Continuing our study on the application of 1,2,4-triazines in organic synthesis [1] we prepared the title compounds as valuable intermediates for metalation reactions leading to functionalized 5,5’-bi-1,2,4-triazines [2].
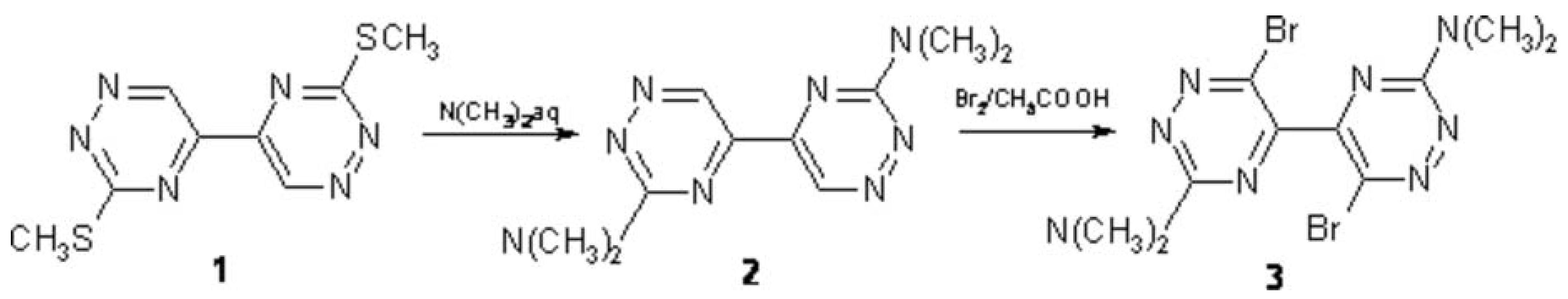
The mixture of 3,3’-bis(methylsulfanyl)-5,5’-bi-1,2,4-triazine (1) [3] (756 mg, 3.0 mmol) and dimethylamine, 40 wt. % solution in water (40 g), was stirred at room temperature for 20 hrs, and then was heated at 70 °C during a period of 30 min. The precipitate was filtered off and it was purified by column chromatography on silica gel (Merck type 60, 230-400 mesh) using a mixture of chloroform/acetone (100:1) as eluent to give 709 mg (96 %) of 3,3’-bis(N,N-dimethylamino)-5,5’-bi-1,2,4-triazine of (2) as a yellow solid.
To a solution 2 (246 mg, 1.0 mmol) in acetic acid (8 mL) the bromine (1.6 g, 10 mmol) was added. The reaction mixture was refluxed for 2 hrs. After that time the reaction mixture was cooled to 20 °C, diluted with water (50 mL) and extracted with chloroform (5 x 25 mL). The organic extract was washed with water (125 mL) and dried over MgSO4. Removal of the solvent in vacuum and purification of the residue by column chromatography on silica gel (Merck type 60, 230-400 mesh) using a mixture of chloroform/acetone (100:1) as eluent gave 222 mg (55 %) of 6,6’-dibromo-3,3’-bis(N,N-dimethylamino)-5,5’-bi-1,2,4-triazine (3) as a yellow solid.
3,3’-Bis(N,N-dimethylamino)-5,5’-bi-1,2,4-triazine (2)
M.p. 223-224 °C
1H NMR (CDCl3, 200 MHz): 3.37 (s, 12H, 4 x CH3), 9.44 (s, 2H, H –triazine).
Anal Calcd. for C10H14N8: C, 48.78; H, 5.69; N, 45.53. Found: C, 48.88; H, 5.57; N, 45.40
6,6’-Dibromo-3,3’-bis(N,N-dimethylamino)-5,5’-bi-1,2,4-triazine (3)
M.p. 122-123 °C
1H NMR(CCl4, 60 MHz): 3.85 (s, 12H, 4 x CH3).
Anal Calcd. for C10H12N8Br2: C, 29.73; H, 2.99; N, 27.73. Found: C, 29.84; H, 2.83; N, 27.63
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- For previous paper in this series, see: Branowska, D.; Rykowski, A. Synlett 2002, 1892–1895.
- Hundsdorf, T.; Neunhoeffer, H. Synthesis 2002, 1800–1805.
- Krass, K.D.; Chen, T.-K.; Paudler, W.W. J. Heterocycl. Chem. 1973, 10, 343–345. [CrossRef]
© 2004 MDPI. All rights reserved
