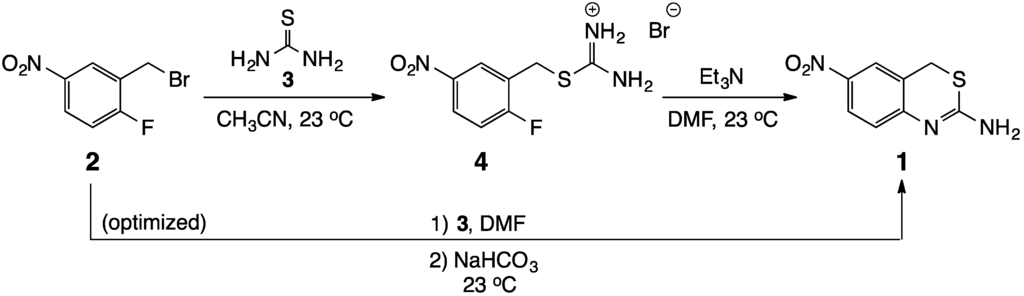
Scheme 1.
Synthesis of 6-nitro-4H-benzo[d][1,3]thiazin-2-amine (1).
Initially, 2 was reacted with 3 in acetonitrile according to the procedure of Lam, et al. [19] to give the isothiouronium salt 4 [20]. The conversion to 1 was then completed by dissolving 4 in N,N-dimethylformamide (DMF) and treating with an equivalent of triethylamine. While successful, this two-step protocol was inefficient, requiring two set-ups and two product isolations, with an overall yield of only 54%. The use of triethylamine as the base in the second step led to emulsions during the final work-up, which contributed to the low yield and made this route more tedious. Thus, we refined the procedure to streamline the synthesis and simplify the isolation of the target heterocycle. In the optimized scheme, the entire process was performed at room temperature in a single reaction vessel by stirring 2 and 3 in DMF solvent for 1 h, followed by treatment with powdered sodium bicarbonate for 3 h. Product isolation involved diluting the crude reaction mixture with water to precipitate the thiazinamine, collecting and washing the solid with water to remove residual base, and drying under vacuum to remove water. The overall yield for the conversion was 80%.
2. Experimental
6-Nitro-4H-benzo[d][1,3]thiazin-2-amine (1)
To a stirred solution of thiourea (3, 1.6 g, 21 mmol) in anhydrous DMF (50 mL) under nitrogen was added 2-fluoro-5-nitrobenzyl bromide (2, 5.0 g, 21 mmol) and the reaction was stirred for 1 h at room temperature. To this solution was added powdered sodium bicarbonate (1.8 g, 21 mmol) and the reaction was stirred for an additional 3 h. The crude reaction was quenched by addition of 150 mL of distilled water and stirring was continued for 30 min. During this time the product precipitated as a bright yellow solid. The solid was collected by filtration through a Buchner funnel and was washed with distilled water. The product was dried under high vacuum at room temperature to give 1 (3.52 g, 80%) as a bright yellow solid. This material was sufficiently pure for most purposes. An analytical sample was obtained by recrystallization from absolute ethanol, mp 228–230 °C. IR (nujol): 3422, 3294, 1656, 1551, 1339 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.13 (d, J = 2.7 Hz, 1H), 8.05 (dd, J = 8.8, 2.7 Hz, 1H), 7.79 (br s, 2H), 7.98 (d, J = 8.8 Hz, 1H), 4.09 (s, 2H); 13C NMR (100 MHz, DMSO-d6): δ 159.1, 153.9, 141.7, 124.2, 123.7, 123.2, 120.9, 28.1. Anal. Calcd for C8H7N3O2S: C, 45.93; H, 3.37; N, 20.08. Found: C, 46.01; H, 3.41; N, 19.97.
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4The supplementary materials and the molfile can be found at http://www.mdpi.com/1422-8599/2016/2/M899.
Acknowledgments
The authors are grateful to NSF (BIR-9512269), the Oklahoma State Regents for Higher Education, the W. M. Keck Foundation, and Conoco, Inc. for funding to establish the Oklahoma Statewide Shared NMR Facility. The College of Arts and Sciences at OSU is also acknowledged for recently providing funds to purchase a new 400 MHz NMR for this facility.
Author Contributions
K.K.G. and J.T.H. optimized the reaction, acquired the spectra, and analyzed the data. R.A.B. designed the synthesis, confirmed the data analysis, and wrote the paper. All of the authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References and Notes
- Compound 2 is prepared in three steps from commercial 2-fluorobenzaldehyde by nitration (NaNO2 in H2SO4), aldehyde reduction (BH3·THF in THF or NaBH4 in EtOH), and conversion to the bromide (PBr3 in Et2O), see Bunce, R.A.; Rogers, D.; Nago, T.; Bryant, S.A. 4H-1-Benzopyrans by a tandem SN2-SNAr reaction. J. Heterocycl. Chem. 2008, 45, 547–550. [Google Scholar] [CrossRef] Compound 2 is also commercially available from Oakwood Products. Inc. [1-(800)-467-3386. Available online: www.oakwoodchemical.com (accessed on 9 May 2016).], though it is rather expensive.
- Bunce, R.A.; Nago, T.; Sonobe, N.; Slaughter, L.M. Benzo-fused heterocycles and carbocycles by intramolecular SNAr and tandem SN2-SNAr reactions. J. Heterocycl. Chem. 2008, 45, 551–557. [Google Scholar] [CrossRef]
- Hall, A.; Farthing, C.N.; Pineiro, J.L.C. Preparation of Fused Aminodihydrothiazine Derivs. Useful as BACE Inhibitors and Amyloid β Inhibitors for Treating Neurodegenerative Disease. World Patent WO 2012098213 A1, 2012. Chem. Abstr. 2012, 157, 261855. [Google Scholar]
- Brodney, M.A.; Butler, C.R.; Helal, C.J.; O'Neill, B.T. Preparation of Hexahydro-pyrano[3,4-d][1,3]thiazin-2-amine Derivatives as BACE Inhibitors Useful in the Treatment of Neurodegenerative Disease. U.S. Patent US20130053373 A1, 2013. Chem. Abstr. 2013, 158, 390009. [Google Scholar]
- Beck, E.M.; Brodney, M.A.; Butler, C.R.; Davoren, J.E.; O'Neill, B.T. Heterocyclic Substituted Hexahydropyrano[3,4-d][1,3]thiazin-2-amine Compounds as Inhibitors of APP, BACE1 and BACE2 and Their Preparation. World Patent WO 2013164730 A1, 2013. Chem. Abstr. 2013, 159, 714182. [Google Scholar]
- Hall, A.; Farthing, C.N.; Eatherton, A.J. Hexahydropyrrolothiazine Compounds as BACE1 Inhibitors and Their Preparation. World Patent WO 2014013076 A1, 2014. Chem. Abstr. 2014, 160, 219225. [Google Scholar]
- Hall, A.; O'Connor, D.M. Preparation of Furo[3,4-d][1,3]thiazinyl Compounds as BACE1 Inhibitors for Treating Neurodegenerative Diseases. British Patent GB 2504615 A1, 2014. Chem. Abstr. 2014, 160, 265100. [Google Scholar]
- Beck, E.M.; Brodney, M.A.; Butler, C.R.; Davoren, J.E. Preparation of Alkyl-substituted hexahydropyrano[3,4-d][1,3]thiazin-2-amine Compounds as BACE1 and BACE2 Inhibitors. World Patent WO 2014045162 A1, 2014. Chem. Abstr. 2014, 160, 515614. [Google Scholar]
- Brodney, M.A.; Beck, E.M.; Butler, C.R.; Davoren, J.E.; O'Neill, B.T. Preparation of Hexahydro-pyrano[3,4-d][1,3]thiazin-2-amine Compounds as BACE1 Inhibitors for Treating Neurological Disorders, Diabetes and Obesity. U.S. Patent US 20140163015 A1, 2014. Chem. Abstr. 2014, 161, 99258. [Google Scholar]
- Brodney, M.A.; Butler, C.R.; Lachapelle, E.A.; O'Neill, B.T. Preparation of Hexahydropyrano[3,4-d][1,3]thiazin-2-amine Compounds as BACE Inhibitors for Treatment of Alzheimers Disease and Other Disorders. World Patent WO 2014097038 A1, 2014. Chem. Abstr. 2014, 161, 171688. [Google Scholar]
- Brodney, M.A.; Butler, C.R.; Beck, E.M.; Davoren, J.E.; LaChapelle, E.A.; O'Neill, B.T. Preparation of Heteroaryl-substituted Hexahydropyrano[3,4-d][1,3]thiazin-2-amine Compounds. U.S. Patent US 20140228356 A1, 2014. Chem. Abstr. 2014, 161, 336389. [Google Scholar]
- Beck, E.M.; Brodney, M.A.; Butler, C.R.; O'Neill, B.T. Preparation of Substituted Phenyl hexahydropyrano[3,4-d][1,3]thiazin-2-amine Compounds Useful in the Treatment of Neurodegenerative Diseases and Diabetes. World Patent WO 2014125397 A1, 2014. Chem. Abstr. 2014, 161, 390000. [Google Scholar]
- Pineiro, J.L.C.; Guerot, C. Preparation of 4a,6,7,7a-Tetrahydro-4H-furo[3,2-d][1,3]thiazin-2-amine Derivatives for the Treatment of Alzheimer-Type Dementia. British Patent GB 2512975 A1, 2014. Chem. Abstr. 2014, 161, 613558. [Google Scholar]
- Brodney, M.A.; Beck, E.M.; Butler, C.R.; Zhang, L.; O'Neill, B.T.; Barreiro, G.; Lachapelle, E.A.; Rogers, B.N. Preparation of N-(2-Amino-6-methyl-4,4a,5,6-tetrahydropyrano[3,4-d][1,3]thiazin-8a(8h)-yl-1,3-thiazol-4-yl) amides as Inhibitors of beta-site Amyloid Precursor Protein Cleaving Enzyme 1 (BACE1). World Patent WO 2015155626 A1, 2015. Chem. Abstr. 2015, 163, 585749. [Google Scholar]
- Thanusu, J.; Kanagarajan, V.; Gopalakrishnan, M. Synthesis, spectral characterization, and in vitro antibacterial and antifungal activities of novel 1,3-thiazine-2-amines comprising morpholine nucleus. J. Enzyme Inhib. Med. Chem. 2010, 25, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Kanagarajan, V.; Gopalakrishnan, M. Domino way toward the synthesis of novel 4-(4-morpholinophenyl)-6-aryl-6H-1,3-thiazin-2-amines under focused microwave irradiation catalyzed by heterogeneous NaHSO4·SiO2 and their in vitro microbiological evaluation. Pharm. Chem. J. 2011, 45, 248–256. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Guernon, J.; Park, H.; Thompson, L.A. Expedient synthesis of furo[2,3-d][1,3]-thiazinamines and pyrano[2,3-d][1,3]thiazinamines from enones and thiourea. J. Org. Chem. 2016, 81, 3386–3390. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Bhowmik, S.; Batra, S. Synthesis of 4-substituted imino-4H-benzo[d][1,3] thiazin-2-amines via palladium-catalysed isocyanide insertion in 2-bromophenylthioureas. RSC Adv. 2014, 4, 41433–41436, and references cited therein. [Google Scholar] [CrossRef]
- Kong, K.H.; Tan, C. K.; Lin, X.; Lam, Y. A versatile thiouronium-based solid-phase synthesis of 1,3,5-triazines. Chem. Eur. J. 2012, 18, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- The intermediate isothiouronium bromide salt was isolated when the first stage of the reaction was performed in anhydrous acetonitrile (room temperature, 24 h, 92%). The spectral data were: IR (nujol): 3302, 3274, 3187, 1656, 1521, 1346 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 9.28 (br s, 4H), 8.52 (dd, J = 6.4, 3.0 Hz, 1H), 8.31 (ddd, J = 9.1, 4.3, 3.0 Hz, 1H), 7.60 (t, J = 9.2 Hz, 1H), 4.70 (s, 2H); 13C NMR (100 MHz, DMSO-d6): δ 168.6, 165.7, 163.1, 144.3, 127.20, 127.15, 126.9, 126.8, 125.3, 125.1, 118.0, 28.4.
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).