Lithium-Induced Modulation of Proliferation and Apoptosis in an In Vitro Model of Colorectal Cancer
Abstract
1. Introduction
2. Results
2.1. Effect of Lithium Salts on Cell Proliferation
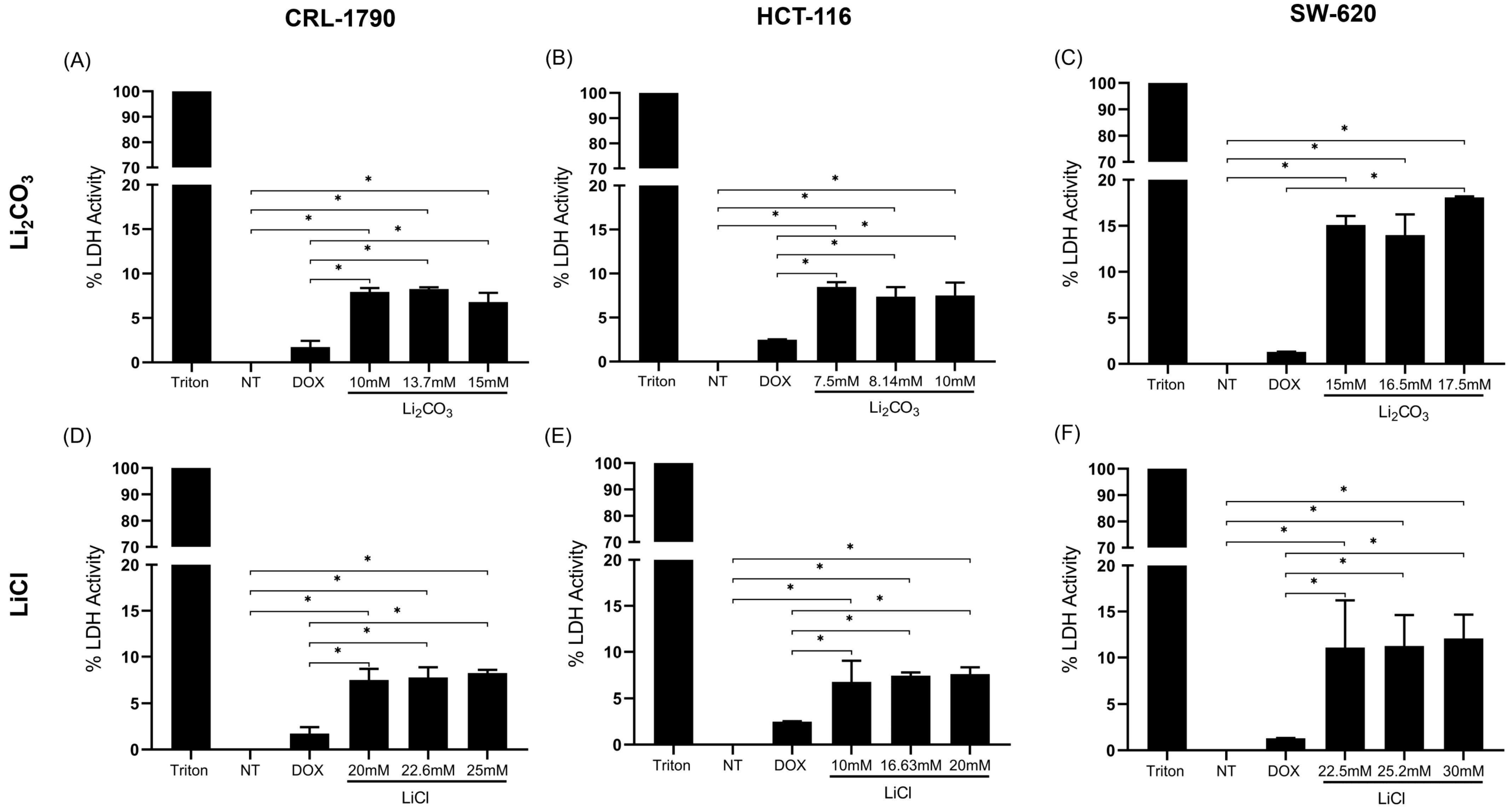
2.2. Effect of Lithium Salts on Cellular Cytotoxicity
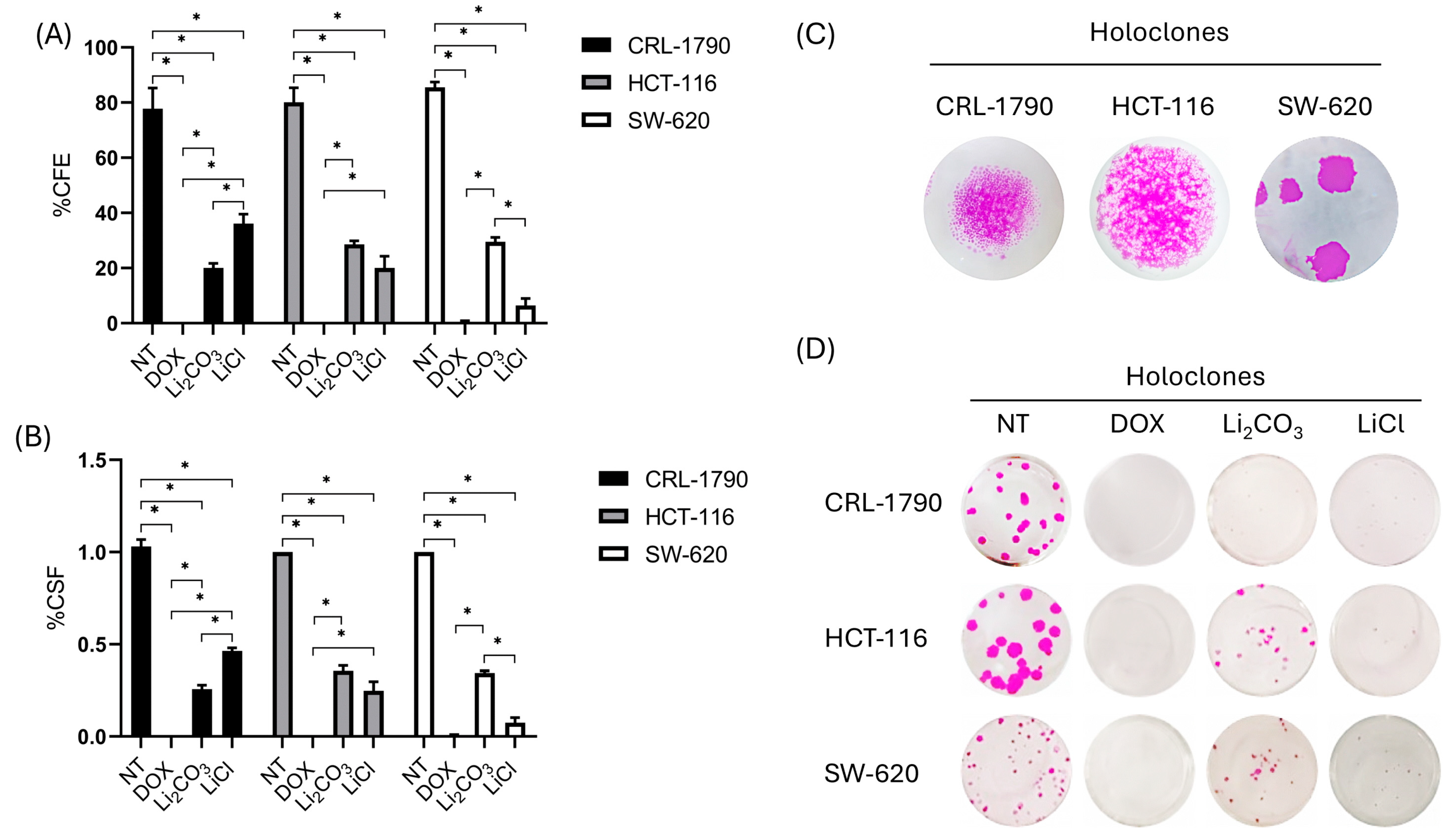
2.3. Long-Term Anti-Proliferative Effects of Lithium Salts
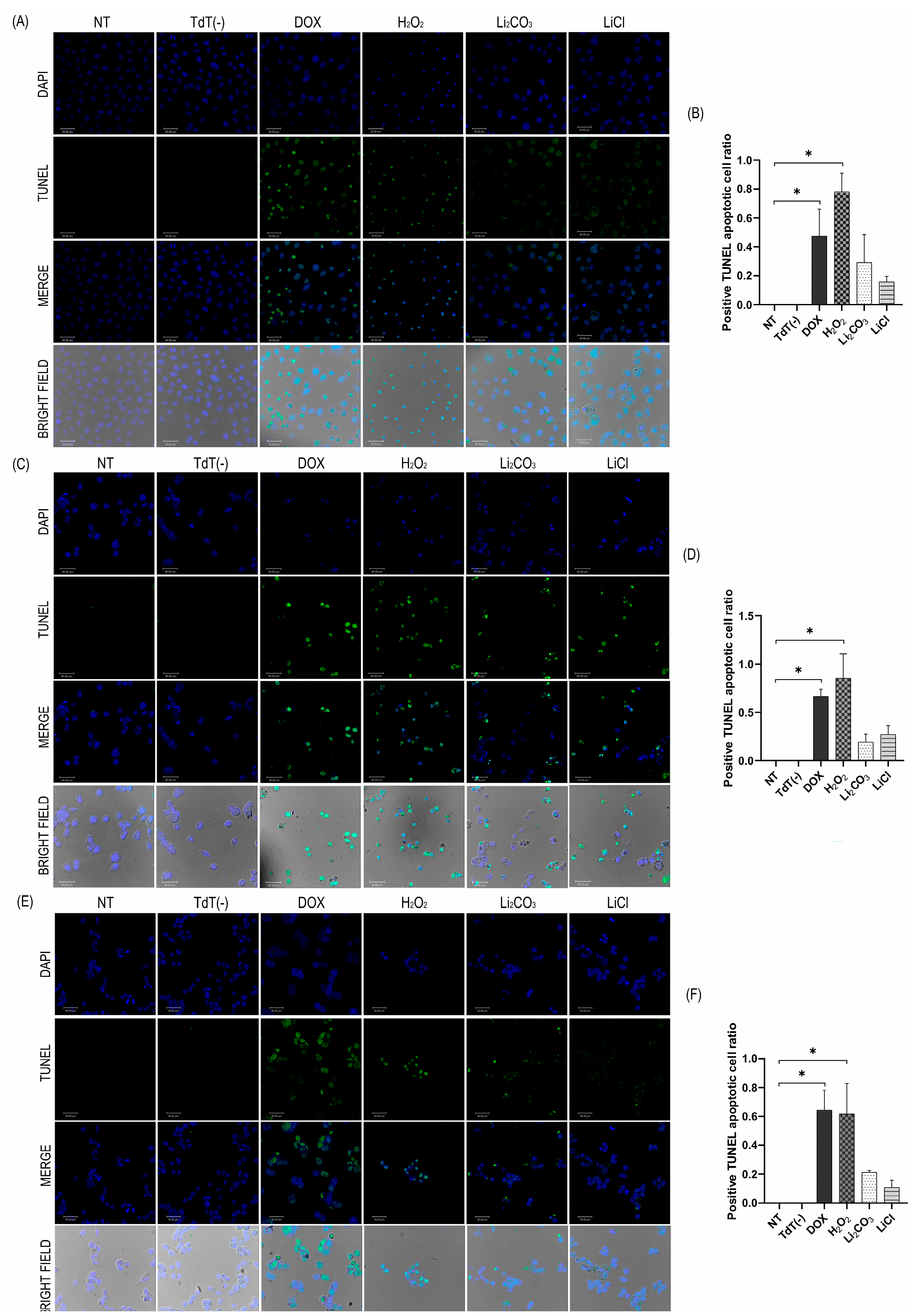
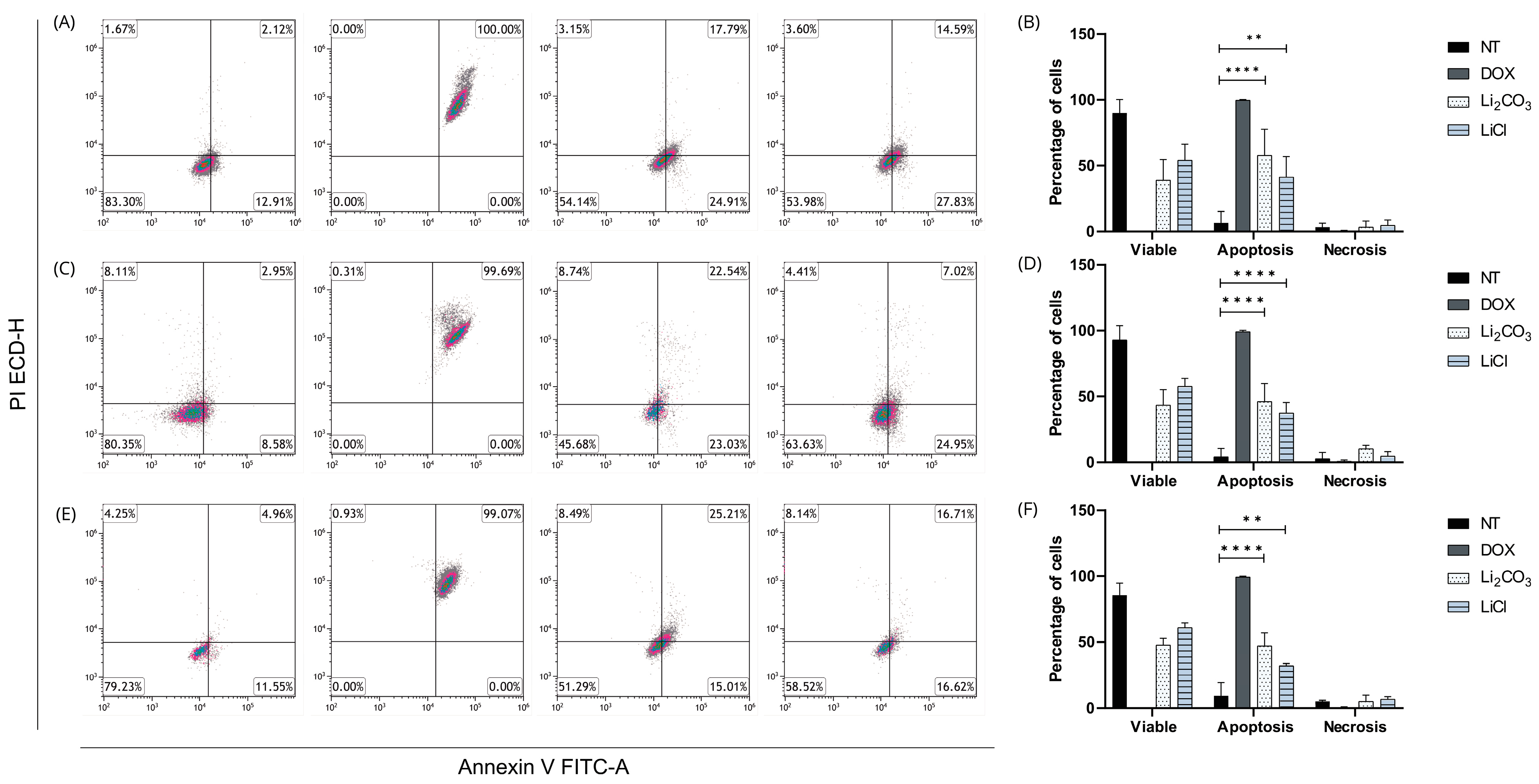
2.4. Apoptotic Effects of Lithium Salts
2.4.1. TUNEL Assay
2.4.2. Annexin V/IP
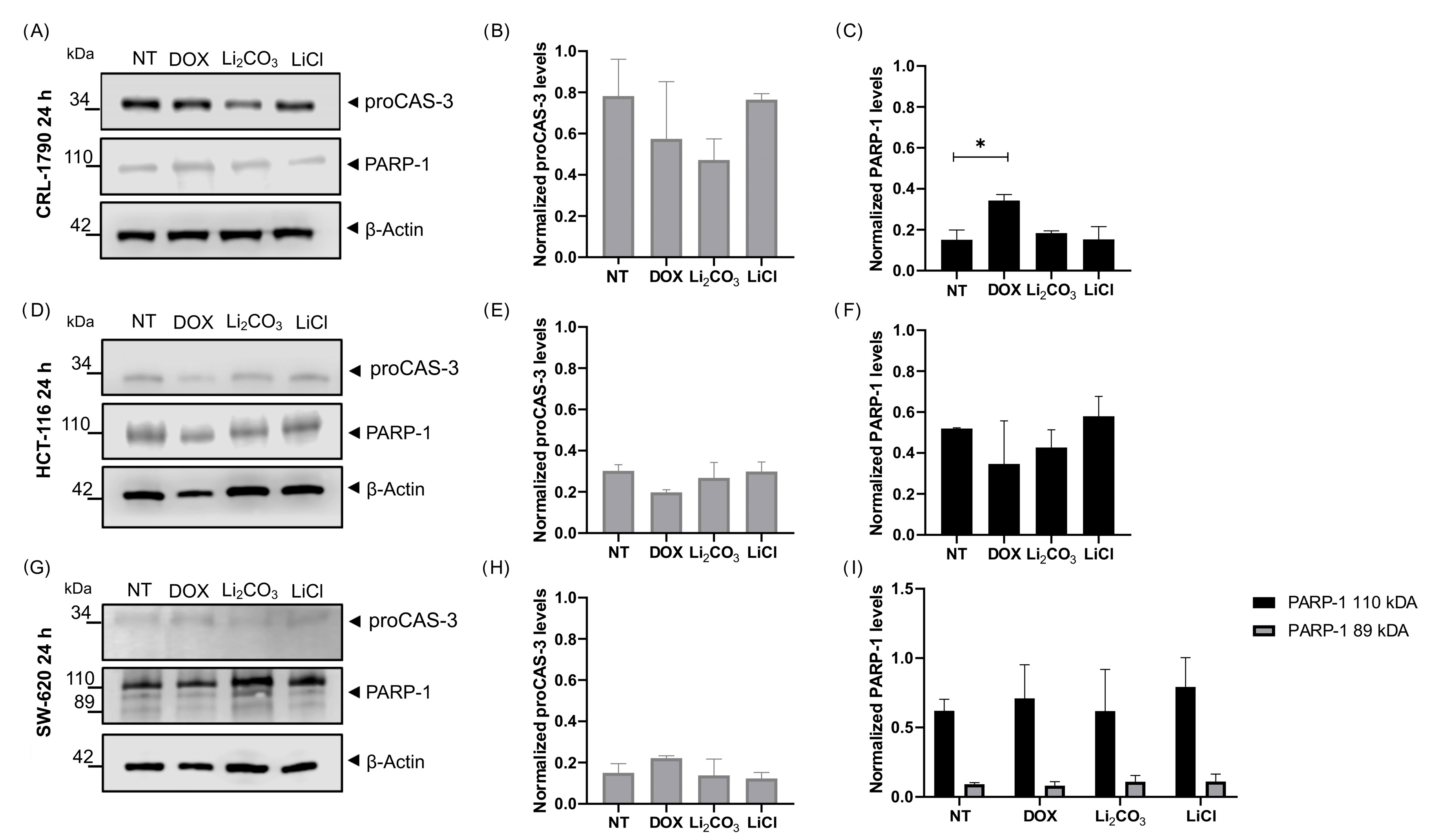
2.4.3. Apoptotic Protein Biomarkers
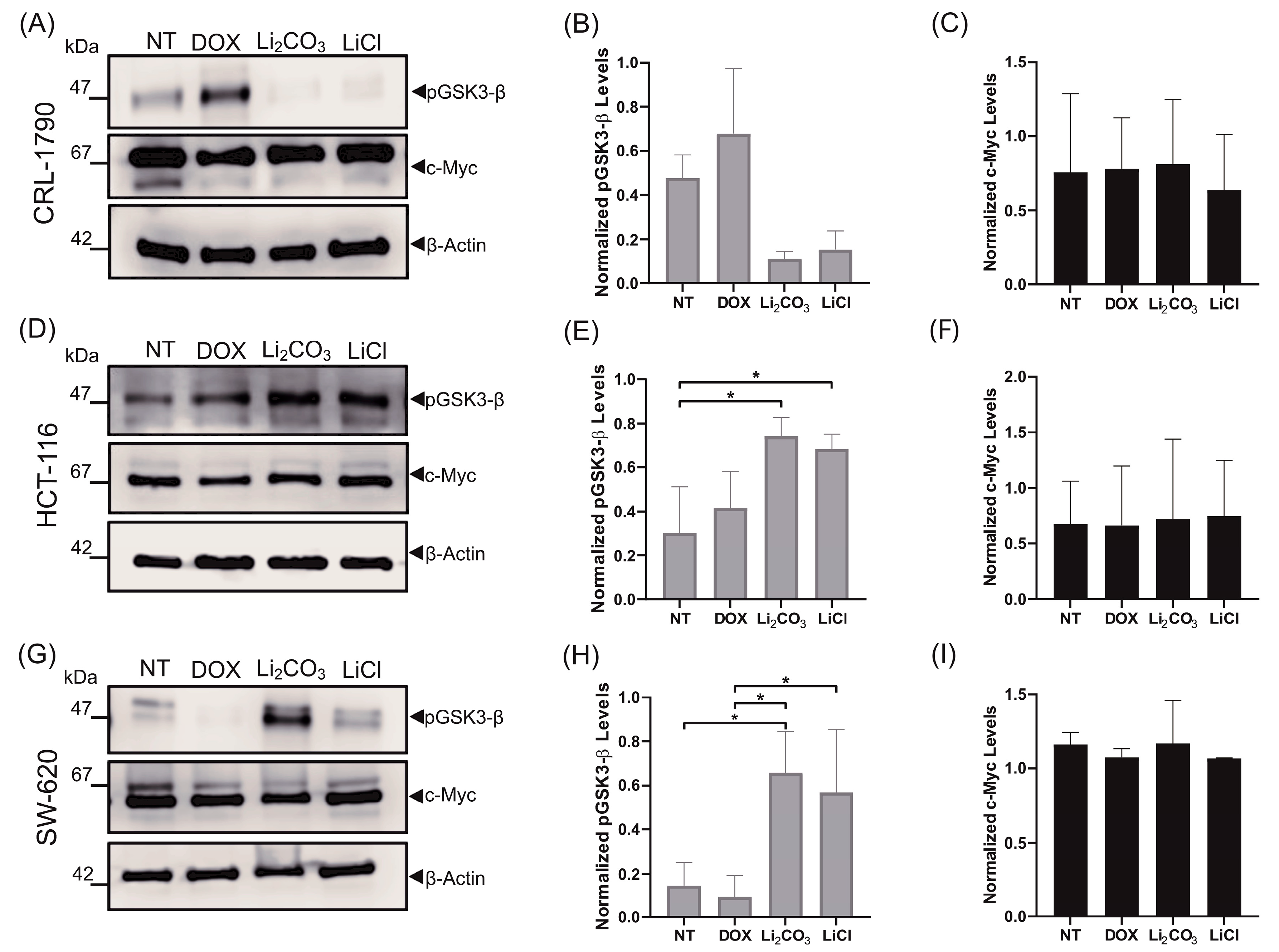
2.5. Effects of Lithium Salts on Proliferation Protein Biomarkers
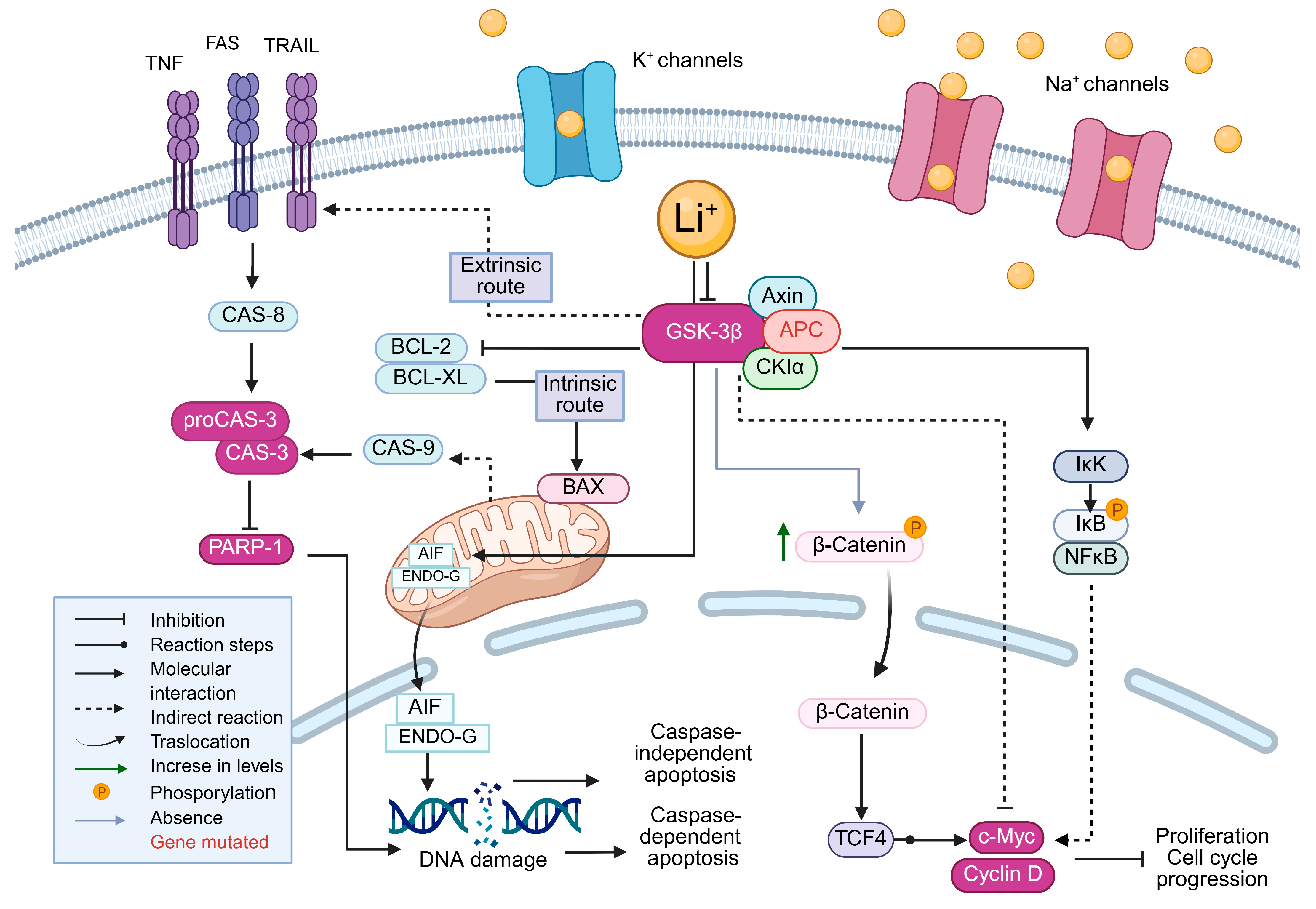
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents
4.3. Cell Viability Assay and IC50 Determination
4.4. Lactate Dehydrogenase (LDH) Release Assay
4.5. Clonogenic Assay
4.6. TUNEL Assay for Detection of Apoptotic DNA Fragmentation
4.7. Annexin V-FITC/PI Flow Cytometry Assay for Apoptosis Detection
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal Cancer |
| Li2CO3 | Lithium Carbonate |
| LiCl | Lithium Chloride |
| CFE | colony-forming efficiency |
| GSK-3β | glycogen synthase kinase-3 beta |
| LDH | lactate dehydrogenase |
| CSF | Clonogenic survival fraction |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP Nick End Labeling |
| ENDOG | endonuclease G |
| AIF | apoptosis-inducing factor |
References
- Fearon, E.R. Molecular Genetics of Colorectal Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 479–507. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jope, R.S.; Johnson, G.V.W. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004, 29, 95–102. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Di, L. Glycogen synthesis and beyond, a comprehensive review of GSK3 as a key regulator of metabolic pathways and a therapeutic target for treating metabolic diseases. Med. Res. Rev. 2022, 42, 946–982. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Shanmugam, I.; Song, J.; Terranova, P.F.; Thrasher, J.B.; Li, B. Lithium suppresses cell proliferation by interrupting E2F–DNA interaction and subsequently reducing S–phase gene expression in prostate cancer. Prostate 2007, 67, 976–988. [Google Scholar] [CrossRef]
- Li, H.; Huang, K.; Liu, X.; Liu, J.; Lu, X.; Tao, K.; Wang, G.; Wang, J. Lithium chloride suppresses colorectal cancer cell survival and proliferation through ROS/GSK-3β/NF-κB signaling pathway. Oxidative Med. Cell. Longev. 2014, 2014, 241864. [Google Scholar] [CrossRef]
- Stamos, J.L.; Weis, W.I. The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-Catenin Signaling and Disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Xue, C.; Chu, Q.; Shi, Q.; Zeng, Y.; Lu, J.; Li, L. Wnt signaling pathways in biology and disease: Mechanisms and therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 106. [Google Scholar] [CrossRef]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a Target of the APC Pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef]
- Chang, S.Y.; Wu, T.H.; Shih, Y.L.; Chen, Y.C.; Su, H.Y.; Chian, C.F.; Lin, Y.-W. SOX1 Functions as a Tumor Suppressor by Repressing HES1 in Lung Cancer. Cancers 2023, 15, 2207. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Riedl, S.J.; Salvesen, G.S. The apoptosome: Signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007, 8, 405–413. [Google Scholar] [CrossRef]
- Khan, H.; Bangar, A.; Grewal, A.K.; Bansal, P.; Singh, T.G. Caspase-mediated regulation of the distinct signaling pathways and mechanisms in neuronal survival. Int. Immunopharmacol. 2022, 110, 108951. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.S.; Melton, D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 1996, 93, 8455–8459. [Google Scholar] [CrossRef]
- He, R.; Du, S.; Lei, T.; Xie, X.; Wang, Y. Glycogen synthase kinase 3β in tumorigenesis and oncotherapy (Review). Oncol. Rep. 2020, 44, 2373–2385. [Google Scholar] [CrossRef]
- Song, P.; Gao, Z.; Bao, Y.; Chen, L.; Huang, Y.; Liu, Y.; Dong, Q.; Wei, X. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J. Hematol. Oncol. 2024, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Novetsky, A.P.; Thompson, D.M.; Zighelboim, I.; Thaker, P.H.; Powell, M.A.; Mutch, D.G.; Goodfellow, P.J. Lithium Chloride and Inhibition of Glycogen Synthase Kinase 3β as a Potential Therapy for Serous Ovarian Cancer. Int. J. Gynecol. Cancer 2013, 23, 361–366. [Google Scholar] [CrossRef] [PubMed]
- García-Acosta, J.C.; Castillo-Montoya, A.I.; Rostro-Alonso, G.O.; Villegas-Vázquez, E.Y.; Quintas-Granados, L.I.; Sánchez-Sánchez, L.; López-Muñóz, H.; Cariño-Calvo, L.; López-Reyes, I.; Bustamante-Montes, L.P.; et al. Unrevealing Lithium Repositioning in the Hallmarks of Cancer: Effects of Lithium Salts (LiCl and Li2CO3) in an In Vitro Cervical Cancer Model. Molecules 2024, 29, 4476. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, T.; Wei, F.; Zhou, Z.; Sun, Y.; Gao, C.; Xu, X.; Zhang, H. Wnt/β-Catenin signaling pathway in hepatocellular carcinoma: Pathogenic role and therapeutic target. Front. Oncol. 2024, 14, 1367364. [Google Scholar] [CrossRef] [PubMed]
- van Staveren, W.C.G.; Solís, D.Y.W.; Hébrant, A.; Detours, V.; Dumont, J.E.; Maenhaut, C. Human cancer cell lines: Experimental models for cancer cells in situ? For cancer stem cells? Biochim. Biophys. Acta (BBA)-Rev. Cancer 2009, 1795, 92–103. [Google Scholar] [CrossRef]
- Ilyas, M.; Tomlinson, I.P.M.; Rowan, A.; Pignatelli, M.; Bodmer, W.F. β-catenin mutations in cell lines established from human colorectal cancers. Proc. Natl. Acad. Sci. USA 1997, 94, 10330–10334. [Google Scholar] [CrossRef]
- Alves, S.; Castro, L.; Fernandes, M.S.; Francisco, R.; Castro, P.; Priault, M.; Chaves, S.R.; Moyer, M.P.; Oliveira, C.; Seruca, R.; et al. Colorectal cancer-related mutant KRAS alleles function as positive regulators of autophagy. Oncotarget 2015, 6, 30787–30802. [Google Scholar] [CrossRef]
- Kaeser, M.D.; Pebernard, S.; Iggo, R.D. Regulation of p53 Stability and Function in HCT116 Colon Cancer Cells. J. Biol. Chem. 2004, 279, 7598–7605. [Google Scholar] [CrossRef]
- Maamer-Azzabi, A.; Ndozangue-Touriguine, O.; Bréard, J. Metastatic SW620 colon cancer cells are primed for death when detached and can be sensitized to anoikis by the BH3-mimetic ABT-737. Cell Death Dis. 2013, 4, e801. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, B.; Zhan, M.; Hua, Z.C. Lithium in Cancer Therapy: Friend or Foe? Cancers 2023, 15, 1095. [Google Scholar] [CrossRef] [PubMed]
- Packiriswamy, N.; Coulson, K.F.; Holcombe, S.J.; Sordillo, L.M. Oxidative stress-induced mitochondrial dysfunction in a normal colon epithelial cell line. World J. Gastroenterol. 2017, 23, 3427–3439. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Taskaeva, I.; Kasatova, A.; Razumov, I.; Bgatova, N.; Taskaev, S. Lithium salts cytotoxicity and accumulation in melanoma cells in vitro. J. Appl. Toxicol. 2024, 44, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Vázquez, E.Y.; Quintas-Granados, L.I.; Cortés, H.; González-Del Carmen, M.; Leyva-Gómez, G.; Rodríguez-Morales, M.; Bustamante-Montes, L.P.; Silva-Adaya, D.; Pérez-Plasencia, C.; Jacobo-Herrera, N.; et al. Lithium: A Promising Anticancer Agent. Life 2023, 13, 537. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; Graveel, C.R.; Zylstra-Diegel, C.R.; Zhong, Z.; Williams, B.O. Wnt/β-catenin Signaling in Normal and Cancer Stem Cells. Cancers 2011, 3, 2050–2079. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Wang, L.; Lee, S. Levistolide A Induces Apoptosis via ROS-Mediated ER Stress Pathway in Colon Cancer Cells. Cell. Physiol. Biochem. 2017, 42, 929–938. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ramushu, P.; Mangoakoane, D.D.; Makola, R.T.; Matsebatlela, T.M. Lithium Induces Oxidative Stress, Apoptotic Cell Death, and G2/M Phase Cell Cycle Arrest in A549 Lung Cancer Cells. Molecules 2025, 30, 1797. [Google Scholar] [CrossRef]
- Quiroz, J.A. Molecular effects of lithium. Mol. Interv. 2004, 4, 259–272. [Google Scholar] [CrossRef]
- Fanali, C. Cancer stem cells in colorectal cancer from pathogenesis to therapy: Controversies and perspectives. World J. Gastroenterol. 2014, 20, 923. [Google Scholar] [CrossRef]
- Baumann, M.; Krause, M.; Hill, R. Clonogens and cancer stem cells. Nat. Rev. Cancer 2008, 8, 990. [Google Scholar] [CrossRef]
- Loh, J.J.; Ma, S. Hallmarks of cancer stemness. Cell Stem Cell 2024, 31, 617–639. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Costabile, V.; Duraturo, F.; Delrio, P.; Rega, D.; Pace, U.; Liccardo, R.; Rossi, G.B.; Genesio, R.; Nitsch, L.; Izzo, P.; et al. Lithium chloride induces mesenchymal-to-epithelial reverting transition in primary colon cancer cell cultures. Int. J. Oncol. 2015, 46, 1913–1923. [Google Scholar] [CrossRef]
- Arnoult, D. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 2003, 22, 4385–4399. [Google Scholar] [CrossRef]
- Luo, Q.; Wu, X.; Zhao, P.; Nan, Y.; Chang, W.; Zhu, X.; Su, D.; Liu, Z. OTUD1 Activates Caspase-Independent and Caspase-Dependent Apoptosis by Promoting AIF Nuclear Translocation and MCL1 Degradation. Adv. Sci. 2021, 8, 2002874. [Google Scholar] [CrossRef]
- Shang, S.; Hua, F.; Hu, Z.W. The regulation of β-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, Y.; Luo, J.; Xu, K.; Tian, P.; Lu, C.; Song, J. Blocking the WNT/β-catenin pathway in cancer treatment:pharmacological targets and drug therapeutic potential. Heliyon 2024, 10, e35989. [Google Scholar] [CrossRef]
- Dang, C.V. MYC on the Path to Cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef]
- Hann, S.R. Role of post-translational modifications in regulating c-Myc proteolysis, transcriptional activity and biological function. Semin. Cancer Biol. 2006, 16, 288–302. [Google Scholar] [CrossRef]
- Daniel, C.J.; Sun, X.X.; Chen, Y.; Zhang, X.; Dai, M.S.; Sears, R.C. Detection of Post-translational Modifications on MYC. In The Myc Gene: Methods and Protocols; Springer: New York, NY, USA, 2021; pp. 69–85. [Google Scholar]
- Maeng, Y.S.; Lee, R.; Lee, B.; Choi SIl Kim, E.K. Lithium inhibits tumor lymphangiogenesis and metastasis through the inhibition of TGFBIp expression in cancer cells. Sci. Rep. 2016, 6, 20739. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, T.R.; Rajendran, S.; O’Reilly, S.; O’Sullivan, G.C.; McKenna, S.L. Lithium Modulates Autophagy in Esophageal and Colorectal Cancer Cells and Enhances the Efficacy of Therapeutic Agents In Vitro and In Vivo. PLoS ONE 2015, 10, e0134676. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, L.; Marinescu, G.; Nazarenko, I.; Thiele, W.; Oberle, C.; Sleeman, J.; Blattner, C. LiCl induces TNF-α and FasL production, thereby stimulating apoptosis in cancer cells. Cell Commun. Signal. 2011, 9, 15. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Q.; Li, H.; Zhao, X.; Zhang, H. LiCl induces apoptosis via CHOP/NOXA/Mcl-1 axis in human choroidal melanoma cells. Cancer Cell Int. 2021, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Lubner, S.J.; Kunnimalaiyaan, M.; Holen, K.D.; Ning, L.; Ndiaye, M.; LoConte, N.K.; Mulkerin, D.L.; Schelman, W.R.; Chen, H. A preclinical and clinical study of lithium in low-grade neuroendocrine tumors. Oncologist 2011, 16, 452–457. [Google Scholar] [CrossRef] [PubMed]
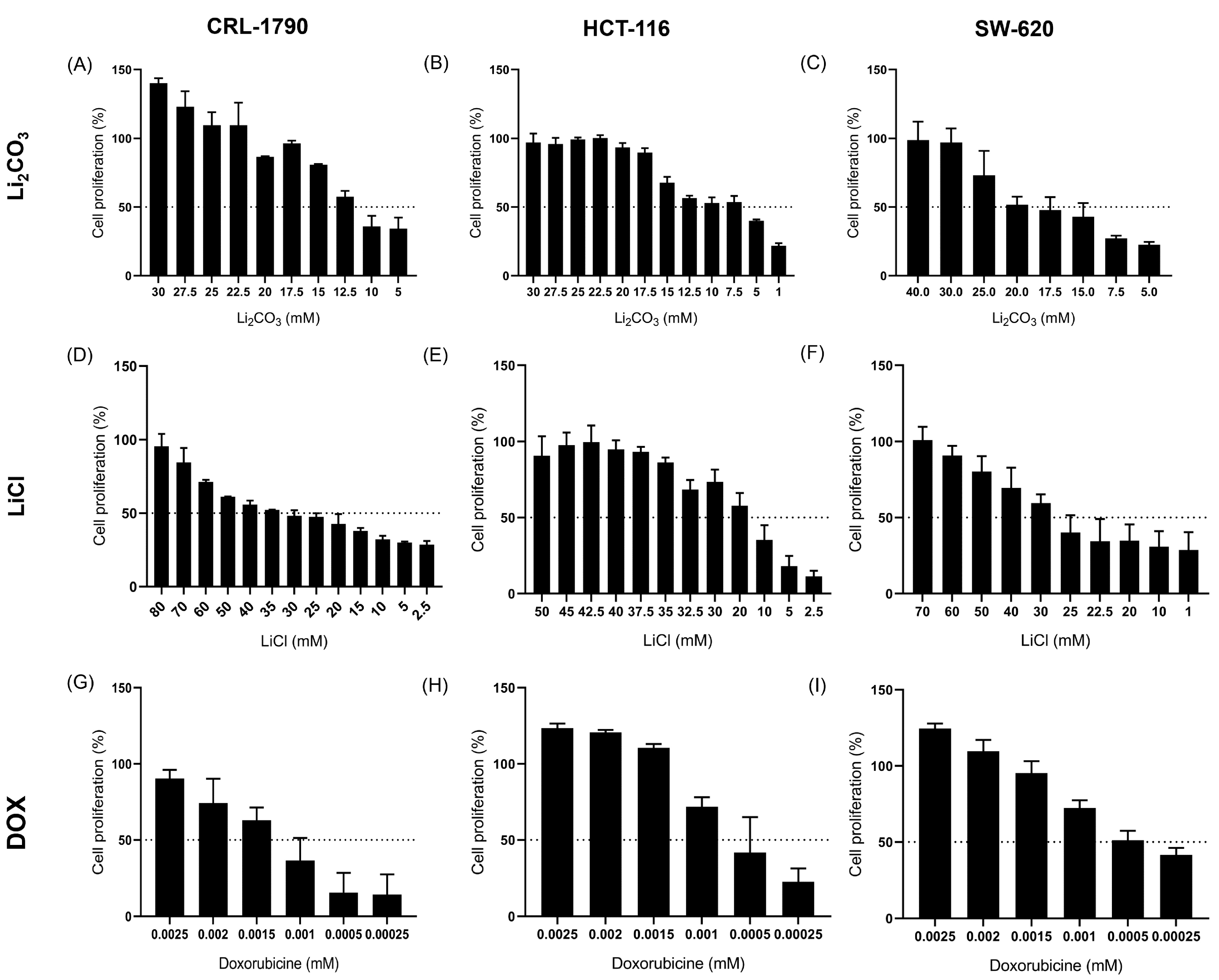
| Cell Line | IC50 Values (mM) | ||
|---|---|---|---|
| Li2CO3 | LiCl | Doxorubucin | |
| CRL-1790 | 13.70 ± 0.62 mM | 22.60 ± 5.60 mM | 11.76 × 10−4 ± 0.83 mM |
| HCT-116 | 8.14 ± 0.81 mM | 16.63 ± 0.06 mM | 7.98 × 10−4 ± 0.77 mM |
| SW-620 | 16.5 ± 0.31 mM | 25.21 ± 1.90 mM | 7.13 × 10−4 ± 0.76 mM |
| Cell Line | LDH Values (%) | ||
|---|---|---|---|
| Li2CO3 | LiCl | Doxorubucin | |
| CRL-1790 | 8.26 ± 0.19% | 7.77 ± 1.08% | 1.72 ± 0.69% |
| HCT-116 | 7.38 ± 1.07% | 7.43 ± 0.33% | 2.49 ± 0.04% |
| SW-620 | 11.7 ± 4.14% | 11.2 ± 3.36% | 1.29 ± 0.02% |
| Cell Lines | Colony-Forming Efficiency (CFE) | Clonogenic Survival Fractions (CSF) | ||||||
|---|---|---|---|---|---|---|---|---|
| Li2CO3 | LICl | DOX | Negative Control | Li2CO3 | LICl | DOX | Negative Control | |
| CRL-1790 | 20.0 ± 1.6% | 36.1 ± 3.4% | 0 | 77.7 ± 7.5% | 0.25 ± 0.02% | 0.46 ± 0.01% | 0 | 1.03 ± 0.03% |
| SW-620 | 29.5 ± 1.5% | 6.4 ± 2.5% | 0.5 ± 0.4% | 85.5 ± 1.9% | 0.34 ± 0.01% | 0.07 ± 0.02% | 0.005 ± 0.004% | 1.00 ± 0.00% |
| HCT-116 | 28.6 ± 1.2% | 20.0 ± 4.3% | 0 | 80.1 ± 5.2% | 0.35 ± 0.01% | 0.24 ± 0.04% | 0 | 1.00 ± 1.20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas-Vázquez, E.Y.; Becerril-Vigueras, X.P.; Leyva-Gómez, G.; Porras-Vázquez, S.A.; Jiménez-Fernández, L.A.; Almanza-Torres, J.M.; Bustamante-Montes, L.P.; Rodríguez-Morales, M.; Trujillo-Condes, V.E.; de la Torre-Núñez, M.; et al. Lithium-Induced Modulation of Proliferation and Apoptosis in an In Vitro Model of Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 11222. https://doi.org/10.3390/ijms262211222
Villegas-Vázquez EY, Becerril-Vigueras XP, Leyva-Gómez G, Porras-Vázquez SA, Jiménez-Fernández LA, Almanza-Torres JM, Bustamante-Montes LP, Rodríguez-Morales M, Trujillo-Condes VE, de la Torre-Núñez M, et al. Lithium-Induced Modulation of Proliferation and Apoptosis in an In Vitro Model of Colorectal Cancer. International Journal of Molecular Sciences. 2025; 26(22):11222. https://doi.org/10.3390/ijms262211222
Chicago/Turabian StyleVillegas-Vázquez, Edgar Yebrán, Ximena Paola Becerril-Vigueras, Gerardo Leyva-Gómez, Samantha Andrea Porras-Vázquez, Luz Aleida Jiménez-Fernández, Jorge Manuel Almanza-Torres, Lilia Patricia Bustamante-Montes, Miguel Rodríguez-Morales, Virgilio Eduardo Trujillo-Condes, Mariana de la Torre-Núñez, and et al. 2025. "Lithium-Induced Modulation of Proliferation and Apoptosis in an In Vitro Model of Colorectal Cancer" International Journal of Molecular Sciences 26, no. 22: 11222. https://doi.org/10.3390/ijms262211222
APA StyleVillegas-Vázquez, E. Y., Becerril-Vigueras, X. P., Leyva-Gómez, G., Porras-Vázquez, S. A., Jiménez-Fernández, L. A., Almanza-Torres, J. M., Bustamante-Montes, L. P., Rodríguez-Morales, M., Trujillo-Condes, V. E., de la Torre-Núñez, M., Tinoco-Torres, B. R., Herrera-Mundo, N., Murillo-González, F. E., Reyes-Hernández, O. D., & Figueroa-González, G. (2025). Lithium-Induced Modulation of Proliferation and Apoptosis in an In Vitro Model of Colorectal Cancer. International Journal of Molecular Sciences, 26(22), 11222. https://doi.org/10.3390/ijms262211222









