Post-Harvest Quality Changes and Molecular Responses of Epidermal Wax in ‘Munage’ Grapes with Botrytis cinerea Infection
Abstract
1. Introduction
2. Results
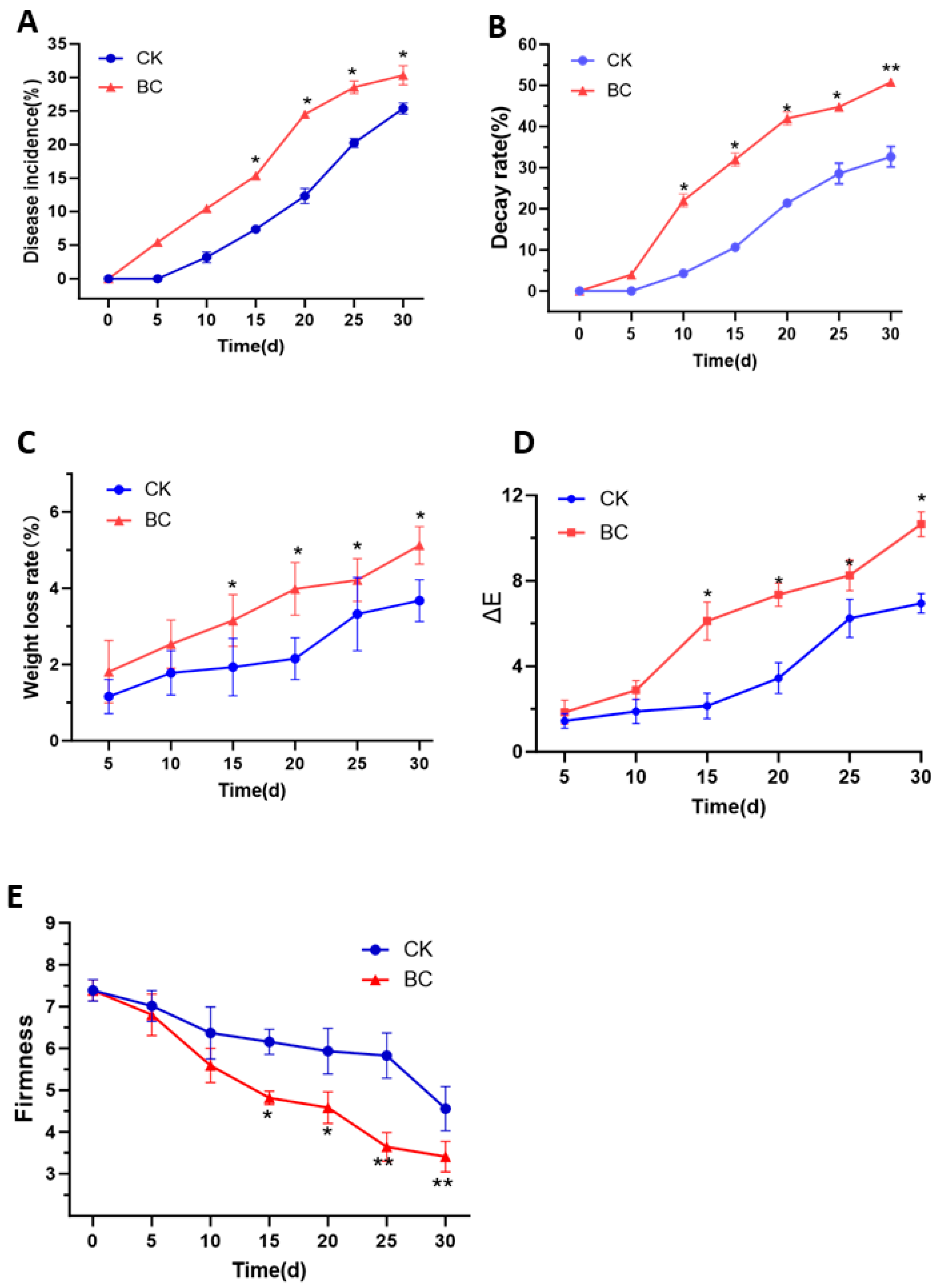
2.1. B. cinerea Infection Decreased the Postharvest Quality in ‘Munage’ Grapes
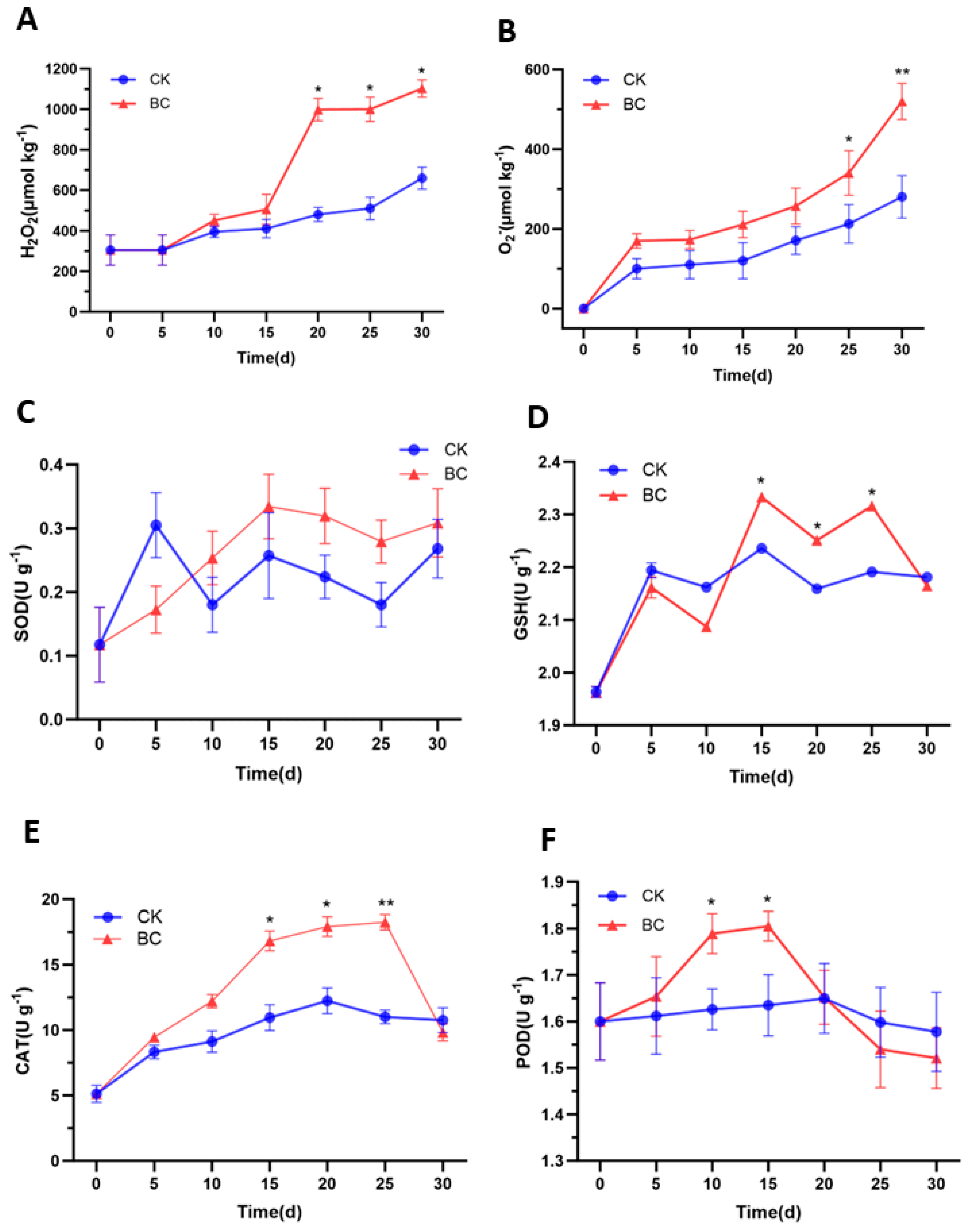
2.2. B. cinerea Infection Changed Active Oxygen and Antioxidant Enzyme Activities of ‘Munage’ Grapes
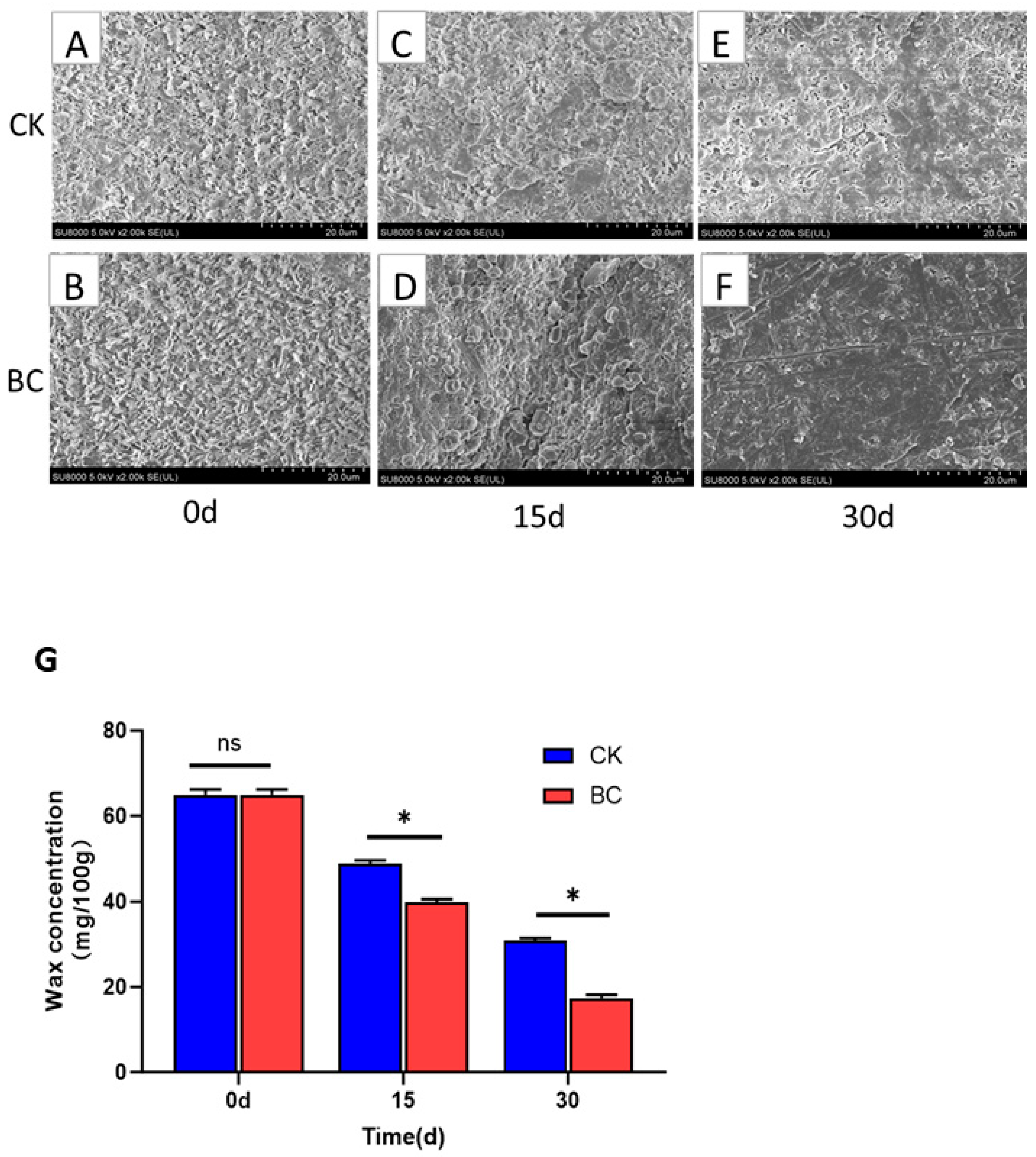
2.3. B. cinerea Infection Changed the Wax Structure and Amount in ‘Munage’ Grapes
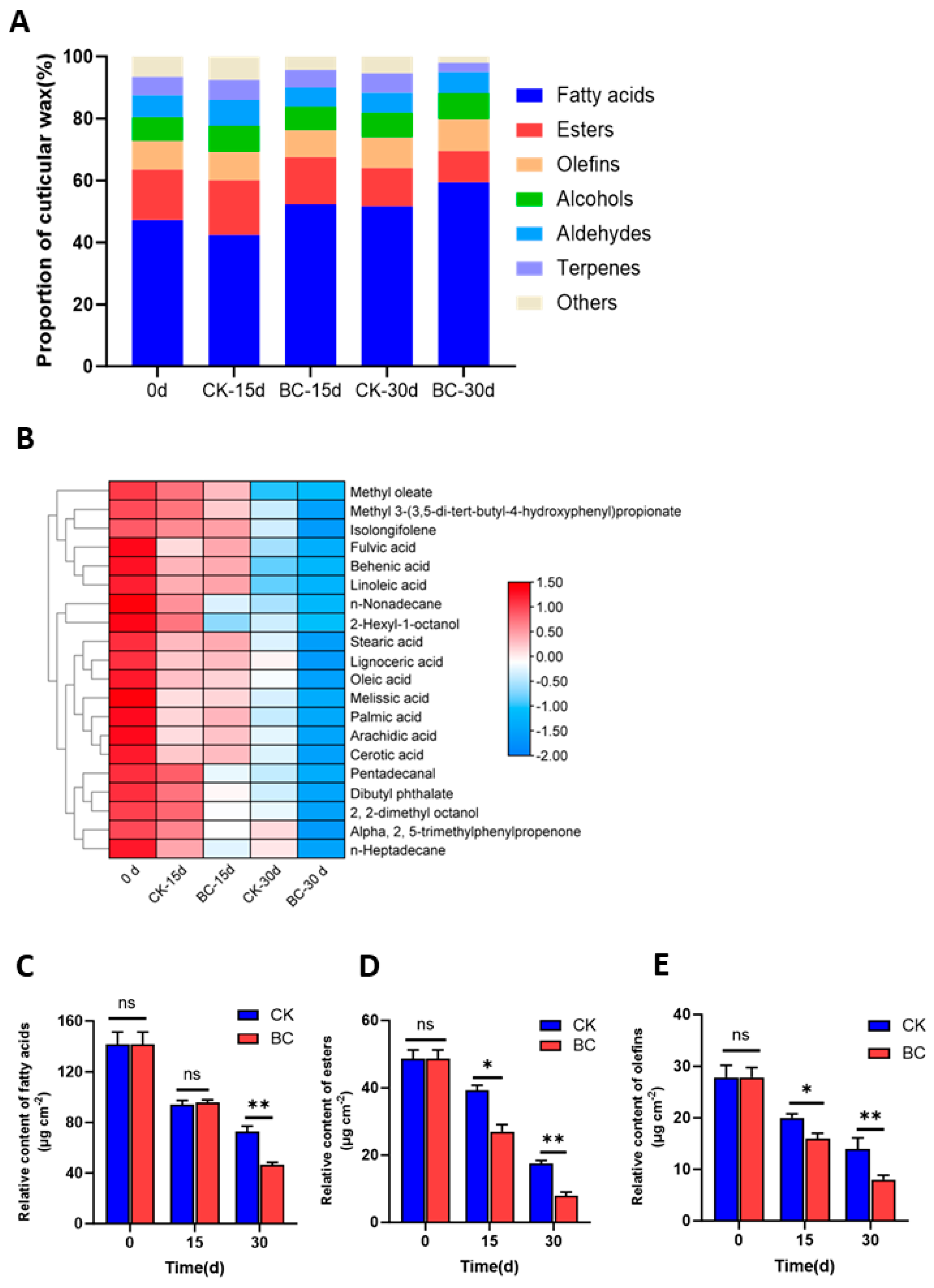
2.4. B. cinerea Infection Decreased the Wax Components in ‘Munage’ Grapes
2.5. Differential Gene Expression Analysis of B. cinerea-Inoculated ‘Munage’ Grapes
2.6. Differential Transcription Factors Analysis of B. cinerea-Inoculated ‘Munage’ Grapes
2.7. Validation of DEGs and TFs
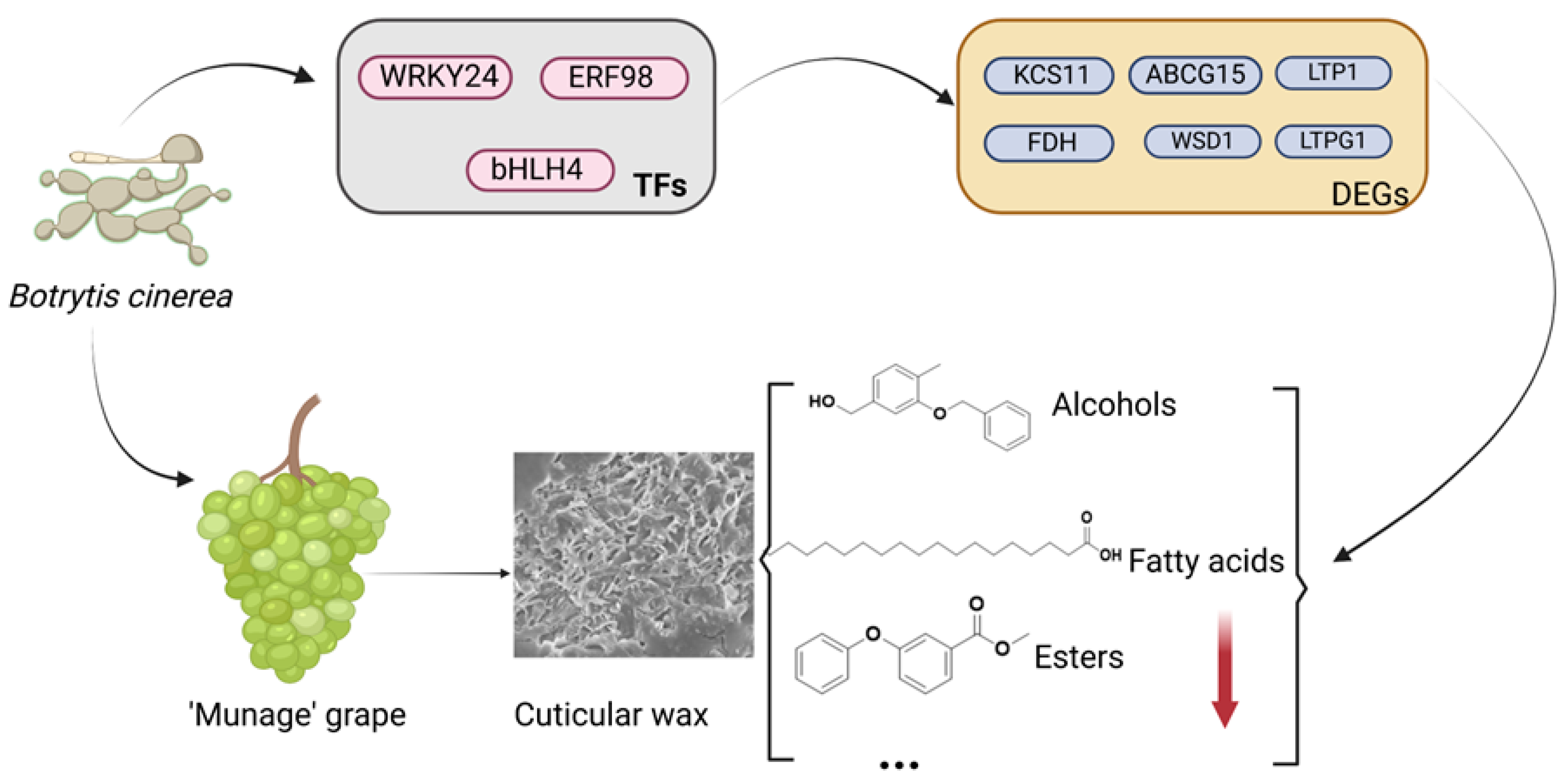
2.8. The Potential Mechanism of B. cinerea-Induced Wax Metabolism in ‘Munage’ Grapes
3. Discussion
4. Materials and Methods
4.1. Grapes and Chemical Substances
4.2. Determination of Postharvest Quality in ‘Munage’ Grapes
4.3. Analysis of Defense Enzyme Activities
4.4. Determination of Wax Microstructure and Total Content
4.5. Analysis of Wax Components in ‘Munage’ Grapes
4.6. RNA Sequencing (RNA-Seq) Analysis
4.7. qRT-PCR Validation
4.8. Statistical Analysis and Correlation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, X.F.; Liu, B.Z.; Maimaitiyiming, R.; Wang, L.; Zhao, L.; Zhang, H.M.; Chen, K.P.; Aihaiti, A. The Effect of Non-Saccharomyces Cerevisiae Torulaspora delbrueckii on the Aroma Composition of Munage Grape Base-Wine and the Mechanism of the Effect. Fermentation 2024, 10, 266. [Google Scholar] [CrossRef]
- Hong, P.J.; Zhang, Z.B.; Zhou, Y.Z.; Lu, X.Q.; Sadeghnezhad, E.; Pang, Q.Q.; Tao, Z.; Cheng, Y.X.; Wang, B.; Jia, H.F. Polygalacturonase inhibiting protein enhances cell wall strength of strawberry fruit for resistance to Botrytis cinerea infection. Sci. Hortic. 2024, 327, 112850. [Google Scholar] [CrossRef]
- Li, Z.X.; Yang, S.; Wang, X.; Liao, Q.H.; Zhang, W.L.; Liu, J.; Liu, G.H.; Tang, J.M. Widely targeted metabolomics analysis reveals the effect of exogenous auxin on postharvest resistance to botrytis cinerea in kiwifruit (Actinidia chinensis L.). Postharvest Biol. Technol. 2023, 195, 112129. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Zhang, X.M.; Zhang, Q.; Chai, S.; Yin, W.; Gao, M.Z.; Wang, X.P. The transcription factors VaERF16 and VaMYB306 interact to enhance resistance of grapevine to Botrytis cinerea infection. Mol. Plant Pathol. 2022, 23, 1415–1432. [Google Scholar] [CrossRef]
- Li, J.; Wei, J.; Tusong, K.; Wu, Z.; Wu, B. Preharvest Botrytis cinerea infection accelerates postharvest spike-stalk browning in ‘Munage’ grape. S. Afr. J. Bot. 2023, 154, 1–10. [Google Scholar] [CrossRef]
- Trivedi, P.; Nguyen, N.; Hykkerud, A.L.; Häggman, H.; Martinussen, I.; Jaakola, L.; Karppinen, K. Developmental and Environmental Regulation of Cuticular Wax Biosynthesis in Fleshy Fruits. Front. Plant Sci. 2019, 10, 431. [Google Scholar] [CrossRef]
- Chu, W.; Gao, H.; Cao, S.; Fang, X.; Chen, H.; Xiao, S. Composition and morphology of cuticular wax in blueberry (Vaccinium spp.) fruits. Food Chem. 2017, 219, 436–442. [Google Scholar] [CrossRef]
- Klavins, L.; Klavins, M. Cuticular wax composition of wild and cultivated northern berries. Foods 2020, 9, 587. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zhang, M.; Ren, X.; Cheng, Y.L.; Peng, X.Y.; Tian, S.; Wang, X.; Xu, L.; Zhang, Y.; Sun, C.; et al. Chemical and thermodynamic analyses of the surface waxes of ‘Korla’ pears: Relationships between the surface waxes and skin greasiness. Postharvest Biol. Technol. 2023, 196, 112156. [Google Scholar] [CrossRef]
- Yang, M.Y.; Ban, Z.J.; Luo, Z.; Wu, B.; Gao, S.; Wang, F.; Belwal, T.; Li, L. The chemical composition and potential role of epicuticular and intracuticular wax in four cultivars of table grapes. Postharvest Biol. Technol. 2021, 173, 111430. [Google Scholar] [CrossRef]
- Arya, G.C.; Sarkar, S.; Manasherova, E.; Aharoni, A.; Cohen, H. The plant cuticle: An ancient guardian barrier set against long-standing rivals. Front. Plant Sci. 2021, 12, 663165. [Google Scholar] [CrossRef]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef]
- Alkan, N.; Friedlander, G.; Ment, D.; Prusky, D.; Fluhr, R. Simultaneous transcriptome analysis of Colletotrichum gloeosporioides and tomato fruit pathosystem reveals novel fungal pathogenicity and fruit defense strategies. New Phytol. 2015, 205, 801–815. [Google Scholar] [CrossRef]
- Liu, R.L.; Zhang, L.P.; Xiao, S.Y.; Chen, H.J.; Han, Y.C.; Niu, B.; Wu, W.J.; Gao, H.Y. Ursolic acid, the main component of blueberry cuticular wax, inhibits Botrytis cinerea growth by damaging cell membrane integrity. Food Chem. 2023, 415, 135753. [Google Scholar] [CrossRef]
- Espana, L.; Heredia-Guerrero, J.A.; Segado, P.; Benítez, J.J.; Heredia, A.; Domínguez, E. Biomechanical properties of the tomato (Solanum lycopersicum) fruit cuticle during development are modulated by changes in the relative amounts of its components. New Phytol. 2014, 202, 790–802. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, R.; Fang, X.; Wu, W.; Han, Y.; Chen, H.; Xu, F.; Gao, H. Botrytis cinerea infection affects wax composition, content and gene expression in blueberry fruit. Postharvest Biol. Technol. 2022, 192, 112020. [Google Scholar] [CrossRef]
- Silva-Moreno, E.; Brito-Echeverría, J.; López, M.; Ríos, J.; Balic, I.; Reinaldo, R. Effect of cuticular waxes compounds from table grapes on growth, germination and gene expression in botrytis cinerea. World J. Microb. Biot. 2016, 32, 74. [Google Scholar] [CrossRef]
- Xing, S.J.; Wang, M.; Zhang, Z.; Yuan, Y.Y.; Song, Z.Y.; Wu, B.; Wei, J. Sulfur dioxide enhances postharvest grape resistance to Botrytis cinerea by promoting glutathione level. Sci. Hortic. 2024, 334, 113295. [Google Scholar] [CrossRef]
- Xue, D.W.; Zhang, X.Q.; Lu, X.L.; Chen, G.; Chen, Z.H. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef]
- Huang, H.; He, X.M.; Sun, Q.M.; Liu, G.M.; Tang, Y.Y.; Sun, J. Differential changes in cuticular wax affect the susceptibility to fruit decay in pitaya after harvest: A cultivar comparative study. Postharvest Biol. Technol. 2024, 210, 112751. [Google Scholar] [CrossRef]
- Wen, H.; Wang, Y.; Wu, B.; Feng, Y.; Dang, Y.; Yang, B.; Ma, X.; Qiao, L. Analysis of wheat wax regulation mechanism by liposome and transcriptome. Front. Genet. 2021, 12, 757920. [Google Scholar] [CrossRef]
- Min, D.; Li, F.; Wang, J.; Fu, X.; Ali, M.; Song, Y.; Ding, J.; Li, X.; Li, M.; Yang, K.; et al. Transcriptome reveals insights into the regulatory mechanism of cuticular wax synthesis in developing apple fruit. Sci. Hortic. 2024, 328, 112891. [Google Scholar] [CrossRef]
- Yang, M.; Xiang, Y.; Luo, Z.; Gao, Y.; Wang, L.; Hu, Q.; Dong, Y.; Qi, M.; Li, D.; Liu, L. Light-responsive transcription factors VvHYH and VvGATA24 mediate wax terpenoid biosynthesis in Vitis vinifera. Plant Physiol. 2024, 196, 1546–1561. [Google Scholar] [CrossRef]
- Dimopoulos, N.; Tindjau, R.; Wong, D.C.J.; Matzat, T.; Haslam, T.; Song, C.; Gambetta, G.A.; Kunst, L.; Castellarin, S.D. Drought stress modulates cuticular wax composition of the grape berry. J. Exp. Bot. 2020, 71, 3126–3141. [Google Scholar] [CrossRef]
- Wan, R.; Guo, C.L.; Hou, X.Q.; Zhu, Y.X.; Gao, M.; Hu, X.Y.; Zhang, S.L.; Jiao, C.; Guo, R.R.; Li, Z. Comparative transcriptomic analysis highlights contrasting levels of resistance of Vitis vinifera and Vitis amurensis to Botrytis cinerea. Hortic. Res. 2021, 8, 1. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Technol. 2014, 87, 103–112. [Google Scholar] [CrossRef]
- Lewandowska, M.; Keyl, A.; Feussner, I. Wax biosynthesis in response to danger: Its regulation upon abiotic and biotic stress. New Phytol. 2020, 227, 698–713. [Google Scholar] [CrossRef]
- Schirra, M.; D’Aquino, S.; Palma, A.; Marceddu, S.; Angioni, A.; Cabras, P.; Scherm, B.; Migheli, Q. Residue level, persistence, and storage performance of citrus fruit treated with fludioxonil. J. Agric. Food Chem. 2005, 53, 6718–6724. [Google Scholar] [CrossRef]
- Chen, M.; Xie, X.; Lin, Q.; Chen, J.; Grierson, D.; Yin, X.; Sun, C.; Chen, K. Differential expression of organic acid degradation-related genes during fruit development of navel oranges (Citrus sinensis) in two habitats. Plant Mol. Biol. Rep. 2013, 31, 1131–1140. [Google Scholar] [CrossRef]
- Huang, H.; Burghardt, M.; Schuster, A.C.; Leide, J.; Lara, I.; Riederer, M. Chemical composition and water permeability of fruit and leaf cuticles of Olea europaea L. J. Agric. Food Chem. 2017, 65, 8790–8797. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Shen, C.; Farnham, M.W.; Ku, K.M. Three-dimensional epicuticular wax on plant surface reduces attachment and survival rate of Salmonella during storage. Postharvest Biol. Technol. 2020, 166, 111197. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, B.; Zhang, J.; Wang, C.; Liu, C.; Liu, Y.; Zhu, X.; Ren, X. Relationships between cuticular waxes and skin greasiness of apples during storage. Postharvest Biol. Technol. 2017, 131, 55–67. [Google Scholar] [CrossRef]
- Curry, E. Effects of 1–MCP applied postharvest on epicuticular wax of apples (Malus domestica Borkh.) during storage. J. Sci. Food Agric 2008, 88, 996–1006. [Google Scholar] [CrossRef]
- Li, N.; Fu, L.; Song, Y.; Li, J.; Xue, X.; Li, S.; Li, L. Wax composition and concentration in jujube (Ziziphus jujuba Mill.) cultivars with differential resistance to fruit cracking. J. Plant Physiol. 2020, 255, 153294. [Google Scholar] [CrossRef]
- Li, N.; Song, Y.; Li, J.; Hao, R.; Feng, X.; Li, L. Transcriptome and genome re-sequencing analysis reveals differential expression patterns and sequence variation in pericarp wax metabolism-related genes in Ziziphus jujuba (Chinese jujube). Sci. Hortic. 2021, 288, 110415. [Google Scholar] [CrossRef]
- Min, D.D.; Li, Z.L.; Fu, X.D.; Wang, J.H.; Li, F.J.; Li, X.A.; Zhang, X.H. Integration of transcriptomic and metabonomic reveals moleculardifferences of sweetness and aroma between postharvest and vine ripenedtomato fruit. Food Control 2022, 139, 109102. [Google Scholar] [CrossRef]
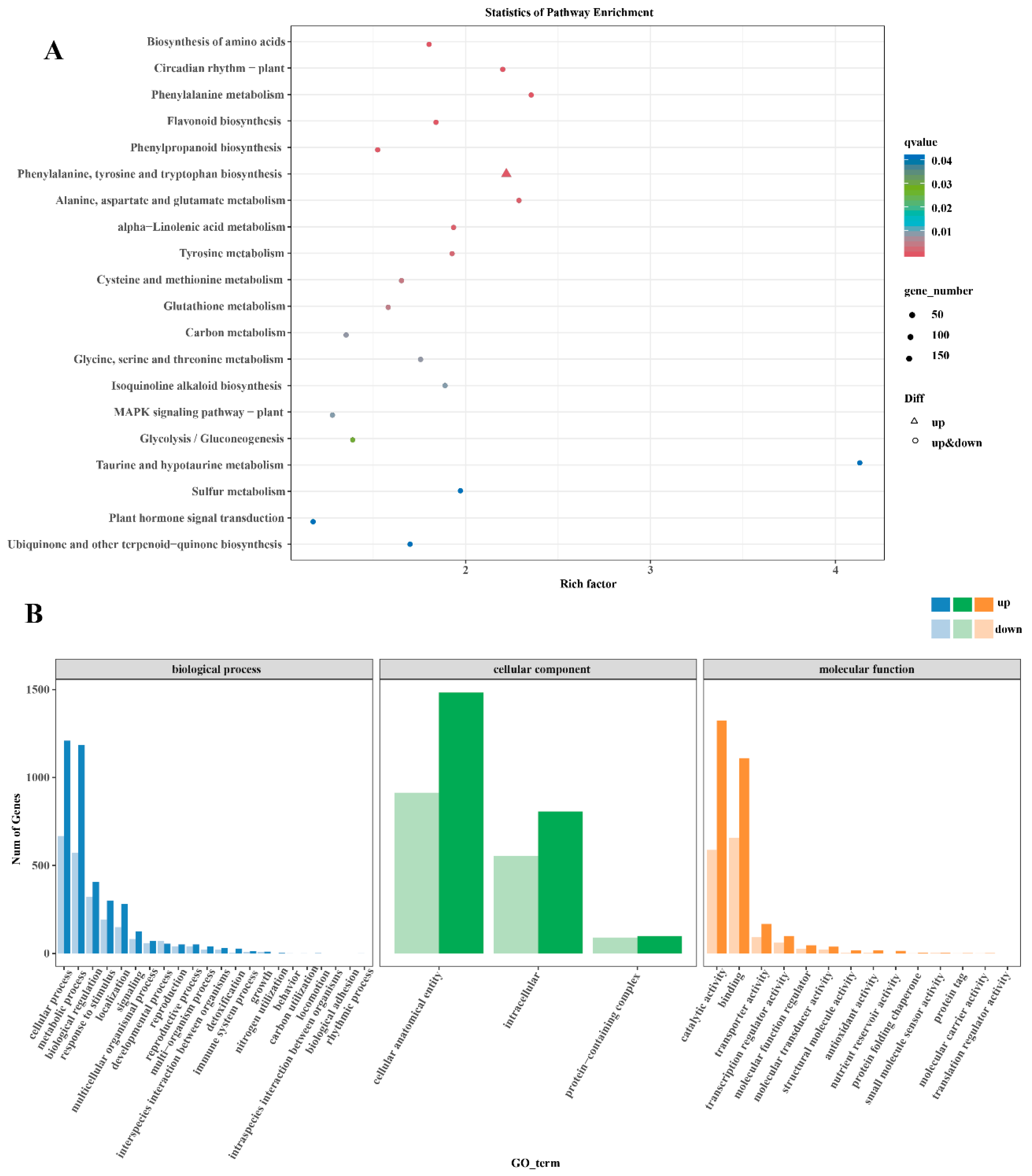


| Types of Participation | Gene ID | Gene | Log2FC | Annotated Gene Function |
|---|---|---|---|---|
| Fatty acid elongation | VIT_18s0001g07640 | KCR1 | −1.37 | Very-long-chain 3-oxoacyl-CoA reductase |
| VIT_04s0008g02250 | FDH | −3.36 | 3-ketoacyl-CoA synthase | |
| VIT_07s0141g00060 | KCS20 | 7.32 | 3-ketoacyl-CoA synthase | |
| VIT_07s0141g00090 | KCS20 | −4.12 | 3-ketoacyl-CoA synthase | |
| VIT_11s0016g04700 | KCS11 | 2.42 | 3-ketoacyl-CoA synthase | |
| VIT_13s0019g01260 | ECR | −1.14 | Very-long-chain enoyl-CoA reductase | |
| VIT_13s0067g03890 | KCS19 | −3.08 | 3-ketoacyl-CoA synthase | |
| VIT_14s0006g02990 | CUT1 | −2.29 | 3-ketoacyl-CoA synthase | |
| VIT_15s0048g02720 | KCS1 | −1.86 | 3-ketoacyl-CoA synthase | |
| VIT_18s0001g12550 | KCS4 | −1.64 | 3-ketoacyl-CoA synthase | |
| VIT_06s0004g04000 | KCS12 | −2.97 | 3-ketoacyl-CoA synthase | |
| Fatty acid synthesis | VIT_00s0233g00060 | KAS3A | 1.16 | 3-oxoacyl-[acyl-carrier-protein] synthase |
| VIT_00s2731g00010 | FATA2 | −1.21 | Oleoyl-acyl carrier protein thioesterase | |
| VIT_03s0063g01880 | LACS2 | −4.39 | Long chain acyl-CoA synthetase | |
| VIT_05s0020g03080 | LACS6 | 1.02 | Long chain acyl-CoA synthetase | |
| VIT_08s0007g07520 | At3g03980 | 4.51 | NADPH-dependent aldehyde reductase-like protein | |
| VIT_08s0007g07530 | At3g03980 | 1.05 | NADPH-dependent aldehyde reductase-like protein | |
| VIT_08s0040g01190 | CLKR27 | 1.65 | 3-oxoacyl-[acyl-carrier-protein] reductase | |
| VIT_08s0040g01200 | CLKR27 | 3.67 | 3-oxoacyl-[acyl-carrier-protein] reductase | |
| VIT_09s0002g04170 | AAE13 | 1.82 | Malonate-CoA ligase | |
| VIT_11s0016g04480 | FATB | 1.31 | Palmitoyl-acyl carrier protein thioesterase | |
| VIT_14s0128g00720 | LACS7 | −1.03 | Long chain acyl-CoA synthetase | |
| VIT_17s0000g01090 | FATB | −8.09 | Palmitoyl-acyl carrier protein thioesterase | |
| VIT_18s0001g04980 | ACC1 | 3.47 | Acetyl-CoA carboxylase | |
| Synthesis of cuticle and wax | VIT_11s0037g01210 | CER3 | 3.14 | Very-long-chain aldehyde decarbonylase |
| NewGene_1726 | MAH1 | −1.02 | Alkane hydroxylase | |
| VIT_00s0207g00010 | AT10 | −3.22 | Acyl transferase | |
| VIT_02s0025g03320 | CYP86A22 | −4.41 | Cytochrome | |
| VIT_05s0020g05040 | PIIF | 1.67 | Wound-induced proteinase inhibitor | |
| VIT_06s0009g03630 | CYP94A1 | −1.25 | Cytochrome | |
| VIT_07s0141g00890 | CYP94A1 | 6.62 | Cytochrome | |
| VIT_07s0141g00920 | CYP94A1 | 4.52 | Cytochrome | |
| VIT_15s0046g02380 | CYP86A2 | 1.27 | Cytochrome | |
| VIT_15s0046g00480 | WSD1 | −1.59 | O-acyltransferase | |
| VIT_15s0046g00520 | WSD1 | −6.13 | O-acyltransferase | |
| VIT_15s0046g00590 | WSD1 | −8.08 | O-acyltransferase | |
| Lipid transport | NewGene_222 | LTPG1 | −1.70 | Non-specific lipid-transfer protein |
| VIT_14s0108g00520 | LTP1 | 1.74 | Non-specific lipid-transfer protein | |
| VIT_06s0061g00230 | ABCG15 | −2.65 | ABC transporter G family member | |
| VIT_09s0002g03550 | ABCG35 | 3.10 | ABC transporter G family member | |
| VIT_09s0002g03560 | ABCG42 | 2.99 | ABC transporter G family member | |
| VIT_09s0002g03570 | ABCG29 | 3.06 | ABC transporter G family member | |
| VIT_11s0016g04540 | ABCG32 | −1.44 | ABC transporter G family member | |
| VIT_13s0019g04600 | ABCG28 | 1.14 | ABC transporter G family member | |
| VIT_13s0067g03750 | ABCG1 | 3.67 | ABC transporter G family member | |
| VIT_16s0039g00010 | ABCG11 | −1.85 | ABC transporter G family member | |
| VIT_07s0005g02430 | ABC1 | 1.01 | Protein ABC transporter |
| TFs ID | TFs | Log2FC | Annotated TF Function |
|---|---|---|---|
| VIT_03s0038g02310 | MYB308 | −1.02 | transcription factor |
| VIT_11s0016g02070 | bHLH41 | 4.96 | transcription factor |
| VIT_05s0049g00500 | ERF98 | 6.83 | ethylene-responsive transcription factor |
| VIT_08s0058g00690 | WRKY24 | 4.31 | transcription factor |
| VIT_19s0014g01680 | TCP4 | −2.16 | transcription factor |
| VIT_03s0038g04450 | bZIP53 | 1.85 | bZIP transcription factor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lv, Y.; Han, T.; Liu, Y.; Jiang, Y. Post-Harvest Quality Changes and Molecular Responses of Epidermal Wax in ‘Munage’ Grapes with Botrytis cinerea Infection. Int. J. Mol. Sci. 2025, 26, 3468. https://doi.org/10.3390/ijms26083468
Wang Y, Lv Y, Han T, Liu Y, Jiang Y. Post-Harvest Quality Changes and Molecular Responses of Epidermal Wax in ‘Munage’ Grapes with Botrytis cinerea Infection. International Journal of Molecular Sciences. 2025; 26(8):3468. https://doi.org/10.3390/ijms26083468
Chicago/Turabian StyleWang, Yu, Yunhao Lv, Tong Han, Yidong Liu, and Ying Jiang. 2025. "Post-Harvest Quality Changes and Molecular Responses of Epidermal Wax in ‘Munage’ Grapes with Botrytis cinerea Infection" International Journal of Molecular Sciences 26, no. 8: 3468. https://doi.org/10.3390/ijms26083468
APA StyleWang, Y., Lv, Y., Han, T., Liu, Y., & Jiang, Y. (2025). Post-Harvest Quality Changes and Molecular Responses of Epidermal Wax in ‘Munage’ Grapes with Botrytis cinerea Infection. International Journal of Molecular Sciences, 26(8), 3468. https://doi.org/10.3390/ijms26083468





