Abstract
Pathogenic GRN variants that reduce progranulin (PGRN) levels cause frontotemporal dementia (FTD). To facilitate model development, we generated induced pluripotent stem cells (iPSCs) from dermal fibroblasts of two family members carrying the GRN c.1009C>T (p.Q337X) pathogenic variant—one symptomatic and one asymptomatic—as well as a non-carrier first-degree relative serving as a genetically matched control. The obtained iPSC lines were validated for pluripotency markers (Nanog, Sox2, Oct4, and TRA1-1-81), genomic integrity, and differentiation potential. The obtained iPSC lines were subsequently directed toward neuroepithelial stem (NES) cells. NES identity was confirmed by the expression of lineage-specific markers, including Nestin and Sox2 (assessed by immunocytochemistry), as well as SOX1, PLAGL1, and MKI67 (evaluated by real-time PCR). Furthermore, GRN mRNA levels were significantly reduced in iPSC and NES lines derived from mutation carriers compared to control cells. The established iPSC and NES cell lines represent a platform for modeling progranulin-deficient FTD. The symptomatic and asymptomatic carrier-derived lines obtained from the same family offer a unique opportunity to study disease progression across clinical phases. The control line, derived from a related (first-degree) non-carrier, minimizes genetic background variability. Their utility of the established cell lines extends to therapeutic drug screening and further differentiation into neuronal, non-neuronal, and organoid models.
1. Introduction
Heterozygous mutations in the GRN gene lead to reduced levels of progranulin (PGRN), causing frontotemporal dementia (FTD) characterized by frontotemporal lobar degeneration (FTLD) with TAR DNA-binding protein 43 (TDP-43) inclusions [,]. On the other hand, very rare homozygous GRN mutations lead to neuronal ceroid lipofuscinosis type 11 (NCL11)—a lysosomal storage disorder with onset in young adulthood, as reviewed by [].
Frontotemporal lobar degeneration is the second most common form of early-onset dementia, typically affecting individuals under the age of 65 []. Heterozygous GRN mutations are found in approximately 13.9% of FTD patients []. These loss-of-function mutations lead to haploinsufficiency of progranulin, a protein involved in lysosomal function, neuronal survival, and neuroinflammation, as reviewed by [].
One such pathogenic variant, rs1598364961 (c.1009C>T, p.Q337X, NM_002087.2), localizes to exon 9 of the GRN gene, which consists of 13 exons. This variant is listed as pathogenic in multiple databases, including ClinVar, and has been repeatedly identified in familial FTD cases since its initial description in 2007 [,,,]. In our previous work, we have characterized skin fibroblast lines derived from a symptomatic patient with familial FTD, carrying the p.Q337X mutation, alongside samples from much younger first-degree relatives: one asymptomatic also harboring the mutation, and a non-carrier serving as a control []. p.Q337X introduces a premature termination codon (PTC), triggering degradation of the aberrant transcript via nonsense-mediated mRNA decay (NMD) and leading to progranulin (PGRN) protein haploinsufficiency and lower mRNA in the carriers’ fibroblasts [].
The current study builds upon these findings by generating and characterizing induced pluripotent stem cell (iPSC) lines and neuroepithelial stem cells (NES) from this family. To our knowledge, no patient-based models with the p.Q337X mutation have been developed so far []. We aim to establish a cellular model system to compare the effects of progranulin deficiency across disease stages (symptomatic vs. asymptomatic), while maintaining similar genetic backgrounds, as all three subjects are first-degree relatives.
2. Results
2.1. Characterization of iPSC Lines from a Family with GRN c.1009C>T (p.Q337X) Pathogenic Variant, and a Healthy First-Degree Relative
We generated iPSC lines from previously characterized dermal fibroblast lines [] derived from a family comprising two members carrying GRN c.1009C>T (p.Q337X) pathogenic variant, a symptomatic one and an asymptomatic one, and a non-carrier first-degree relative. The patient designated as P1 was diagnosed with familial FTD; the subject P2 (two decades younger) remained asymptomatic (as of 2025). The ages of the subjects have been omitted to protect personal confidential information.
The obtained iPSC lines fulfilled the criteria listed in Table 1 and illustrated in Figure 1, Figure 2 and Figure 3.

Table 1.
Characterization of the generated iPSC lines.
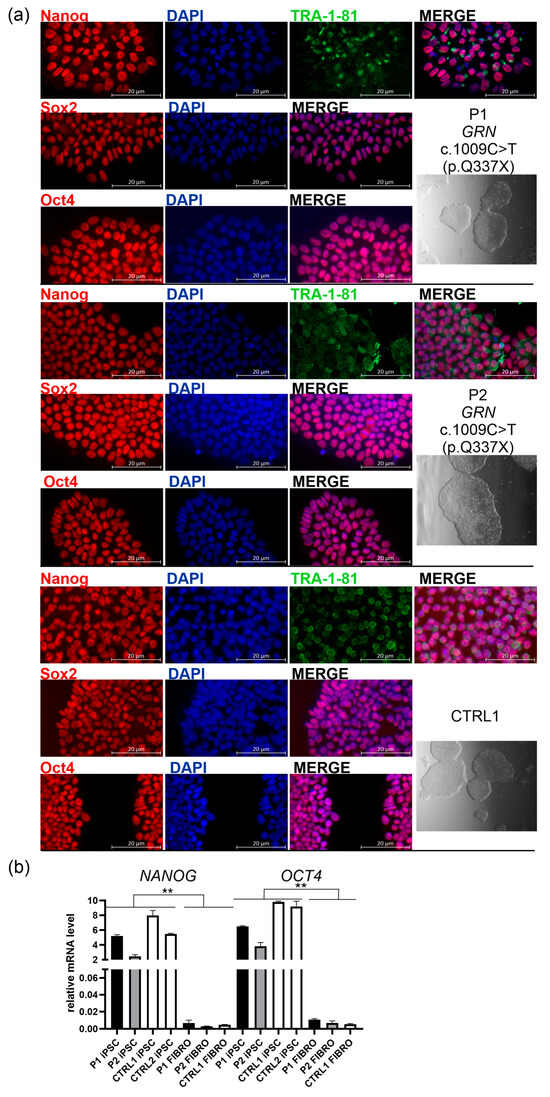
Figure 1.
Characterization of induced pluripotent stem cell (iPSC) lines derived from two carriers of the pathogenic GRN variant c.1009C>T (p.Q337X): a symptomatic patient (P1), an asymptomatic carrier (P2), and a non-carrier first-degree relative (CTRL1); (a) right panel: all iPSC lines form colonies with well-defined boundaries, as visualized using Leica Integrated Modulation Contrast (IMC); greyscale images are shown below the corresponding cell line names; left panels: all iPSC lines showed positive immunostaining for pluripotency markers: Nanog or Sox2 or Oct4 (red), and TRA1-1-81 (green), and DAPI (blue). The figure displays split and merged fluorescence images acquired from either two (blue and red) or three channels: blue (DAPI), green (Alexa Fluor 488-conjugated secondary antibody), and red (Alexa Fluor 555-conjugated secondary antibody). Scale bar: 20 μm. The corresponding larger views, from which (a) images were cropped, are presented in Figure S1 in the Supplementary Materials; (b) All iPSC lines: P1, P2, CTRL1 (non-carrier first-degree relative), CTRL2 (non-carrier unrelated control) express pluripotency markers, NANOG and OCT4, compared to respective fibroblast cells designated as P1 FIBRO, P2 FIBRO, and CTRL1 FIBRO; p was calculated in two-tailed t-test, ** p < 0.001.
The established lines formed colonies with the typical morphology of iPSCs, characterized by well-defined boundaries as visualized by Leica Integrated Modulation Contrast (IMC) (Figure 1a, right panel: the greyscale image below the line name). These colonies expressed key pluripotency markers, including Nanog, Sox2, Oct4, and TRA-1-81 (Figure 1a, left panels; the corresponding larger views, from which the highlighted rectangles were cropped, are presented in Figure S1). TRA-1-81 is an epitope on the Podocalyxin-like protein 1 (PODXL) expressed on the surface of pluripotent stem cells. The high expression of NANOG and OCT4, core pluripotency transcription factors, maintaining the undifferentiated state and self-renewal capacity of iPSC, was further validated at the mRNA level and contrasted with over 1000-fold lower expression of these markers in the corresponding fibroblast lines derived from P1, P2, and CTRL1 (Figure 1b, Table 1).
To assess the purity of the obtained iPSC lines (P1, P2, and CTRL1), we quantified Nanog- and DAPI-positive nuclei in fluorescence images acquired using a Zeiss Axioexaminer.Z1 microscope (Carl Zeiss Microscopy GmbH, Oberkochen, Germany). More than 18K cells were analyzed per line confirming the high purity of our colonies (see Table 2).

Table 2.
Purity of the obtained iPSC lines P1, P2, and CTRL1 based on quantification of Nanog- and DAPI-positive nuclei.
To verify the differentiation potential of the obtained iPSC lines into the three germ layers, the cells were subjected to in vitro embryoid body (EB) formation, followed by analysis of ectodermal, endodermal, and mesodermal markers (Figure 2, Table 1, Figure S2 in the Supplementary Materials provides the corresponding split channels for detailed visualization). For ectoderm, β3-Tubulin (TUJ1) and Nestin were used. To better visualize the borders between the embryonic germ layers a co-staining using markers specific to both endo/ectodermal or meso/ectodermal lineages was used (Figure 2a). Endodermal marker Sox17 (SRY-Box Transcription Factor 17) was co-visualized with ectodermal marker Nestin, DAPI stained the nucleus. Among the endodermal markers, SOX17 is considered the most specific and reliable marker for definitive endoderm in embryoid bodies derived from iPSCs. SOX17 is expressed during the earliest stages of definitive endoderm formation and is not typically found in mesodermal or ectodermal lineages []. We also performed staining with NCAM1 (Neural Cell Adhesion Molecule 1) that is expressed in both neuroectoderm and paraxial mesoderm []. NCAM1 was visualized with ectodermal marker MAP2 (Microtubule-Associated Protein 2) to show the distinction between these two germ layers. Staining with CXCR4 (C-X-C chemokine receptor type 4) in EBs was performed to identify mesendodermal or cardiogenic mesoderm populations [] (Figure 2a). CXCR4 was co-visualized with DAPI.
The differentiation potential of P1, P2, and CTRL1 iPSC lines into the three germ layers was assessed in parallel using the STEMdiff ™ Trilineage Differentiation Kit (STEMCELL Technologies, Vancouver, BC, Canada). Unlike the spontaneous and heterogeneous formation of 3D embryoid bodies, which typically requires over 21 days, this approach employs defined media to induce directed differentiation into ectoderm, mesoderm, and endoderm within 5–7 days. To confirm differentiation of P1, P2, and CTRL1 iPSC lines into a homogeneous mesodermal population, CXCR4 staining was performed as recommended in the protocol of the STEMdiff ™ Trilineage Differentiation Kit. CXCR4 staining revealed both membrane-bound and cytoplasmic localization (Supplementary Materials, Figure S3). This pattern is consistent with CXCR4′s role as a membrane-bound G protein-coupled receptor (GPCR) that resides on the plasma membrane and undergoes constitutive endocytosis []. In addition, mRNA analyses showed significantly higher expression of TBXT mRNA, which encodes the T Brachyury transcription factor (a mesodermal marker), and increased expression of FOXA2, which encodes Forkhead Box A2 (an endodermal marker), in embryoid bodies (EBs) derived from P1, P2, and CTRL2, compared to their respective iPSC lines (Figure 2b).
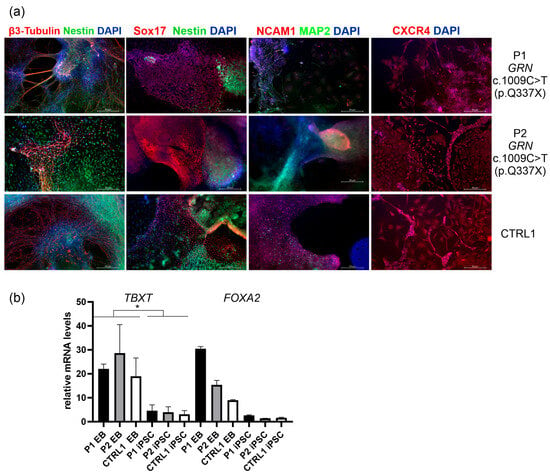
Figure 2.
(a) In vitro differentiation of iPSCs into embryoid bodies (EB) and the analysis of the markers of the three germ layers (ectoderm, mesoderm and endoderm) by immunocytochemistry. For ectoderm, β3-Tubulin (TUJ1) (secondary antibody Alexa Flour 555 red) and Nestin (secondary antibody Alexa Flour 488 green) were used. To better visualize the borders between the embryonic germ layers a co-staining using markers specific to both endo/ectodermal or meso/ectodermal lineages was used. Endodermal marker Sox17 (SRY-Box Transcription Factor 17) was co-visualized with ectodermal marker Nestin (secondary antibody Alexa Flour 488 green), DAPI stained the nucleus (blue). NCAM1 (Neural Cell Adhesion Molecule 1) is expressed in both neuroectoderm and paraxial mesoderm []. NCAM1 (secondary antibody Alexa Flour 555 red) was visualized with ectodermal marker MAP2 (Microtubule-Associated Protein 2; secondary antibody Alexa Flour 488 green). CXCR4 (C-X-C chemokine receptor type 4) identifies mesendodermal or cardiogenic mesoderm populations []. CXCR4 was co-visualized with DAPI. Scale bar: 50 μm. The corresponding split images acquired from different fluorescence channels are provided in Figure S2 in the Supplementary Materials. Original microscopy images are available in a single ZIP archive as Supplementary Materials. (b) mRNA analyses in embryoid bodies (EBs) derived from P1, P2, and CTRL2 revealed significantly higher expression of TBXT mRNA (encoding the T Brachyury transcription factor, a mesodermal marker) and FOXA2 mRNA (encoding Forkhead Box A2, an endodermal marker) compared to their respective iPSC lines. p was calculated in two-tailed t-test, * p < 0.05.
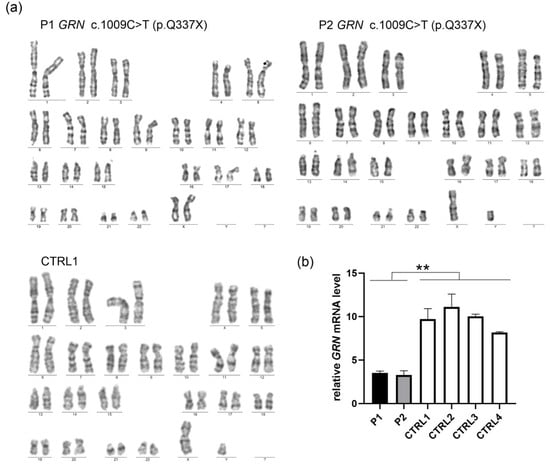
Figure 3.
(a) The established iPSC lines—P1, P2, and CTRL1—exhibited a normal diploid karyotype (b) iPSCs derived from symptomatic (P1) and asymptomatic (P2) carriers of pathogenic GRN variant c.1009C>T (p.Q337X) had decreased mRNA GRN levels, compared to the lines obtained from a non-carrier with wild-type sequence (CTRL1) and other unrelated control iPSCs (CTRL2-CTRL4). p was calculated in a two-tailed t-test, ** p < 0.001.
Mycoplasma contamination was excluded in all iPSC samples (Table 1). No residual expression of Sendai virus-derived reprogramming vectors was detected by real-time PCR analysis, with early-passage iPSCs—still expressing viral sequences—serving as positive controls (Table 1 and Supplementary Materials, Figure S4). Karyotype analysis by G-banding revealed normal chromosomal complements for all samples, with no detectable structural or numerical abnormalities (Figure 3a). Short tandem repeat (STR) profiling verified that the iPSC lines were genetically identical to the original donor fibroblasts, previously characterized by Gaweda-Walerych et al. []. Due to ethical considerations, STR profiling results are not disclosed to protect the personal information of cell line donors.
The presence of the pathogenic variant c.1009C>T was confirmed in iPSC lines derived from mutation carriers (P1 and P2), while the non-carrier line (CTRL1) retained the wild-type sequence, as determined by Sanger sequencing (Figure S5). Furthermore, iPSCs derived from both symptomatic (P1) and asymptomatic (P2) carriers of the GRN pathogenic variant c.1009C>T (p.Q337X) exhibited reduced GRN mRNA levels compared to the non-carrier line derived from a first-degree relative (CTRL1), and other unrelated control iPSCs (CTRL2-CTRL4) (Figure 3b).
2.2. Characterization of Neuroepithelial Stem Cells (NES) Lines from a Symptomatic Patient with GRN c.1009C>T (p.Q337X) Pathogenic Variant, Asymptomatic Carrier (P2) of Pathogenic GRN Variant c.1009C>T (p.Q337X), and a Healthy First-Degree Relative (CTRL1)
We have further generated NES lines from the established iPSC lines (P1, P2, CTRL1, and CTRL2—an unrelated control subject). For neural induction, the dual SMAD inhibition method was adapted using hNoggin (a BMP4 inhibitor), SB431542 (a TGFβ inhibitor), and CHIR99021 (a GSK3β inhibitor), as described before [,].
To confirm successful neural induction from iPSCs, NES cell lines were verified for the expression of protein markers such as Nestin and Sox2 by immunofluorescence (Figure 4a) []. In addition, the expression of other NES markers: SOX1 (an early neural lineage marker indicating commitment to neuroectodermal fate), PLAGL1 (associated with neural stem cell regulation), and MKI67 (proliferation marker) was confirmed by RT-PCR, based on previously published data [,,,,,,] (Figure 4b). For comparison in the RT-PCR analyses, and to highlight differences between the identity states of pluripotency, neuroepithelial stem cells, and terminal neuronal differentiation, we included one neuronal line designated CTRL5 NEU.
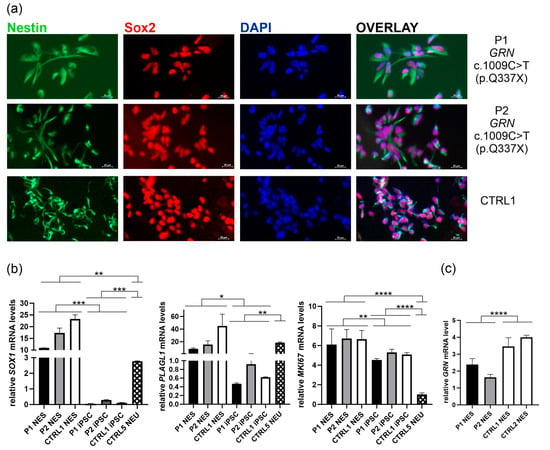
Figure 4.
Characterization of neuroepithelial stem cells (NES) derived from the established iPSCs P1, P2, and CTRL1 (a) All lines express characteristic NES markers such as Nestin (green) and Sox2 (red); nuclei were stained with DAPI (blue); The upper panel: symptomatic patient (P1 carrying pathogenic GRN variant c.1009C>T (p.Q337X); The middle panel: asymptomatic carrier of pathogenic GRN variant c.1009C>T (p.Q337X) (P2); The lower panel: a non-carrier first-degree relative (CTRL1); Scale bar: 20 μm. (b) mRNA expression of NES-related markers (SOX1, PLAGL1, and MKI67) in the obtained NES lines (P1, P2, CTRL1), compared to respective iPSC lines, and a control neuronal line CTRL5 NEU; (c) lower GRN mRNA expression in P1 and P2 NES lines than CTRL1 and CTRL2 NES lines; P was calculated in two-tailed t-test, * p < 0.05, ** p < 0.001, *** p < 0.001, **** p < 0.0001.
To generate this neuronal line, we used iPSC line IIMCBi002-A, derived from an unrelated control subject, which has been previously characterized in detail by Liszewska et al. []. We first used the IIMCBi002-A iPSC line to generate a NES line, which was subsequently differentiated into neuronal line CTRL5 NEU over a 6-week culture period [,] (see Section 4).
The mRNA expression of SOX1, PLAGL1, and MKI67 in NES lines was compared to respective iPSC lines designated as P1 iPSC, P2 iPSC, and CTRL1 iPSC, and neuronal line CTRL5 NEU (Figure 4b). These juxtapositions clearly demonstrated the successful transition from pluripotency through neuroepithelial stem cell identity to terminal neuronal differentiation. As expected, SOX1 mRNA level was the highest in NES, low in neuronal line, and almost absent in iPSCs (Figure 4b, left panel). PLAGL1 expression was also low in iPSCs, while similar levels were observed in NES and neuronal line CTRL5 NEU (Figure 4b, middle panel) []. Consistent with its role in proliferation, MKI67 mRNA expression levels were the highest in NES lines, slightly but significantly lower in iPSCs, and very low in the neuronal line (Figure 4b, right panel).
We also confirmed lower GRN mRNA levels in NES lines derived from carriers of GRN c.1009C>T (p.Q337X) pathogenic variant, compared to the control first-degree relative (CTRL1) and unrelated control NES line (CTRL2) (Figure 4c).
3. Discussion
In this study, we successfully generated iPSC lines from a family with pathogenic GRN variant c.1009C>T (p.Q337X), and further used them to obtain neuroepithelial stem cells. The generated iPSC and NES lines exhibited expression of markers specific to their respective developmental stages (Figure 1, Figure 2 and Figure 4). Moreover, as expected, GRN mRNA levels were markedly reduced in both iPSC and NES lines derived from mutation carriers compared to control cells (Figure 3 and Figure 4), consistent with previous analyses of dermal fibroblast lines from this family [], and published data [].
Immunostaining for four iPSC markers demonstrated nuclear localization of Nanog, Sox2, and Oct4, and membrane staining of TRA-1-81. These findings, together with quantitative analysis of NANOG and OCT4 mRNA levels, confirmed successful reprogramming and maintenance of pluripotency (Figure 1). In addition, microscopic image analysis revealed the high purity (>90%) of the obtained iPSC cultures (Table 2). Embryoid body differentiation further confirmed the ability of the iPSCs to generate derivatives of the three germ layers. Likewise, the results presented in Figure 4b further support the proliferative status of our iPSC lines and distinguish them from NES, based on the mRNA expression profile of MKI67, a marker of cellular proliferation, and SOX1, a neural stem cell-specific marker. However, a limitation of our study is that we did not perform a genome-wide transcriptomic assay, such as PluriTest, which generates a pluripotency score—reflecting the degree of similarity to the transcriptomic signature of pluripotent stem cells based on comparison with extensive reference datasets—and a novelty score, which identifies abnormalities that may compromise oncogenic safety.
To characterize neuroepithelial stem cells, five markers were used: Nestin and Sox2 (immunofluorescence), and SOX1, MKI67, and PLAGL1 (RT-PCR). The results of SOX1, PLAGL1, and MKI67 mRNA expression levels in the obtained NES lines, compared to their respective iPSC lines, and a control neuronal cell line, align with previously published data [,,,,]. Consistent with its role in early neural specification, SOX1 was markedly upregulated in NES and the neuronal line CTRL5 NEU, while only residual expression was detected in iPSC lines (Figure 4b, left panel) [,]. This is in agreement with prior studies identifying SOX1 as a neural stem/progenitor marker activated during early neuroectodermal differentiation [,]. Plag1 has been identified as a regulator of neurogenic potential in mouse neural progenitor cells [,,]. PLAGL1 expression, previously reported to be low in human embryonic stem cells (ESCs) and to increase upon differentiation into cortical neural cells [,], exhibited a similar pattern in our analysis. PLAGL1 levels were low in iPSCs, but significantly elevated in NES and CTRL5 NEU lines (Figure 4b, middle panel), supporting its previously described role in neural lineage specification [].
In line with its function as a proliferation marker, MKI67 mRNA expression was highest in NES lines, moderate in iPSCs (but significantly lower than in NES), and very low in the post-mitotic neuronal line CTRL5 NEU (Figure 4b, right panel). This expression trend reflects the proliferative dynamics of NES cells during lineage commitment and expansion [].
c.1009C>T pathogenic variant present in generated iPSCs and NES lines is considered rare among GRN mutations (between 1–4%) [,]. To date, among a cohort of 97 unrelated patients with frontotemporal lobar degeneration characterized by TAR DNA-binding protein 43-kDa-positive inclusions (FTLD-TDP), fifty distinct GRN mutations have been identified []. Notably, the c.1009C>T pathogenic variant was detected in two individuals in this study [].
To date, neuronal lines have been generated from iPSCs derived from FTD patients with heterozygous and homozygous GRN pathogenic variants, including p.A9D, p. S116X, p.T272SfsX, p.R493X, IVS1 + 5G>C [,,,,], reviewed in [,]. In addition, one organoid model has been developed based on iPSCs from FTD and NCL11 patients bearing the c.900_901dupGT GRN mutation []. Other human organoid models were based on GRN silencing, reviewed in []. To our knowledge, patient-based models with GRN c.1009C>T (p.Q337X) mutation have not been developed so far [].
Taken together, our study provides the first patient-derived iPSC and NES models carrying the rare GRN c.1009C>T (p.Q337X) mutation. These models offer a valuable platform for investigating the molecular and cellular consequences of progranulin haploinsufficiency. Future differentiation into neuronal and glial subtypes, or the development of brain organoids, may reveal mechanisms underlying lysosomal dysfunction, neuroinflammation, and neurodegeneration in FTLD-TDP, thereby advancing our understanding of PGRN deficiency-related pathogenesis and guiding potential therapeutic strategies.
4. Materials and Methods
4.1. Reprogramming Fibroblasts into iPSCs
All cell cultures were maintained in a 37 °C incubator with 5% CO2. Primary skin fibroblasts were cultured as previously described [,]. Induced pluripotent stem cells (iPSCs) were generated by transducing 5 × 105 fibroblasts using the Sendai virus-based CytoTune-iPS 2.0 Reprogramming Kit (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer’s instructions. The Yamanaka factors (OSKM) were delivered at multiplicities of infection (MOI) of 5 (Oct4), 3 (Sox2), and 5 (Klf4/c-Myc). After approximately three weeks following viral transduction, colonies exhibiting typical iPSC morphology started to emerge. Individual colonies were manually picked and expanded in E8 medium (Thermo Fisher Scientific, Gibco, Waltham, MA, USA) on vitronectin-coated plates (Thermo Fisher Scientific). The cells were passaged every 3–4 days using 0.5 mM EDTA (Thermo Fisher Scientific, Invitrogen) with addition of 10 μM Y-27632 ROCK inhibitor (Enzo Biochem Inc., Farmingdale, NY, USA). The resulting iPSCs were validated for the expression of pluripotency markers by immunostaining and RT-PCR. Comprehensive characterization of each iPSC line was performed after passage 15. CTRL2–CTRL4 iPSC lines used in Figure 3b were generated from fibroblasts without GRN mutation derived from control individuals, unrelated to P1, P2, and CTRL1 subjects.
4.2. Real-Time-PCR (RT-PCR) Analysis
RNA was extracted using QIAzol Lysis Reagent (Qiagen, Manchester, UK) according to standard protocol, and subsequently reverse transcribed using the NG dART RT cDNA synthesis kit (EURx Molecular Biology Products, Gdansk, Poland; cat. no. E0801) with a mixture of random hexamer primers and oligo(dT) primers. Quantitative real-time PCR analysis was conducted with RT HS-PCR Mix SYBR (A&A BIOTECHNOLOGY, Gdansk, Poland) using a StepOne Plus system (Applied Biosystems, Foster City, CA, USA). Changes in gene expression were determined with the ∆Ct method using GAPDH levels for normalization, as described previously []. To confirm the elimination of the reprogramming vector, iPSC lines were analyzed for the presence of Sendai virus (SeV) genome, along with pluripotency markers NANOG and OCT4. Embryoid bodies obtained from P1, P2, and CTRL2 were analyzed for TBXT and FOXA2, mesodermal and endodermal markers, respectively. NES lines (P1, P2, CTRL1) were analyzed for the expression of NES-related markers (SOX1, PLAGL1, and MKI67), compared to respective iPSC lines. GRN mRNA levels were analyzed as described previously []. Primers [,,,] are listed in Supplementary Materials in Table S1.
4.3. Karyotype Analysis
Chromosomal analysis was performed in the Institute of Psychiatry and Neurology in Warsaw using G-banding staining. Over twenty metaphases were analyzed for each sample. Cytogenetic analysis and karyotyping were performed with a light microscope Nikon Eclipse 50i (Nikon Corporation, Tokyo, Japan) integrated with LUCIA Cytogenetics Imaging Software ver. 3.1. A detailed description of the procedure is provided in the Supplementary Materials.
4.4. Sanger Sequencing and Authentication of iPSC Lines—Short Tandem Repeat (STR) Profiling
Sanger sequencing was performed on DNA extracted from iPSC lines to confirm the presence/absence of the c.1009C>T pathogenic variant, following previously published protocols []. To confirm that the obtained iPSC lines were genetically identical to the original donor fibroblasts, STR profiling was performed using the PowerPlex® Fusion 6C System (Promega Corporation, Madison, WI, USA), which targets 27 STR loci plus Amelogenin. PCR products were electrophoresed on a 3500 Genetic Analyzer (Life Technologies Corporation, Carlsbad, CA, USA) and analyzed using GeneMapper ID-X software ver. 1.6 (Life Technologies Corporation, Carlsbad, CA, USA). STR profiling was done for the respective fibroblast–iPSC pairs in the Genomics Core Facility, Malopolska Centre of Biotechnology at Jagiellonian University, Gronostajowa 7A street, 30-387 Kraków. 14 loci recommended by the American National Standards Institute were reported in the final STR profiling report.
4.5. Mycoplasma Testing
The cell lines (fibroblasts and iPSCs) were mycoplasma-negative in the PCR Mycoplasma kit (MP Biomedicals LLC, Irvine, CA, USA).
4.6. Embryoid Body Formation
To confirm that the obtained iPSC lines can differentiate into the three germ layers, a modified “Embryoid Body (EB) formation using Essential 6™ Medium protocol” was applied (www.lifetechnologies.com/protocols, last accessed on 1 October 2025). Briefly, iPSCs were detached with EDTA and transferred into 6-well culture plate (Thermo Fisher Scientific, Gibco, Waltham, MA, USA) coated with Anti-Adherence Rinsing Solution (STEMCELL Technologies, catalog # 07010) and cultured in E8 medium with ROCK inhibitor for 24 h. From the second day, E6 medium was used and exchanged every other day. The cells formed three-dimensional floating aggregates, so-called embryoid bodies (EBs). After ten days in culture, the EBs were transferred onto 24-well cell culture plates containing coverslips pre-coated with matrigel (1:250 in E6 medium). The EBs attached to the matrigel surface and differentiated. After 10–14 days, cells were fixed with 4% paraformaldehyde and stained with germ layer-specific markers (Table 1) using an immunofluorescence procedure. RNA extraction from embryoid bodies (EBs) obtained from P1, P2, and CTRL2 was performed using QIAzol Lysis Reagent (Qiagen, Manchester, UK) according to the manufacturer’s protocol.
4.7. Generation of Neuroepithelial Stem Cells (NES) and Neuronal Differentiation
The obtained iPSCs (P1, P2, CTRL1, and CTRL2) were differentiated into neuroepithelial stem cells according to published protocols [,]. A detailed description of the procedure is provided in the Supplementary Materials. NES identity was confirmed by the formation of rosette-like clusters and expression of protein markers such as Nestin and Sox2 was confirmed by immunofluorescence [,]. In addition, mRNA expression of NES markers, such as SOX1, PLAGL1, and MKI67, was analyzed by RT-PCR.
In this study, the iPSC line IIMCBi002-A, previously characterized in detail [], was used. This iPSC line was derived from an unrelated control subject and was kindly provided by Dr. Ewa Liszewska from the Laboratory of Molecular and Cellular Neurobiology at the International Institute of Molecular and Cell Biology in Warsaw, Poland. We have generated NES line from the iPSC IIMCBi002-A line and it was subsequently differentiated into neurons over a 6-week culture period following the withdrawal of EGF and bFGF2, using Neurobasal medium supplemented with B27 (Thermo Fisher Scientific, Gibco) supplemented with Culture One supplement (Thermo Fisher Scientific, Gibco) during first week of differentiation [,]. The obtained neuronal line was designated as CTRL5 NEU, and was used in RT-PCR analysis of NES markers, SOX1, PLAGL1, and MKI67 in Figure 4b.
4.8. Immunofluorescence
Cells were fixed in 4% formaldehyde for 10–15 min, washed three times with PBS, then permeabilized for 5 min. in 0.3% Triton X-100 in PBS, washed three times with PBS, and incubated for 1 h in blocking buffer comprised of PBS with 0.05% Tween-20, and 5% normal goat serum (NGS). Primary antibodies (Table S2) were diluted in antibody dilution buffer consisting of PBS with 5% NGS, 0.05% Tween-20, and 0.02% sodium azide, then added to cells and incubated overnight at 4 °C. After washing three times, cells were incubated with diluted secondary antibodies for 1 h at room temperature in the dark. After washing three more times, nuclei were counterstained with DAPI and the coverslides were mounted on microscopy slides. Immunostained cells were imaged using fluorescent microscope Zeiss Axioexaminer.Z1 (Carl Zeiss Microscopy GmbH, Oberkochen, Germany).
4.9. Assessment of iPSC Line Purity
To evaluate the purity of the obtained iPSC lines, fluorescence images of Nanog- and DAPI-stained nuclei were acquired using a Zeiss Axio Examiner.Z1 microscope and analyzed with Cellpose (version 3.1.1.1) and ImageJ (version 1.54p). A detailed description of the procedure is provided in the Supplementary Materials.
4.10. Statistical Analysis
For RT-PCR experiments, the relative values obtained from different iPSC or NES lines were used to calculate means, standard deviations, and statistical significance. The two-tailed unpaired t-test with Welch’s correction was used (GraphPad Prism 6.0). p < 0.05 was considered significant.
5. Conclusions
The iPSC and NES lines generated from a family with a pathogenic progranulin (GRN) variant c.1009C>T (p.Q337X) offer a valuable resource for studying early molecular changes, identifying therapeutic targets, and developing patient-specific models such as neurons, glia, and brain organoids. Our work contributes to a deeper understanding of PGRN deficiency-linked FTD pathogenesis and supports the development of precision medicine approaches for neurodegenerative diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262311242/s1.
Author Contributions
Conceptualization, K.G.-W. and M.W. (Michalina Wężyk); methodology, M.W. (Michalina Wężyk), K.G.-W., S.G.-Z. and J.S.; software J.S.; validation, M.W. (Michalina Wężyk), K.G.-W., S.G.-Z. and J.S.; formal analysis, K.G.-W., S.G.-Z. and J.S.; investigation, K.G.-W.,A.F., S.G.-Z., N.M., A.C., E.L., M.W. (Michalina Wężyk), M.W. (Marta Woźniak), and J.S.; resources, M.W. (Michalina Wężyk) and K.G.-W.; data curation, K.G.-W., M.W. (Michalina Wężyk), E.L., N.M., A.C., A.F., S.G.-Z., M.W. (Michalina Wężyk) and J.S.; writing—original draft preparation, K.G.-W.; writing—review and editing, K.G.-W., M.W. (Michalina Wężyk), A.F., S.G.-Z., J.S., N.M. and A.C.; visualization, K.G.-W.; supervision, K.G.-W. and M.W. (Michalina Wężyk); project administration, K.G.-W. and M.W. (Michalina Wężyk); funding acquisition, M.W. (Michalina Wężyk). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EU Joint Program—Neurodegenerative Disease Research (EU JPND; JPco-fuND2), project no. 2021/03/Y/NZ5/00112, entitled “Pre-symptomatic synaptic disorders in Alzheimer’s disease”. Project partner in a consortium coordinated by Prof. Christophe Mulle—University of Bordeaux, Partners: Prof. Ann Brinkmalm—Univeristy of Gothenburg, Dr. Matthijs Verhage—CNCR/Vrije Universiteit of Amsterdam, Dr. Gabor Tamas—University of Szeged, Dr. Michalina Wężyk—Mossakowski Medical Research Institute PAS; 1 April 2022–31 March 2026, and Medical Research Agency, ID: 2024/ABM/03/KPO/KPOD.07.07-IW.07-0105/24-00, TITLE “Innovative platform of organoid modelling and therapy testing in human neurologic and oncologic diseases”; 1 January 2025–31 March 2026.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bioethics Committee of the Central Clinical Hospital of the Ministry of Interior Affairs and Administration (currently National Medical Institute of the Ministry of the Interior and Administration) in Warsaw (protocol code 108/2017, date of approval 8 November 2017), and the Bioethical Committee of the Medical University of Gdansk (protocol code NKB.B.N/244/2017, date of approval 29 June 2017) and Bioethics Committee at the Regional Medical Chamber in Gdansk (protocol code KB-G5/2023, date of approval 24 October 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used in this study include STR profiling of human DNA samples, and thus cannot be openly shared due to personal data protection. The age of the subjects have been omitted to protect confidentiality and is available upon request.
Acknowledgments
We thank the participants of the study and the neurologists for the diagnosis. We would like to thank Ewa Liszewska from the Laboratory of Molecular and Cellular Neurobiology at the International Institute of Molecular and Cell Biology in Warsaw, Poland, for kindly providing the iPSC line IIMCBi002-A.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| bFGF | Basic Fibroblast Growth Factor |
| BMP4 | Bone Morphogenetic Protein 4 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| EB | embryoid body |
| EGF | Epidermal Growth Factor |
| FOXA2 | Forkhead Box A2 |
| FTD | frontotemporal dementia |
| FTLD | frontotemporal lobar degeneration |
| FTLD-TDP | frontotemporal lobar degeneration with TDP-43–positive inclusions |
| GRN | Progranulin gene |
| GPCR | G protein-coupled receptor |
| GSK3β | Glycogen Synthase Kinase 3 Beta |
| hNoggin | Human Noggin |
| IMC | Integrated Modulation Contrast |
| iPSCs | induced pluripotent stem cells |
| KOSR | KnockOut Serum Replacement |
| MOI | multiplicity of infection |
| MAP2 | Microtubule-Associated Protein 2 |
| MKI67 | Marker Of Proliferation Ki-67 |
| Nanog | Nanog Homeobox |
| NCAM1 | Neural Cell Adhesion Molecule 1 |
| NCL11 | Neuronal Ceroid Lipofuscinosis type 11 |
| NES | neuroepithelial stem cells |
| NGS | normal goat serum |
| NMD | nonsense-mediated mRNA decay |
| Oct4 | Octamer-Binding Protein 4 |
| OSKM | Oct4, Sox2, Klf4, cMyc |
| PLAGL1 | PLAG1 Like Zinc Finger 1 |
| PGRN | progranulin |
| PODXL | Podocalyxin-like protein 1 |
| PTC | premature termination codon |
| RT-PCR | Real-time PCR |
| SOX1 | SRY-Box Transcription Factor 1 |
| Sox2 | SRY-Box Transcription Factor 2 |
| Sox17 | SRY-Box Transcription Factor 17 |
| STR | Short tandem repeat |
| TBXT | T Brachyury Transcription Factor, T-Box Transcription Factor T |
| TDP-43 | TAR DNA-binding protein 43 |
| TGFβ | Transforming Growth Factor-beta |
References
- Baker, M.; Mackenzie, I.R.; Pickering-Brown, S.M.; Gass, J.; Rademakers, R.; Lindholm, C.; Snowden, J.; Adamson, J.; Sadovnick, A.D.; Rollinson, S.; et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006, 442, 916–919. [Google Scholar] [CrossRef]
- Cruts, M.; Gijselinck, I.; van der Zee, J.; Engelborghs, S.; Wils, H.; Pirici, D.; Rademakers, R.; Vandenberghe, R.; Dermaut, B.; Martin, J.J.; et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006, 442, 920–924. [Google Scholar] [CrossRef]
- Gaweda-Walerych, K.; Aragona, V.; Lodato, S.; Sitek, E.J.; Narozanska, E.; Buratti, E. Progranulin deficiency in the brain: The interplay between neuronal and non-neuronal cells. Transl. Neurodegener. 2025, 14, 18. [Google Scholar] [CrossRef]
- Nuytemans, K.; Franzen, S.; Broce, I.J.; Caramelli, P.; Ellajosyula, R.; Finger, E.; Gupta, V.; Illan-Gala, I.; Loi, S.M.; Morhardt, D.; et al. Gaps in biomedical research in frontotemporal dementia: A call for diversity and disparities focused research. Alzheimers Dement. 2024, 20, 9014–9036. [Google Scholar] [CrossRef]
- Pottier, C.; Ren, Y.; Perkerson, R.B., 3rd; Baker, M.; Jenkins, G.D.; van Blitterswijk, M.; DeJesus-Hernandez, M.; van Rooij, J.G.J.; Murray, M.E.; Christopher, E.; et al. Genome-wide analyses as part of the international FTLD-TDP whole-genome sequencing consortium reveals novel disease risk factors and increases support for immune dysfunction in FTLD. Acta Neuropathol. 2019, 137, 879–899. [Google Scholar] [CrossRef]
- Van Deerlin, V.M.; Wood, E.M.; Moore, P.; Yuan, W.; Forman, M.S.; Clark, C.M.; Neumann, M.; Kwong, L.K.; Trojanowski, J.Q.; Lee, V.M.; et al. Clinical, genetic, and pathologic characteristics of patients with frontotemporal dementia and progranulin mutations. Arch. Neurol. 2007, 64, 1148–1153. [Google Scholar] [CrossRef]
- Chen-Plotkin, A.S.; Martinez-Lage, M.; Sleiman, P.M.; Hu, W.; Greene, R.; Wood, E.M.; Bing, S.; Grossman, M.; Schellenberg, G.D.; Hatanpaa, K.J.; et al. Genetic and clinical features of progranulin-associated frontotemporal lobar degeneration. Arch. Neurol. 2011, 68, 488–497. [Google Scholar] [CrossRef]
- Yu, C.E.; Bird, T.D.; Bekris, L.M.; Montine, T.J.; Leverenz, J.B.; Steinbart, E.; Galloway, N.M.; Feldman, H.; Woltjer, R.; Miller, C.A.; et al. The spectrum of mutations in progranulin: A collaborative study screening 545 cases of neurodegeneration. Arch. Neurol. 2010, 67, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gijselinck, I.; Van Broeckhoven, C.; Cruts, M. Granulin mutations associated with frontotemporal lobar degeneration and related disorders: An update. Hum. Mutat. 2008, 29, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Gaweda-Walerych, K.; Walerych, D.; Berdynski, M.; Buratti, E.; Zekanowski, C. Parkin Levels Decrease in Fibroblasts With Progranulin (PGRN) Pathogenic Variants and in a Cellular Model of PGRN Deficiency. Front. Mol. Neurosci. 2021, 14, 676478. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Rodriguez, R.T.; Wang, J.; Ghodasara, A.; Kim, S.K. Targeting SOX17 in human embryonic stem cells creates unique strategies for isolating and analyzing developing endoderm. Cell Stem Cell 2011, 8, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.H.; Chakraborty, D.; Vieira, C.P.; Prasain, N.; Li Calzi, S.; Fortmann, S.D.; Hu, P.; Banno, K.; Jamal, M.; Huang, C.; et al. Specific mesoderm subset derived from human pluripotent stem cells ameliorates microvascular pathology in type 2 diabetic mice. Sci. Adv. 2022, 8, eabm5559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Foudi, A.; Geay, J.F.; Berthebaud, M.; Buet, D.; Jarrier, P.; Jalil, A.; Vainchenker, W.; Louache, F. Intracellular localization and constitutive endocytosis of CXCR4 in human CD34+ hematopoietic progenitor cells. Stem Cells 2004, 22, 1015–1029. [Google Scholar] [CrossRef]
- Calvo-Garrido, J.; Winn, D.; Maffezzini, C.; Wedell, A.; Freyer, C.; Falk, A.; Wredenberg, A. Protocol for the derivation, culturing, and differentiation of human iPS-cell-derived neuroepithelial stem cells to study neural differentiation in vitro. STAR Protoc. 2021, 2, 100528. [Google Scholar] [CrossRef]
- Wężyk, M.; Szybinska, A.; Wojsiat, J.; Szczerba, M.; Day, K.; Ronnholm, H.; Kele, M.; Berdynski, M.; Peplonska, B.; Fichna, J.P.; et al. Overactive BRCA1 Affects Presenilin 1 in Induced Pluripotent Stem Cell-Derived Neurons in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 175–202. [Google Scholar] [CrossRef]
- Chung, S.H.; Marzban, H.; Aldinger, K.; Dixit, R.; Millen, K.; Schuurmans, C.; Hawkes, R. Zac1 plays a key role in the development of specific neuronal subsets in the mouse cerebellum. Neural Dev. 2011, 6, 25. [Google Scholar] [CrossRef]
- Sakai, H.; Fujii, Y.; Kuwayama, N.; Kawaji, K.; Gotoh, Y.; Kishi, Y. Plag1 regulates neuronal gene expression and neuronal differentiation of neocortical neural progenitor cells. Genes Cells 2019, 24, 650–666. [Google Scholar] [CrossRef]
- Valente, T.; Junyent, F.; Auladell, C. Zac1 is expressed in progenitor/stem cells of the neuroectoderm and mesoderm during embryogenesis: Differential phenotype of the Zac1-expressing cells during development. Dev. Dyn. 2005, 233, 667–679. [Google Scholar] [CrossRef]
- Rraklli, V.; Sodersten, E.; Nyman, U.; Hagey, D.W.; Holmberg, J. Elevated levels of ZAC1 disrupt neurogenesis and promote rapid in vivo reprogramming. Stem Cell Res. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Varrault, A.; Dantec, C.; Le Digarcher, A.; Chotard, L.; Bilanges, B.; Parrinello, H.; Dubois, E.; Rialle, S.; Severac, D.; Bouschet, T.; et al. Identification of Plagl1/Zac1 binding sites and target genes establishes its role in the regulation of extracellular matrix genes and the imprinted gene network. Nucleic Acids Res. 2017, 45, 10466–10480. [Google Scholar] [CrossRef] [PubMed]
- Liszewska, E.; Majchrowicz, L.; Krogulec, E.; Kotulska, K.; Kaczmarek, L.; Kalita, K.; Dobrzyn, A.; Jaworski, J. Establishment of two hiPSC lines (IIMCBi001-A and IIMCBi002-A) from dermal fibroblasts of healthy donors and characterization of their cell cycle. Stem Cell Res. 2021, 52, 102225. [Google Scholar] [CrossRef]
- Zhang, M.; Ngo, J.; Pirozzi, F.; Sun, Y.P.; Wynshaw-Boris, A. Highly efficient methods to obtain homogeneous dorsal neural progenitor cells from human and mouse embryonic stem cells and induced pluripotent stem cells. Stem Cell Res. Ther. 2018, 9, 67. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, E.C.; Lee, J.Y.; Lee, M.R.; Shim, J.W.; Oh, J.S. Neuronal Cell Differentiation of iPSCs for the Clinical Treatment of Neurological Diseases. Biomedicines 2024, 12, 1350. [Google Scholar] [CrossRef]
- Li, W.; Sun, W.; Zhang, Y.; Wei, W.; Ambasudhan, R.; Xia, P.; Talantova, M.; Lin, T.; Kim, J.; Wang, X.; et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl. Acad. Sci. USA 2011, 108, 8299–8304. [Google Scholar] [CrossRef] [PubMed]
- Venere, M.; Han, Y.G.; Bell, R.; Song, J.S.; Alvarez-Buylla, A.; Blelloch, R. Sox1 marks an activated neural stem/progenitor cell in the hippocampus. Development 2012, 139, 3938–3949. [Google Scholar] [CrossRef] [PubMed]
- Gasperoni, J.G.; Tran, S.C.; Grommen, S.V.H.; De Groef, B.; Dworkin, S. The Role of PLAG1 in Mouse Brain Development and Neurogenesis. Mol. Neurobiol. 2024, 61, 5851–5867. [Google Scholar] [CrossRef]
- Almeida, S.; Zhang, Z.; Coppola, G.; Mao, W.; Futai, K.; Karydas, A.; Geschwind, M.D.; Tartaglia, M.C.; Gao, F.; Gianni, D.; et al. Induced pluripotent stem cell models of progranulin-deficient frontotemporal dementia uncover specific reversible neuronal defects. Cell Rep. 2012, 2, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Xu, Y.F.; Dickey, C.A.; Buratti, E.; Baralle, F.; Bailey, R.; Pickering-Brown, S.; Dickson, D.; Petrucelli, L. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J. Neurosci. 2007, 27, 10530–10534. [Google Scholar] [CrossRef]
- Raitano, S.; Ordovas, L.; De Muynck, L.; Guo, W.; Espuny-Camacho, I.; Geraerts, M.; Khurana, S.; Vanuytsel, K.; Toth, B.I.; Voets, T.; et al. Restoration of progranulin expression rescues cortical neuron generation in an induced pluripotent stem cell model of frontotemporal dementia. Stem Cell Rep. 2015, 4, 16–24. [Google Scholar] [CrossRef]
- Lee, C.W.; Stankowski, J.N.; Chew, J.; Cook, C.N.; Lam, Y.W.; Almeida, S.; Carlomagno, Y.; Lau, K.F.; Prudencio, M.; Gao, F.B.; et al. The lysosomal protein cathepsin L is a progranulin protease. Mol. Neurodegener. 2017, 12, 55. [Google Scholar] [CrossRef]
- Valdez, C.; Wong, Y.C.; Schwake, M.; Bu, G.; Wszolek, Z.K.; Krainc, D. Progranulin-mediated deficiency of cathepsin D results in FTD and NCL-like phenotypes in neurons derived from FTD patients. Hum. Mol. Genet. 2017, 26, 4861–4872. [Google Scholar] [CrossRef]
- Lines, G.; Casey, J.M.; Preza, E.; Wray, S. Modelling frontotemporal dementia using patient-derived induced pluripotent stem cells. Mol. Cell Neurosci. 2020, 109, 103553. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.R.; Martins, S.; Cammarata, G.; Martins, M.; Cardoso, A.M.; Almeida, M.R.; do Carmo Macario, M.; Santana, I.; Peca, J.; Cardoso, A.L. Generation and Characterization of Novel iPSC Lines from a Portuguese Family Bearing Heterozygous and Homozygous GRN Mutations. Biomedicines 2022, 10, 1905. [Google Scholar] [CrossRef]
- Gaweda-Walerych, K.; Sitek, E.J.; Narozanska, E.; Wężyk, M.; Brockhuis, B.; Zekanowski, C.; Slawek, J. Functional characterization of a novel progranulin mutation in a patient with progressive nonfluent aphasia. Neurobiol. Aging 2018, 72, 186.e9–186.e12. [Google Scholar] [CrossRef] [PubMed]
- Gaweda-Walerych, K.; Mohagheghi, F.; Zekanowski, C.; Buratti, E. Parkinson’s disease-related gene variants influence pre-mRNA splicing processes. Neurobiol. Aging 2016, 47, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Vanderheijden, C.; Yakkioui, Y.; Vaessen, T.; Santegoeds, R.; Temel, Y.; Hoogland, G.; Hovinga, K. Developmental gene expression in skull-base chordomas and chondrosarcomas. J. Neurooncol. 2025, 172, 249–256. [Google Scholar] [CrossRef]
- Falk, A.; Koch, P.; Kesavan, J.; Takashima, Y.; Ladewig, J.; Alexander, M.; Wiskow, O.; Tailor, J.; Trotter, M.; Pollard, S.; et al. Capture of neuroepithelial-like stem cells from pluripotent stem cells provides a versatile system for in vitro production of human neurons. PLoS ONE 2012, 7, e29597. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).