Synthesis of 2-hydroxy-3-methoxy-5-(4-methoxyphenylazo) benzaldehyde. A new aldehyde for the preparation of biologically active molecules.
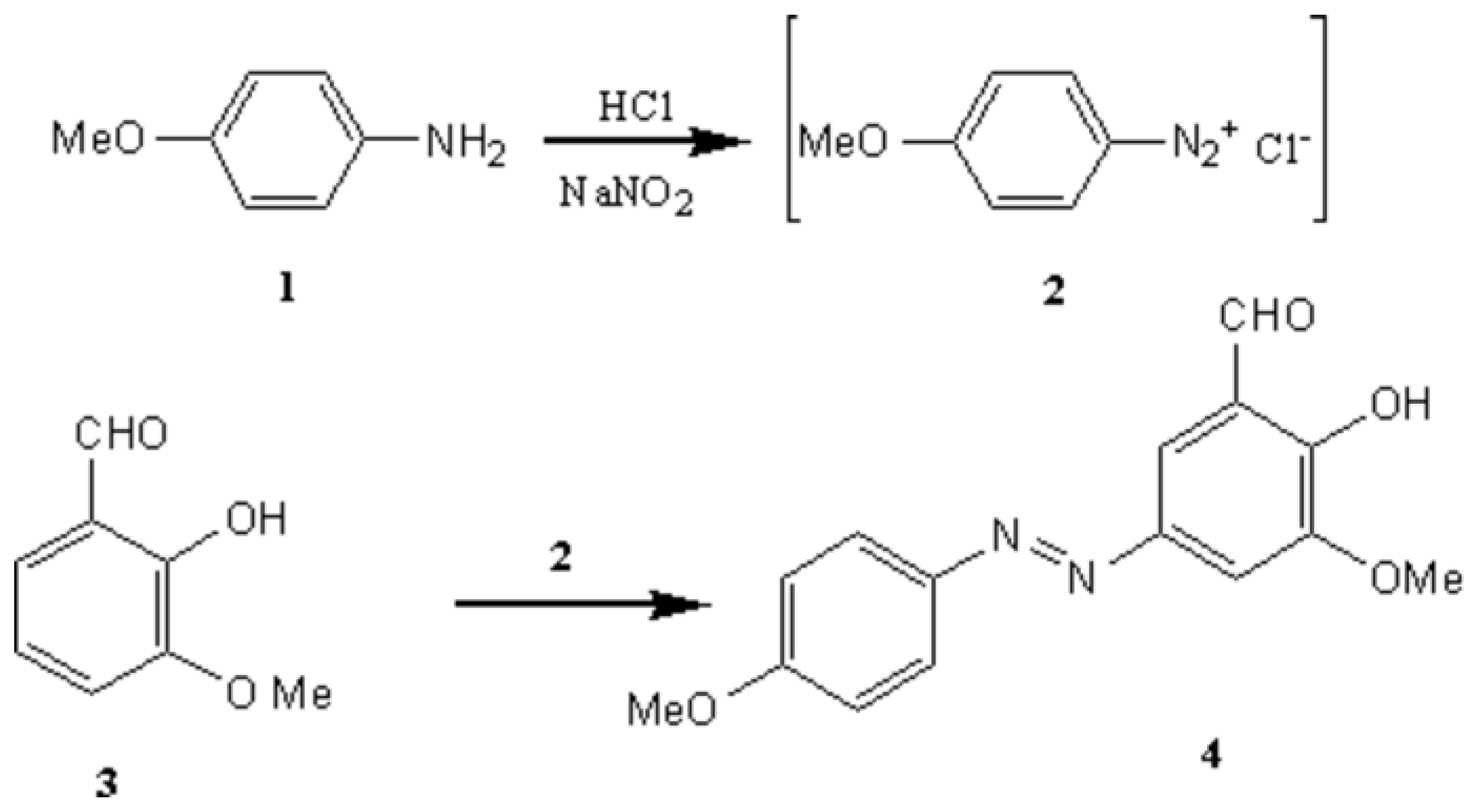
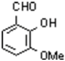 , 19.8), 135 (MeOC6 H4N2, 40.3),107 (MeOC6 H4, 100), 92 (C6H4O, 20.3).
, 19.8), 135 (MeOC6 H4N2, 40.3),107 (MeOC6 H4, 100), 92 (C6H4O, 20.3).Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Pathak, P.; Jolly, V.S.; Sharma, K.P. Orient. J. Chem. 2000, 16(1), 161–162.
- Pathak, P.; Jolly, V.S.; Sharma, K.P. Orient. J.Chem. 2000, 16(3), 493–494.
- Alka, Pradhan; Vinod, Pradhan; Jolly, V.S. Orient. J. Chem. 1994, 10, 73–74.
- Jolly, V. S.; Pathak, M.; Jain, R. J .Indian. Chem .Soc 1993, 70, 505–507.
- Samadhiya, S.; Halve, A. Orient. J. Chem. 2001, 17(1), 119–122.
- Halve, A.; Goyal, A. Orient. J. Chem. 1996, 12(1), 87–88.
- Furniss, B. S.; Hannaferd, A.J.; Rogers, V.; Smith, P.W.G.; Tatchell, A.R. Vogel's Textbook of Practical Organic Chemistry, 4th ed.; Longman Inc.: New York, 1981; p. 716. [Google Scholar]
- Samples Availability: Available from MDPI.
© 2004 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Esmaeilbeig, A.R.; Zarei, M. Synthesis of 2-hydroxy-3-methoxy-5-(4-methoxyphenylazo) benzaldehyde. A new aldehyde for the preparation of biologically active molecules. Molbank 2004, 2004, M371. https://doi.org/10.3390/M371
Jarrahpour AA, Esmaeilbeig AR, Zarei M. Synthesis of 2-hydroxy-3-methoxy-5-(4-methoxyphenylazo) benzaldehyde. A new aldehyde for the preparation of biologically active molecules. Molbank. 2004; 2004(1):M371. https://doi.org/10.3390/M371
Chicago/Turabian StyleJarrahpour, A. A., A. R. Esmaeilbeig, and M. Zarei. 2004. "Synthesis of 2-hydroxy-3-methoxy-5-(4-methoxyphenylazo) benzaldehyde. A new aldehyde for the preparation of biologically active molecules." Molbank 2004, no. 1: M371. https://doi.org/10.3390/M371
APA StyleJarrahpour, A. A., Esmaeilbeig, A. R., & Zarei, M. (2004). Synthesis of 2-hydroxy-3-methoxy-5-(4-methoxyphenylazo) benzaldehyde. A new aldehyde for the preparation of biologically active molecules. Molbank, 2004(1), M371. https://doi.org/10.3390/M371



