Preclinical Assessment in Transgenic NOD Mice of a Novel Immunotherapy for Type 1 Diabetes: Lipoplexes Down-Modulate the Murine C1858T Ptpn22 Variant In Vitro
Abstract
1. Introduction
2. Results
2.1. Transfection of the R619W-Ptpn22 Variant in the L929 Mouse Fibroblast Cell Line
2.2. siRNAs Are Delivered in the R619W-L929 Cell Line by a Commercial Transfection System and Efficiently Block Variant Ptpn22 Expression
2.3. Efficient Silencing of Variant Ptpn22 in R619W-L929 Cells by siRNA Delivery Using Lipoplexes
3. Discussion
4. Materials and Methods
4.1. siRNA Design
4.2. Liposome/Lipoplex Preparation
4.3. Generation of R619W-Transfected L929 Murine Fibroblast Cell Line
4.4. L929 Cell Culture
4.5. Mutant Ptpn22-Gene Silencing in a Transfected L929 Fibroblast Line (R619W-L929)
4.5.1. siRNA Transfection Using a Commercial Transfection System
4.5.2. Custom Liposome Transfection Protocol of R619W-L929 Fibroblast Line
4.5.3. qRT-PCR
- R619W forward (R619W-forward): 5′-TCCCCTCCGAATAGTGCTGA-3′;
- R619W reverse (R619W-reverse): 5′-CATTCAGGGAGTGGCGG-3′.
- GAPDH forward (GAPDH-FW): 5′-ATTCAACGGCACAGTCAAGG-3′;
- GAPDH reverse (GAPDH-RV): 5′-ATCATAAACATGGGGGCATC-3′.
4.6. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ptpn22 | Protein tyrosine phosphatase N22 |
| Lyp | Lymphoid phosphatase |
| NOD | Non-Obese Diabetic mice |
| siRNA | Small interfering RNA |
| T1D | Type 1 Diabetes |
| ATD | Autoimmune Thyroid Disease |
| Ct | Cycle Threshold |
| APS3v | Autoimmune Polyglandular Syndrome type 3 variant |
| L-T4 | Levothyroxine |
| Arg | Arginine |
| Trp | Tryptophan |
| SAM | Siglec 10 sialoside mimetic |
| CRISPR-Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) associated protein 9 (Cas9) |
| RISC | RNA-Induced Silencing Complex |
| s/a | sense/antisense |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| DOPE | Phospholipids [1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine |
| DOTAP | N-[1-(2,3-Dioleoyloxy) propyl]-N, N, N-trimethylammonium chloride |
| DSPE-PEG2000-Maleimide | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- [maleimide (polyethylene glycol)-2000] (ammonium salt) |
| Min. | Minutes |
| ATCC | American Type Culture Collection |
| DMEM HG | Dulbecco’s Modified Eagle Medium High Glucose |
| % | Percentage |
| OCT | Optimal cutting temperature |
| IVIS | In vivo imaging system |
References
- Redondo, M.J.; Morgan, N.G. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2023, 9, 542–554. [Google Scholar] [CrossRef]
- Dittmar, M.; Kahaly, G.J. Genetics of the autoimmune polyglandular syndrome type 3 variant. Thyroid 2010, 20, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Johnson, R.K.; Stene, L.C. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020, 8, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Rapini, N.; Schiaffini, R.; Fierabracci, A. Immunotherapeutic Strategies for the Prevention and Treatment of Distinct Stages of Type 1 Diabetes: An Overview. Int. J. Mol. Sci. 2020, 21, 2103. [Google Scholar] [CrossRef]
- Woittiez, N.J.C.; Roep, B.O. Impact of disease heterogeneity on treatment efficacy of immunotherapy in Type 1 diabetes: Different shades of gray. Immunotherapy 2015, 7, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Palombi, M.; Fierabracci, A. The putative role of the C1858T polymorphism of protein tyrosine phosphatase PTPN22 gene in autoimmunity. Autoimmun. Rev. 2013, 12, 717–725. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Wu, P.-D.; Zhang, Y.-L.; Fang, P.-F.; Wu, F.-F.; He, X.-F. The Association between PTPN22 SNPs and Susceptibility to Type 1 Diabetes: An Updated Meta-Analysis. PLoS ONE 2025, 20, e0321624. [Google Scholar] [CrossRef]
- Žak, R.; Navasardyan, L.; Hunák, J.; Martinů, J.; Heneberg, P. PTPN22 intron polymorphism rs1310182 (c.2054-852T>C) is associated with type 1 diabetes mellitus in patients of Armenian descent. PLoS ONE 2023, 18, e0286743. [Google Scholar] [CrossRef]
- Fichna, M.; Małecki, P.P.; Żurawek, M.; Furman, K.; Gębarski, B.; Fichna, P.; Ruchała, M. Genetic variants and risk of endocrine autoimmunity in relatives of patients with Addison’s disease. Endocr. Connect. 2023, 12, e230008. [Google Scholar] [CrossRef]
- Pasha, U.; Nisar, H.; Nisar, H.; Abid, R.; Ashraf, N.M.; Sadaf, S. Molecular dynamic simulations unravel the underlying impact of missense mutation in autoimmunity gene PTPN22 on predisposition to rheumatoid arthritis. J. Interferon Cytokine Res. 2023, 43, 121–132. [Google Scholar] [CrossRef]
- Vang, T.; Congia, M.; Macis, M.D.; Musumeci, L.; Orrú, V.; Zavattari, P.; Nika, K.; Tautz, L.; Taskén, K.; Cucca, F.; et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat. Genet. 2005, 37, 1317–1319. [Google Scholar] [CrossRef]
- Anderson, W.; Barahmand-Pour-Whitman, F.; Linsley, P.S.; Cerosaletti, K.; Buckner, J.H.; Rawlings, D.J. PTPN22 R620W gene editing in T cells enhances low-avidity TCR responses. eLife 2023, 12, e81577. [Google Scholar] [CrossRef]
- Belcastro, E.; Cudini, A.; Mezzani, I.; Petrini, S.; D’Oria, V.; Schiaffini, R.; Scarsella, M.; Russo, A.L.; Fierabracci, A. Effect of the autoimmune-associated genetic variant PTPN22 R620W on neutrophil activation and function in patients with insulin-dependent diabetes mellitus. Front. Immunol. 2025, 16, 1554570. [Google Scholar] [CrossRef]
- Orozco, R.C.; Marquardt, K.; Pratumchai, I.; Shaikh, A.F.; Mowen, K.; Domissy, A.; Sherman, L.A. Autoimmunity-associated allele of tyrosine phosphatase gene PTPN22 enhances anti-viral immunity. PLoS Pathog. 2024, 20, e1012095. [Google Scholar] [CrossRef]
- Bottini, N.; Musumeci, L.; Alonso, A.; Rahmouni, S.; Nika, K.; Rostamkhani, M.; MacMurray, J.; Meloni, G.F.; Lucarelli, P.; Pellacchia, M.; et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat. Genet. 2004, 36, 337–338. [Google Scholar] [CrossRef]
- Perri, V.; Pellegrino, M.; Ceccacci, F.; Scipioni, A.; Petrini, S.; Gianchecchi, E.; Lo Russo, A.; De Santis, S.; Mancini, G.; Fierabracci, A. Use of short interfering RNA delivered by cationic liposomes to enable efficient down-regulation of PTPN22 gene in human T lymphocytes. PLoS ONE 2017, 12, e0175784. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, M.; Ceccacci, F.; Petrini, S.; Scipioni, A.; De Santis, S.; Cappa, M.; Mancini, G.; Fierabracci, A. Exploiting novel tailored immunotherapies of type 1 diabetes: Short interfering RNA delivered by cationic liposomes enables efficient down-regulation of variant PTPN22 gene in T lymphocytes. Nanomedicine 2019, 18, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Belcastro, E.; Accardo, A.; Sandomenico, A.; Pagliarosi, O.; Rosa, E.; Petrini, S.; Conti, L.A.; Giorda, E.; Corsetti, T.; et al. Preparation and In Vitro Evaluation of RITUXfab-Decorated Lipoplexes to Improve Delivery of siRNA Targeting C1858T PTPN22 Variant in B Lymphocytes. Int. J. Mol. Sci. 2021, 23, 408. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Belcastro, E.; Ceccacci, F.; Petrini, S.; Conti, L.A.; Pagliarosi, O.; Giorda, E.; Sennato, S.; Schiaffini, R.; Wang, P.; et al. Improvement of Lipoplexes With a Sialic Acid Mimetic to Target the C1858T PTPN22 Variant for Immunotherapy in Endocrine Autoimmunity. Front. Immunol. 2022, 13, 838331. [Google Scholar] [CrossRef]
- Büll, C.; Heise, T.; Adema, G.J.; Boltje, T.J. Sialic Acid Mimetics to Target the Sialic Acid-Siglec Axis. Trends Biochem. Sci. 2016, 41, 519–531. [Google Scholar] [CrossRef]
- Rillahan, C.D.; Schwartz, E.; McBride, R.; Fokin, V.V.; Paulson, J.C. Click and pick: Identification of sialoside analogues for siglec-based cell targeting. Angew. Chem. Int. Ed. Engl. 2012, 51, 11014–11018. [Google Scholar] [CrossRef]
- Sennato, S.; Paldino, G.; Bombelli, C.; Mezzani, I.; Petrini, S.; Delfino, D.V.; Fiorentino, F.; Marianecci, C.; Ciogli, A.; Rotili, D.; et al. Optimization of Lipoplexes Functionalized with a Sialic Acid Mimetic (F9-PEG) to Target the C1858T PTPN22 Variant for Preclinical Assessment of a Novel Immunotherapy in Endocrine Autoimmunity. Pharmaceutics 2025, 17, 710. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, J.; Silva Lima, B. Regulatory acceptance of animal models of disease to support clinical trials of medicines and advanced therapy medicinal products. Eur. J. Pharmacol. 2015, 759, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bernard, N.F.; Ertug, F.; Margolese, H. High incidence of thyroiditis and anti-thyroid autoantibodies in NOD mice. Diabetes 1992, 41, 40–46. [Google Scholar] [CrossRef]
- Beales, P.E.; Castri, F.; Valiant, A.; Rosignoli, G.; Buckley, L.; Pozzilli, P. Adrenalitis in the non-obese diabetic mouse. Autoimmunity 2002, 35, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Thayer, T.C.; Wen, L.; Wong, F.S. Mouse Models of Autoimmune Diabetes: The Nonobese Diabetic (NOD) Mouse. Methods Mol. Biol. 2020, 2128, 87–92. [Google Scholar] [CrossRef]
- Lin, X.; Pelletier, S.; Gingras, S.; Rigaud, S.; Maine, C.J.; Marquardt, K.; Dai, Y.D.; Sauer, K.; Rodriguez, A.R.; Martin, G.; et al. CRISPR-Cas9-Mediated Modification of the NOD Mouse Genome with Ptpn22R619W Mutation Increases Autoimmune Diabetes. Diabetes 2016, 65, 2134–2138. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Live-cell imaging to compare the transfection and gene silencing efficiency of calcium phosphate nanoparticles and a liposomal transfection agent. Gene Ther. 2017, 24, 282–289. [Google Scholar] [CrossRef]
- Golde, W.; Gollobin, P.; Rodriguez, L. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim. 2005, 34, 39–43. [Google Scholar] [CrossRef]
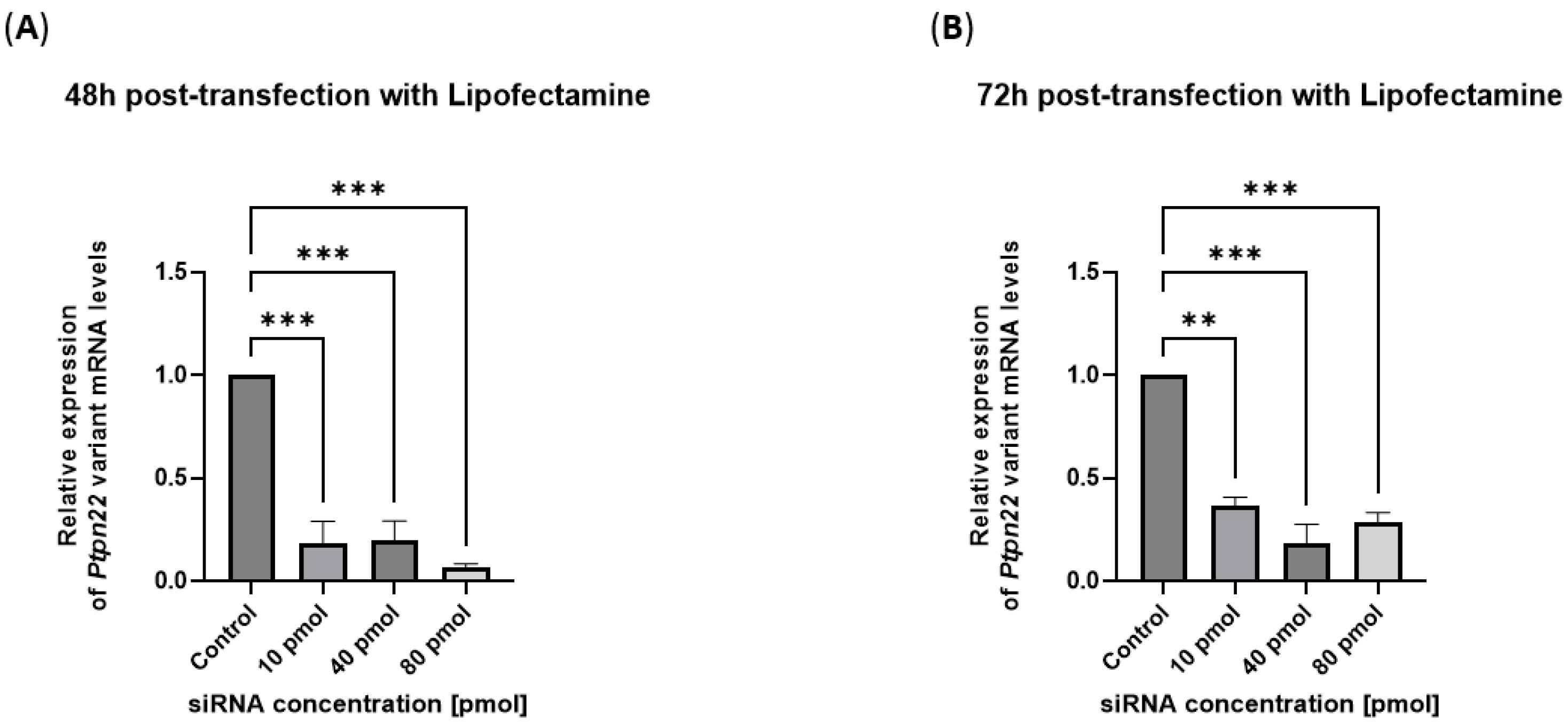
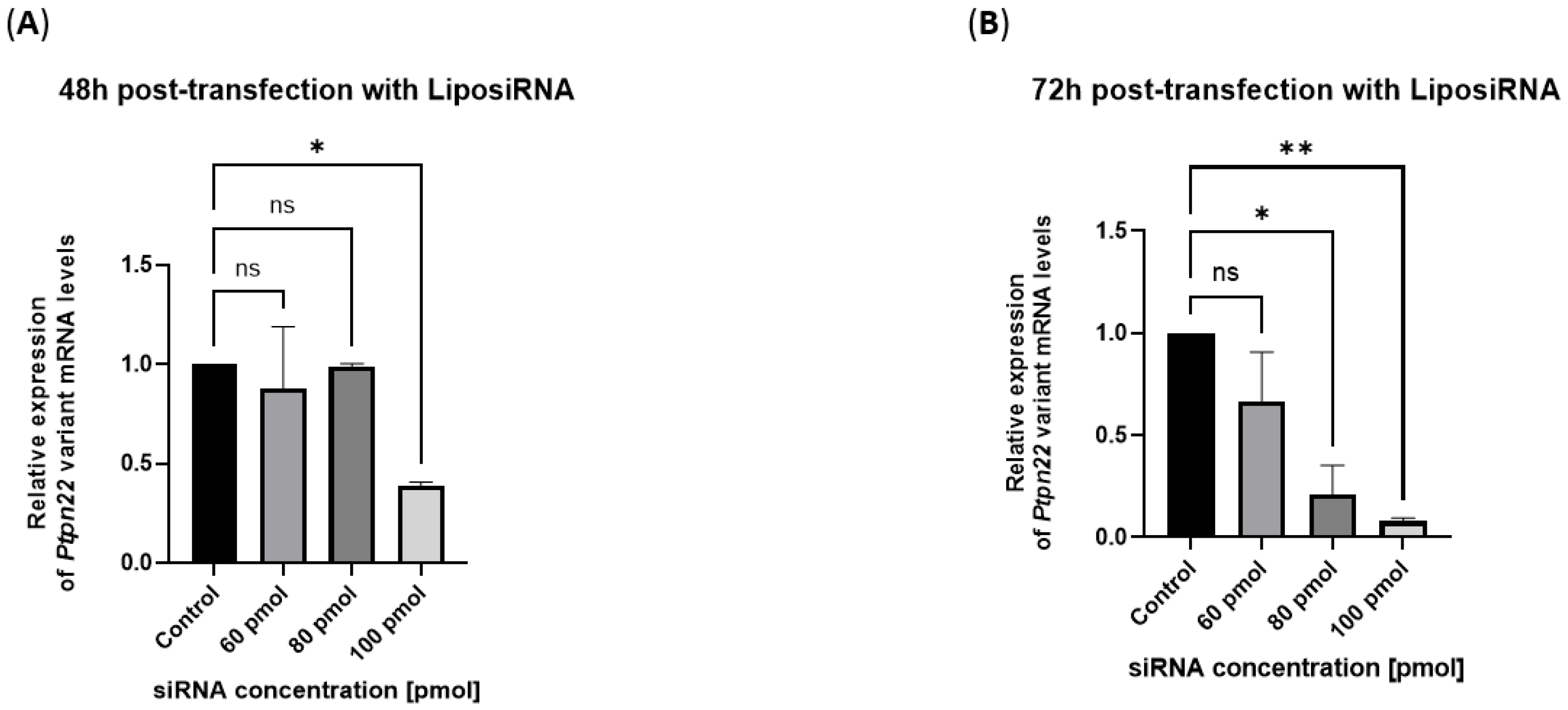
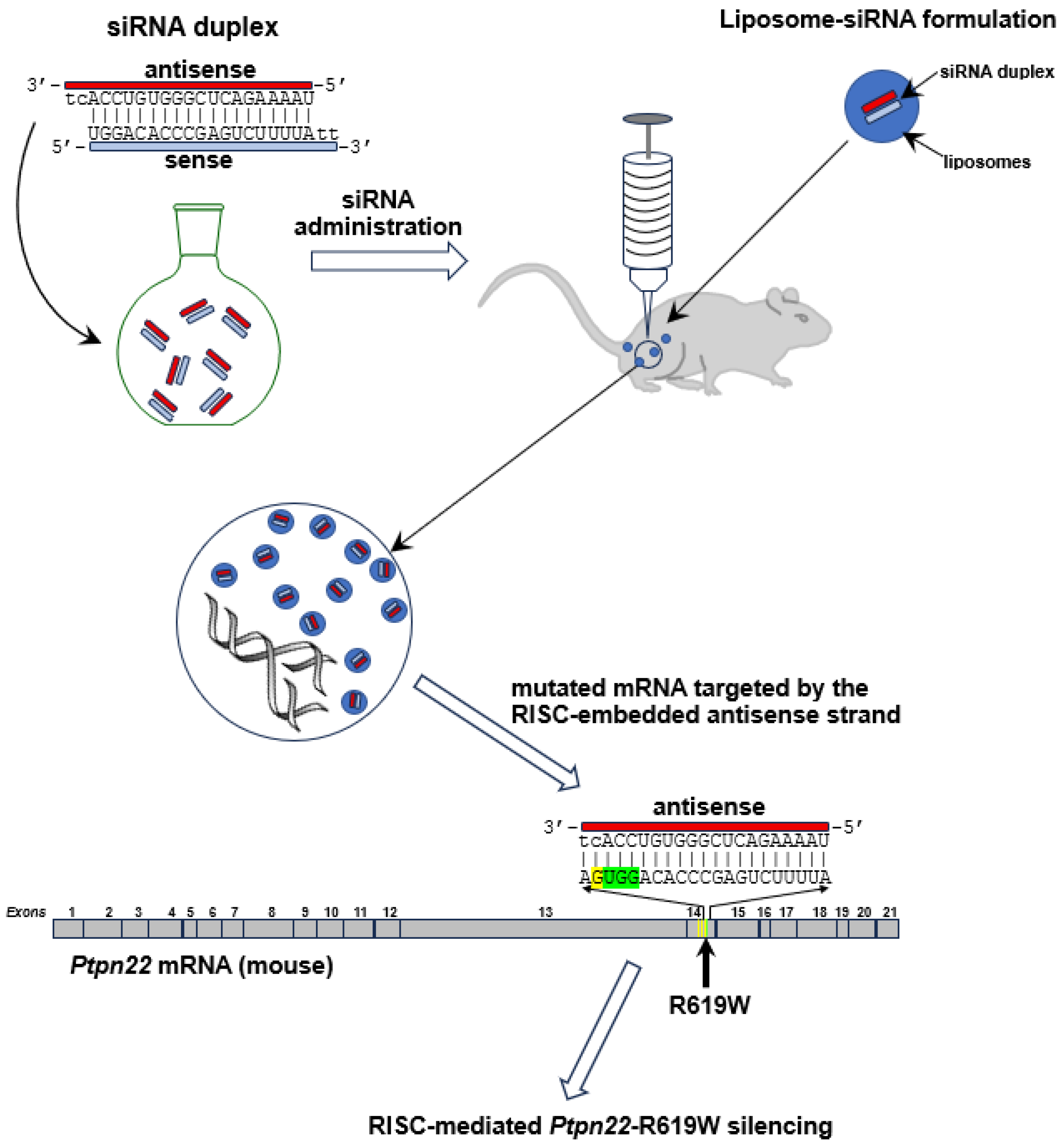
| Sample ID | Ptpn22 Ct | β-Actin Ct | ΔCt | ΔΔCt | Fold Change (2−ΔΔCt) |
|---|---|---|---|---|---|
| L929-EV | 36.86 | 16.99 | −19.60 | 0.00 | 1.00 |
| L929-EV | 36.93 | 17.09 | |||
| L929-EV | 36.98 | 17.35 | |||
| L929-Ptpn22-R619W | 26.43 | 17.10 | −10.46 | −10.46 | 1409.15 |
| L929-Ptpn22-R619W | 26.21 | 17.14 | |||
| L929-Ptpn22-R619W | 26.24 | 17.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzani, I.; Accardo, A.; Bellacchio, E.; Fais, L.; Diaferia, C.; Fierabracci, A. Preclinical Assessment in Transgenic NOD Mice of a Novel Immunotherapy for Type 1 Diabetes: Lipoplexes Down-Modulate the Murine C1858T Ptpn22 Variant In Vitro. Int. J. Mol. Sci. 2025, 26, 11241. https://doi.org/10.3390/ijms262311241
Mezzani I, Accardo A, Bellacchio E, Fais L, Diaferia C, Fierabracci A. Preclinical Assessment in Transgenic NOD Mice of a Novel Immunotherapy for Type 1 Diabetes: Lipoplexes Down-Modulate the Murine C1858T Ptpn22 Variant In Vitro. International Journal of Molecular Sciences. 2025; 26(23):11241. https://doi.org/10.3390/ijms262311241
Chicago/Turabian StyleMezzani, Irene, Antonella Accardo, Emanuele Bellacchio, Luca Fais, Carlo Diaferia, and Alessandra Fierabracci. 2025. "Preclinical Assessment in Transgenic NOD Mice of a Novel Immunotherapy for Type 1 Diabetes: Lipoplexes Down-Modulate the Murine C1858T Ptpn22 Variant In Vitro" International Journal of Molecular Sciences 26, no. 23: 11241. https://doi.org/10.3390/ijms262311241
APA StyleMezzani, I., Accardo, A., Bellacchio, E., Fais, L., Diaferia, C., & Fierabracci, A. (2025). Preclinical Assessment in Transgenic NOD Mice of a Novel Immunotherapy for Type 1 Diabetes: Lipoplexes Down-Modulate the Murine C1858T Ptpn22 Variant In Vitro. International Journal of Molecular Sciences, 26(23), 11241. https://doi.org/10.3390/ijms262311241









