As part of our research programme we have synthesized the title compounds as valuable intermediates for the preparation of acyclonucleosides-biologically active molecules. The starting material 1 was obtained according to the reported procedure [1] and title compounds were obtained using N-Bromosuccinimide (NBS) as brominating agent.
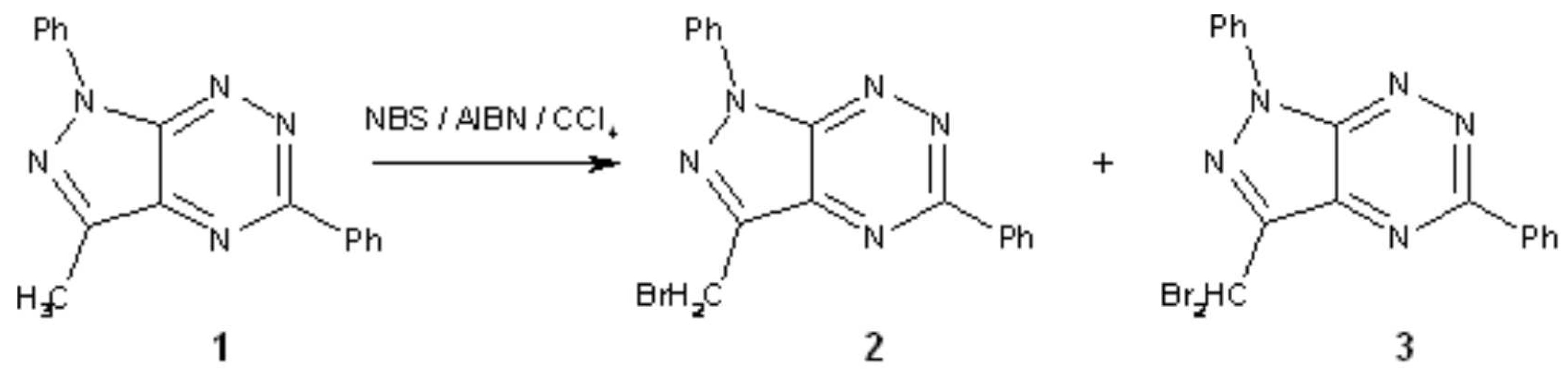
To a solution of 1 (72 mg, 0.25 mmol) in CCl4 (10 ml) NBS (178 mg, 1mmol) and Azobis(isobutyronitrile) (AIBN) (17 mg, 0.1 mmol) were added. The mixture was refluxed for 4.5 h. The solvent was evaporated in vacuo and the residue was purified by column silica gel chromatography (silica gel 230-400 mesh, CHCl3/n-hexane mixture 1:1) to give 99 mg (0.27 mmol, 54%) of 2 and 63 mg (0.14 mmol, 28%) of 3.
3-Bromomethyl-1,5-Diphenyl-1H-Pyrazolo[4,3-e][1,2,4]Triazine (2)
Melting Point: 185-187oC
1H-NMR (200 MHz, CDCl3): δ= 8.67-8.72 (m, 2H); 8.41-8.46 (m, 2H); 7.55-7.65 (m, 5H); 7.42-7.46 (m, 1H); 5.02 (s, 2H).
IR (KBr, cm-1): 3032; 1595; 1500; 1421; 1213; 1109; 1080; 753; 689.
MS- EI (m/z, %): 365 (6) [M+]; 339 (32); 337 (32); 286 (14); 259 (29); 258 (100); 218 (20); 155 (51); 115 (17); 77 (18).
HR-MS (EI, 70eV) Calculated for C17H1279BrN5: 365.02761. Found: 365.02685.
3-Dibromomethyl-1,5-Diphenyl-1H-Pyrazolo[4,3-e][1,2,4]Triazine (3)
Melting Point: 200-202oC
1H-NMR (200 MHz, CDCl3): δ= 8.70-8.75 (m, 2H); 8.41-8.46 (m, 2H); 7.57-7.66 (m, 5H); 7.44-7.48 (m, 1H); 7.18 (s, 1H).
IR (KBr, cm-1): 2922; 1593; 1500, 1420; 1108; 775; 690.
MS- EI (m/z, %): 445 (10) [M+]; 419 (29); 417 (58); 415 (29); 366 (23); 364 (22); 339 (32); 338 (100); 337 (32); 336 (98); 258 (37); 235 (38); 233 (40); 218 (14); 155 (10); 77 (30).
HR-MS- EI: Calculated for C17H1279Br81BrN5: 444.93607. Found: 444.93751.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References
- Rykowski, A.; Mojzych, M.; Karczmarzyk, Z. Heterocycles 2000, 53, 2175.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.