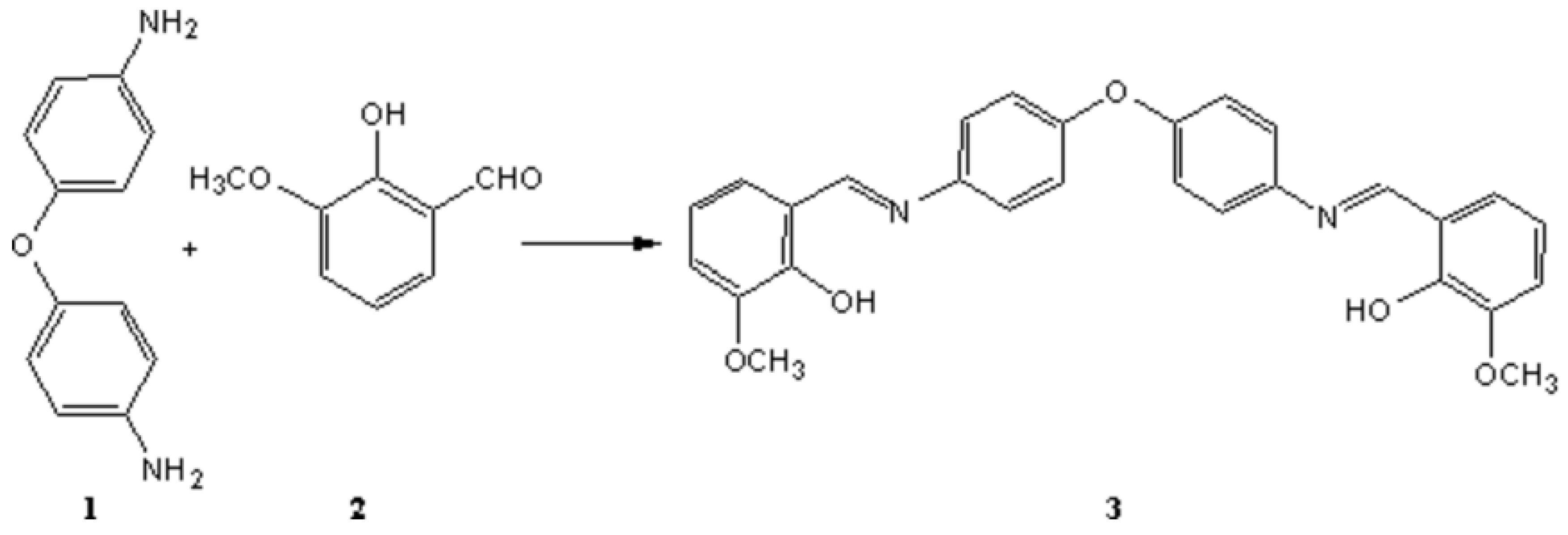
A mixture of 4,4'-diaminodiphenyl ether 1 (1.00 g, 5.00 mmol), o-vanillin 2 (1.52 g, 10.00 mmol) in methanol (40.00 mL) was stirred at room temperature for one hour to give an orange precipitate. Then it was filtered and washed with methanol to give the pure imine 3 (2.27 g, 97.00 %).
m.p. 134-136 °C.
IR (KBr) (cm−1): 1620.4(C=N), 3050-3300 (OH)
1H-NMR (DMSO) (250 MHz) δ(ppm): 3.87 (6H, s, 2OCH3), 6.59-7.40 (14H, m, 4Ph), 8.59 (2H, s, N=CH), 13.61 (2H, s, OH)
13C-NMR (DMSO) (62.90 MHz) δ(ppm): 56.60 (OCH3), 115.29-134.1 (aromatic carbons), 161.96 (C=N)
MS (m/z, %): 468(M+, 21.5), 406 (HOPh(O)C=NPhOPHN=CPh), 17.5), 242 (CH3OPhOHC=NPh(O), 6), 150 (CH3OPhOHC=N, 18.7), 136 (CH3OPhOHCH, 6.3), 123 (CH3OPhOH, 10), 119(Ph(O)N=CH, 5.1), 76 (PhH), 43 (C-OCH3, 100), 41 (C=C-OH, 94).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
The authors thank the Shiraz University Research Council for financial support (Grant No. 81-SC-1540-C220).
- Sample Availability: Available from MDPI.
© 2004 MDPI. All rights reserved.