State of the Art of CAR-NK Cell Therapy in Multiple Myeloma: A Comprehensive Review of Cell Sources and Target Antigens
Abstract
1. Introduction
2. Biological Distinctions Between NK Cells and T Cells
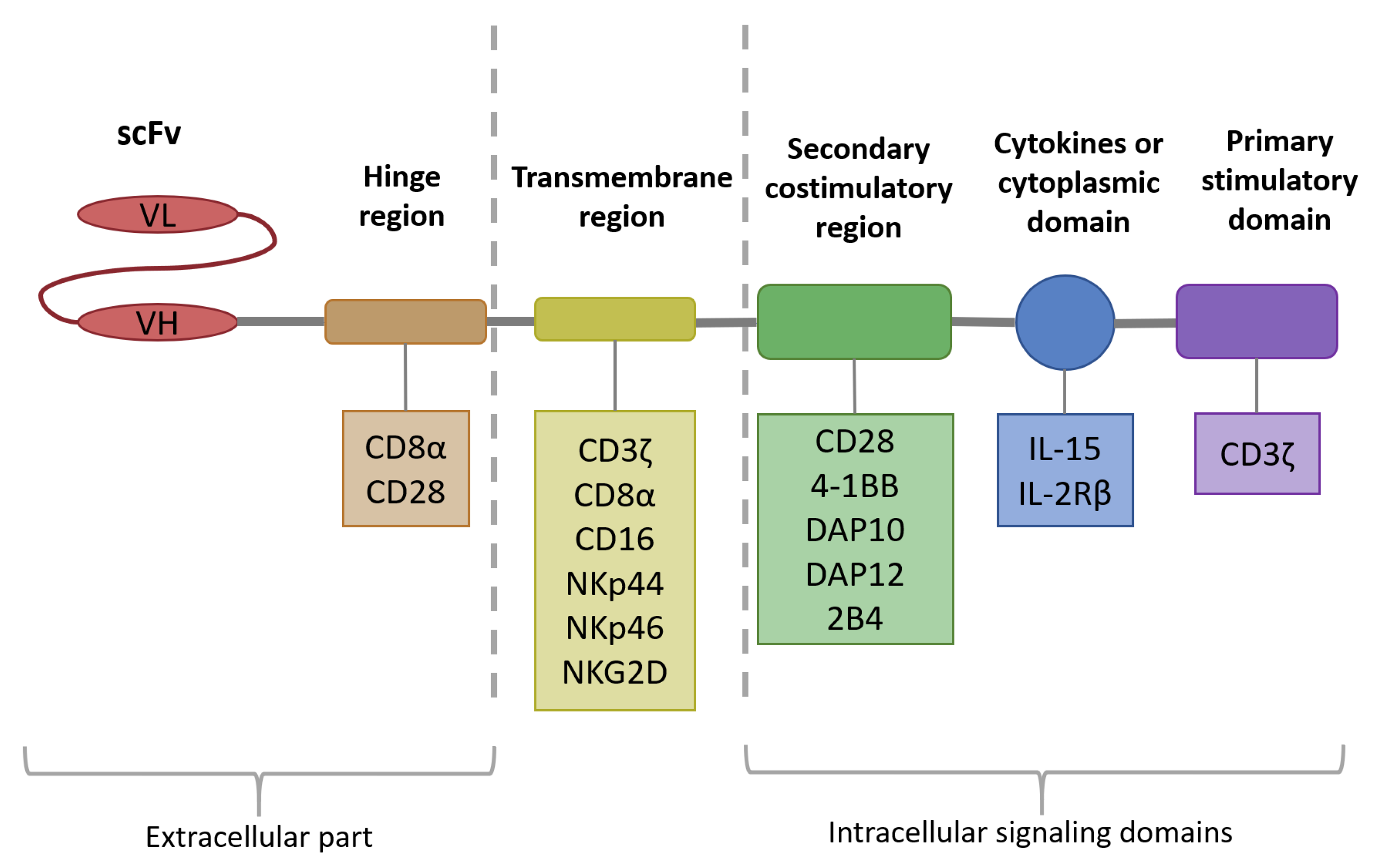
3. Chimeric Antigen Receptor Technology as One of the Strategies to Improve NK Cell Anti-Multiple Myeloma Response
3.1. Intracellular Signaling Domain
3.2. Transmembrane Domain
3.3. Hinge Region
4. NK Cells Versus T Cells: Distinct Advantages for CAR-Based Immunotherapy
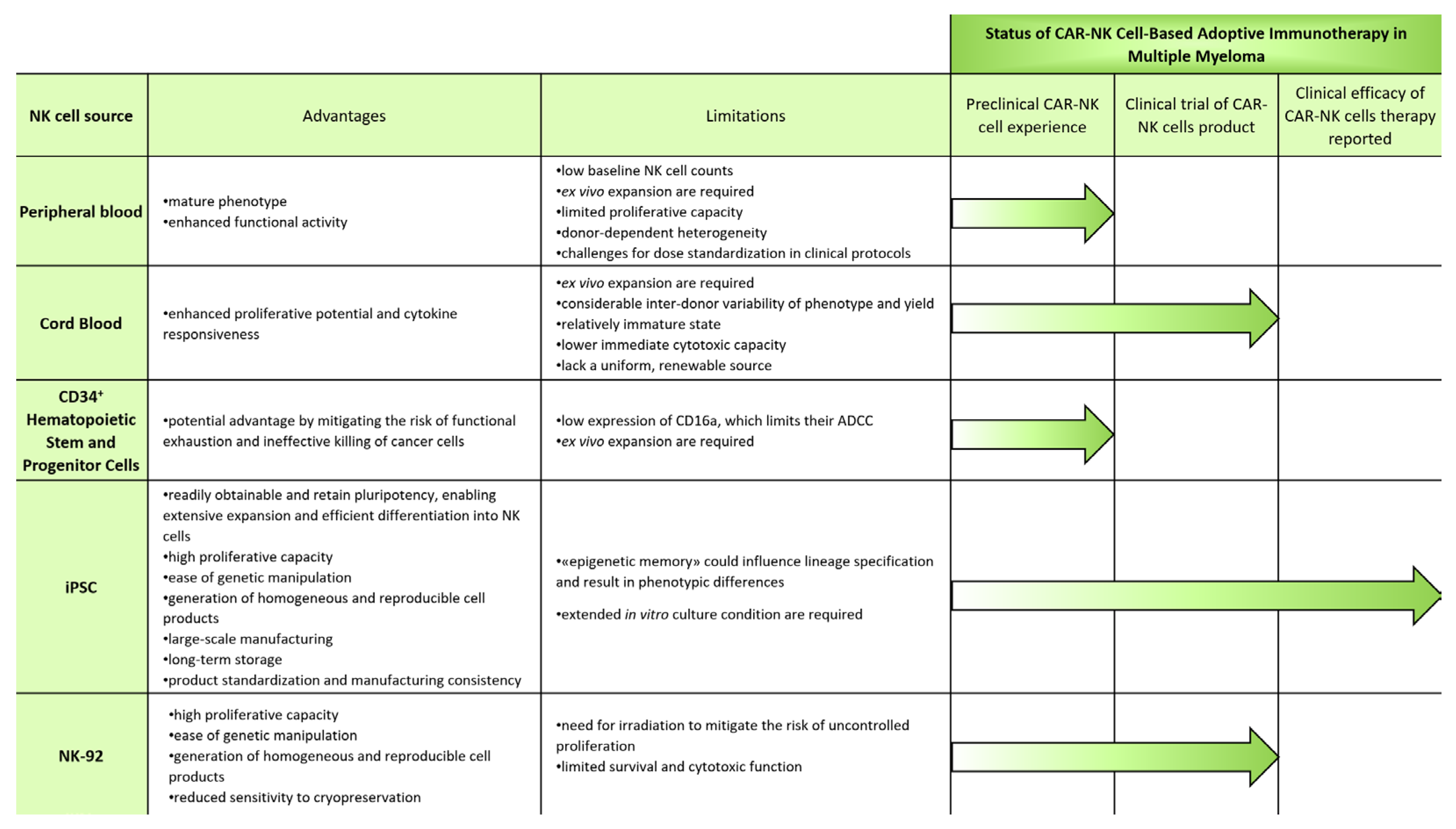
5. Sources for CAR-NK-Cell-Based Adoptive Immunotherapy in Multiple Myeloma
5.1. Blood
5.1.1. Peripheral Blood
5.1.2. Cord Blood
5.2. Stem and Progenitor Cells
5.2.1. CD34+ Hematopoietic Stem and Progenitor Cells
5.2.2. iPSCs
5.3. NK Cell Lines
NK-92
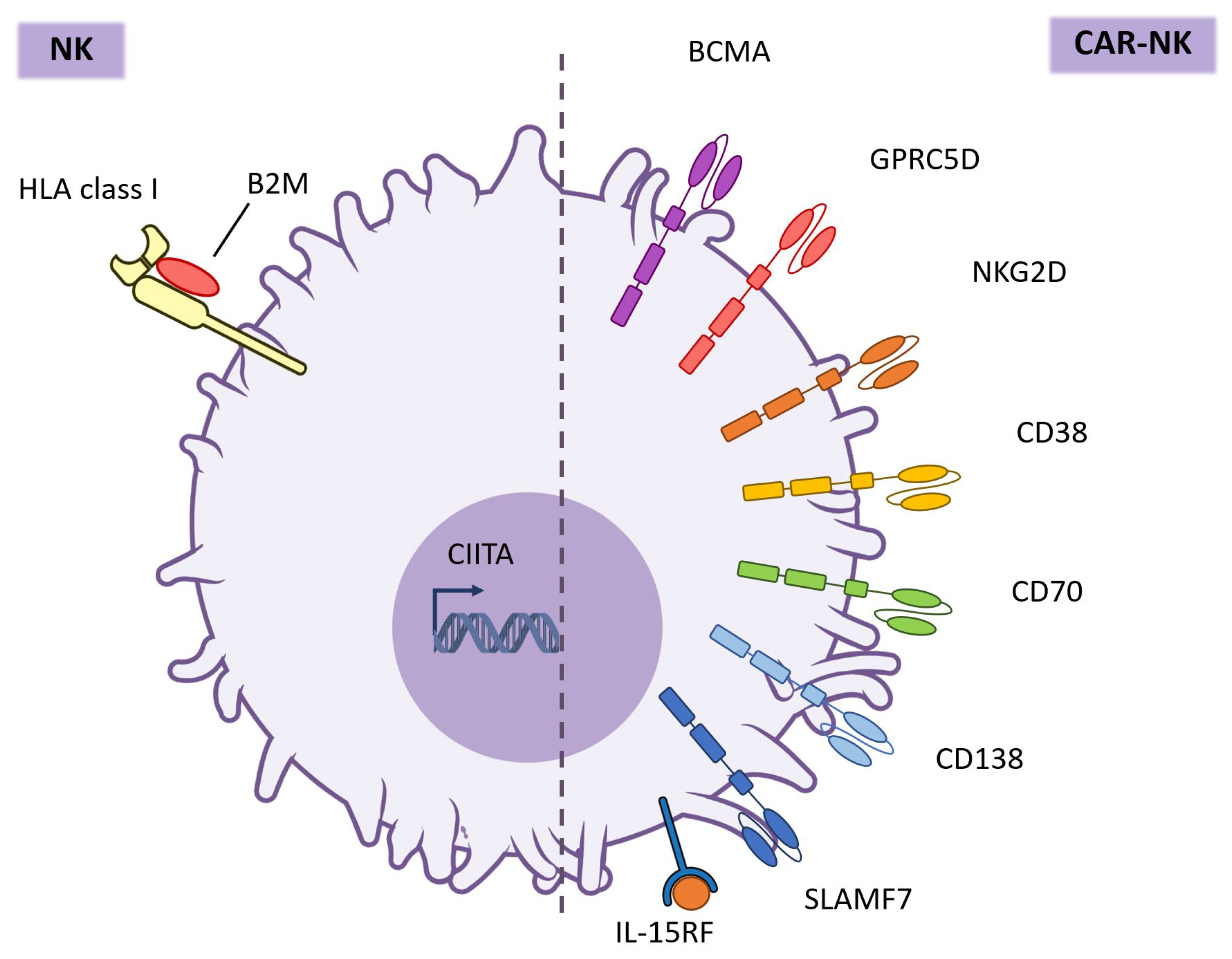
6. Targets for CAR-NK Cell Therapy in Multiple Myeloma
6.1. BCMA
6.2. BCMA-Based Dual-Target
6.2.1. BCMA and CD19
6.2.2. BCMA and GPRC5D
6.2.3. BCMA and CXCR4
6.3. GPRC5D
6.4. NKG2D
6.5. CD38
6.6. CD70
6.7. CD138
6.8. SLAMF7
7. Translation Barriers
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MM | Multiple myeloma |
| CAR | Chimeric antigen receptor |
| CRS | Cytokine release syndrome |
| NK | Natural killer |
| GvHD | Graft-versus-host disease |
| PB | Peripheral blood |
| CB | Cord blood |
| iPSC | Induced pluripotent stem cell |
| BCMA | B-cell maturation antigen |
| ICANS | Immune effector cell–associated neurotoxicity syndrome |
| TAA | Tumor-associated antigens |
| MHC | Major histocompatibility complex |
| scFv | Single-chain variable fragment |
| TMD | Transmembrane domain |
| DAP10 | DNAX-activating protein 10 |
| DAP12 | DNAX-activating protein 12 |
| NCT | Number clinical trial |
| ADCC | Antibody-dependent cellular cytotoxicity |
| ESCs | Embryonic stem cells |
| HSPC | Hematopoietic stem and progenitor cells |
| ASH | American Society of Hematology |
| CMC | Chemistry, Manufacturing, and Controls |
References
- Malard, F.; Neri, P.; Bahlis, N.J.; Terpos, E.; Moukalled, N.; Hungria, V.T.M.; Manier, S.; Mohty, M. Multiple Myeloma. Nat. Rev. Dis. Prim. 2024, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple Myeloma: 2024 Update on Diagnosis, Risk-Stratification, and Management. Am. J. Hematol. 2024, 99, 1802–1824. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, L.; Lin, X.; Fan, Z.; Jia, M.; Lin, C.; Zhu, M.; Teng, H.; Chen, G. Global, Regional and National Epidemiological Trends of Multiple Myeloma from 1990 to 2021: A Systematic Analysis of the Global Burden of Disease Study 2021. Front. Public Health 2025, 13, 1527198. [Google Scholar] [CrossRef]
- Pinto, V.; Bergantim, R.; Caires, H.R.; Seca, H.; Guimarães, J.E.; Vasconcelos, M.H. Multiple Myeloma: Available Therapies and Causes of Drug Resistance. Cancers 2020, 12, 407. [Google Scholar] [CrossRef]
- Vu, S.H.; Pham, H.H.; Pham, T.T.P.; Le, T.T.; Vo, M.C.; Jung, S.H.; Lee, J.J.; Nguyen, X.H. Adoptive NK Cell Therapy-a Beacon of Hope in Multiple Myeloma Treatment. Front. Oncol. 2023, 13, 1275076. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Ahmadieh-Yazdi, A.; Vicidomini, R.; Poondla, N.; Tanzadehpanah, H.; Dirbaziyan, A.; Mahaki, H.; Manoochehri, H.; Kalhor, N.; Dama, P. CAR T Therapies in Multiple Myeloma: Unleashing the Future. Cancer Gene Ther. 2024, 31, 667–686. [Google Scholar] [CrossRef]
- Gagelmann, N.; Riecken, K.; Wolschke, C.; Berger, C.; Ayuk, F.A.; Fehse, B.; Kröger, N. Development of CAR-T Cell Therapies for Multiple Myeloma. Leukemia 2020, 34, 2317–2332. [Google Scholar] [CrossRef]
- Rendo, M.J.; Joseph, J.J.; Phan, L.M.; DeStefano, C.B. CAR T-Cell Therapy for Patients with Multiple Myeloma: Current Evidence and Challenges. Blood Lymphat. Cancer Targets Ther. 2022, 12, 119–136. [Google Scholar] [CrossRef]
- Dagar, G.; Gupta, A.; Masoodi, T.; Nisar, S.; Merhi, M.; Hashem, S.; Chauhan, R.; Dagar, M.; Mirza, S.; Bagga, P.; et al. Harnessing the Potential of CAR-T Cell Therapy: Progress, Challenges, and Future Directions in Hematological and Solid Tumor Treatments. J. Transl. Med. 2023, 21, 449, Erratum in J. Transl. Med. 2023, 21, 571. [Google Scholar] [CrossRef]
- Clara, J.A.; Childs, R.W. Harnessing Natural Killer Cells for the Treatment of Multiple Myeloma. Semin. Oncol. 2022, 49, 69–85. [Google Scholar] [CrossRef]
- Khawar, M.B.; Sun, H. CAR-NK Cells: From Natural Basis to Design for Kill. Front. Immunol. 2021, 12, 707542. [Google Scholar] [CrossRef]
- Jørgensen, L.V.; Christensen, E.B.; Barnkob, M.B.; Barington, T. The Clinical Landscape of CAR NK Cells. Exp. Hematol. Oncol. 2025, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, G.; Rudensky, A.Y. Interactions between Innate and Adaptive Lymphocytes. Nat. Rev. Immunol. 2014, 14, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A Guide to Antigen Processing and Presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef]
- Kretschmer, L.; Fuchs, N.; Busch, D.H.; Buchholz, V.R. Picking up Speed: Cell Cycle Regulation during Effector CD8+ T Cell Differentiation. Med. Microbiol. Immunol. 2023, 212, 253–260, Correction in Med. Microbiol. Immunol. 2023, 212, 261–262. https://doi.org/10.1007/s00430-023-00772-x. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, F.; Zheng, F. Stem Cell like Memory T Cells: A New Paradigm in Cancer Immunotherapy. Clin. Immunol. 2022, 241, 109078. [Google Scholar] [CrossRef]
- Mace, E.M. Human Natural Killer Cells: Form, Function, and Development. J. Allergy Clin. Immunol. 2023, 151, 371–385. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, H.; Jounaidi, Y. Comprehensive Snapshots of Natural Killer Cells Functions, Signaling, Molecular Mechanisms and Clinical Utilization. Signal Transduct. Target. Ther. 2024, 9, 302. [Google Scholar] [CrossRef]
- Yao, P.; Liu, Y.G.; Huang, G.; Hao, L.; Wang, R. The Development and Application of Chimeric Antigen Receptor Natural Killer (CAR-NK) Cells for Cancer Therapy: Current State, Challenges and Emerging Therapeutic Advances. Exp. Hematol. Oncol. 2024, 13, 118. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, J. Emerging Roles of CAR-NK Cell Therapies in Tumor Immunotherapy: Current Status and Future Directions. Cell Death Discov. 2024, 10, 318. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Peng, L.; Sferruzza, G.; Yang, L.; Zhou, L.; Chen, S. CAR-T and CAR-NK as Cellular Cancer Immunotherapy for Solid Tumors. Cell. Mol. Immunol. 2024, 21, 1089–1108. [Google Scholar] [CrossRef] [PubMed]
- Leivas, A.; Rio, R.; Mateos, R.; Paciello, M.L.; Garcia-Ortiz, A.; Fernandez, L.; Perez-Martinez, A.; Anthony Lee, D.; Powell, D.J.; Valeri, A.; et al. NKG2D-CAR Transduced Primary Natural Killer Cells Efficiently Target Multiple Myeloma Cells. Blood 2018, 132 (Suppl. 1), 590. [Google Scholar] [CrossRef]
- Ren, Q.; Zu, Y.; Su, H.; Lu, Q.; Xiang, B.; Luo, Y.; Zhang, J.; Song, Y. Single VHH-Directed BCMA CAR-NK Cells for Multiple Myeloma. Exp. Hematol. Oncol. 2023, 12, 10–13. [Google Scholar] [CrossRef]
- Park, E.; Mun, H.J.; Seo, E.; Hwang, S.; Lee, J.H.; Song, S.; Sung, H.; Kim, H.Y.; Kwon, M.J. CAR NK92 Cells Targeting BCMA Can Effectively Kill Multiple Myeloma Cells Both In Vitro and In Vivo. Biomedicines 2024, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. DAP10- and DAP12-Associated Receptors in Innate Immunity. Immunol. Rev. 2009, 227, 150–160. [Google Scholar] [CrossRef]
- Upshaw, J.L.; Arneson, L.N.; Schoon, R.A.; Dick, C.J.; Billadeau, D.D.; Leibson, P.J. NKG2D-Mediated Signaling Requires a DAP10-Bound Grb2-Vav1 Intermediate and Phosphatidylinositol-3-Kinase in Human Natural Killer Cells. Nat. Immunol. 2006, 7, 524–532. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Han, Z.; Wang, Y. Does a Natural Killer Need a CAR? Front. Immunol. 2025, 16, 1606126. [Google Scholar] [CrossRef]
- Wrona, E.; Borowiec, M.; Potemski, P. CAR-NK Cells in the Treatment of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 5899. [Google Scholar] [CrossRef]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human IPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-Tumor Activity. Cell Stem Cell 2018, 23, 181–192.e5. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Q.; Zhong, M.; Wang, Z.; Chen, Z.; Zhang, Y.; Xing, H.; Tian, Z.; Tang, K.; Liao, X.; et al. 2B4 Costimulatory Domain Enhancing Cytotoxic Ability of Anti-CD5 Chimeric Antigen Receptor Engineered Natural Killer Cells against T Cell Malignancies. J. Hematol. Oncol. 2019, 12, 49. [Google Scholar] [CrossRef]
- Van den Eynde, A.; Gehrcken, L.; Verhezen, T.; Lau, H.W.; Hermans, C.; Lambrechts, H.; Flieswasser, T.; Quatannens, D.; Roex, G.; Zwaenepoel, K.; et al. IL-15-Secreting CAR Natural Killer Cells Directed toward the Pan-Cancer Target CD70 Eliminate Both Cancer Cells and Cancer-Associated Fibroblasts. J. Hematol. Oncol. 2024, 17, 8. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Page, A.; Chuvin, N.; Valladeau-Guilemond, J.; Depil, S. Development of NK Cell-Based Cancer Immunotherapies through Receptor Engineering. Cell. Mol. Immunol. 2024, 21, 315–331. [Google Scholar] [CrossRef]
- Hudecek, M.; Sommermeyer, D.; Kosasih, P.L.; Silva-Benedict, A.; Liu, L.; Rader, C.; Jensen, M.C.; Riddell, S.R. The Nonsignaling Extracellular Spacer Domain of Chimeric Antigen Receptors Is Decisive for in Vivo Antitumor Activity. Cancer Immunol. Res. 2015, 3, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kanapuru, B.; George, B.; Lin, X.; Xu, Z.; Bryan, W.W.; Pazdur, R.; Theoret, M.R. FDA Approval Summary: Idecabtagene Vicleucel for Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2022, 28, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, K.; Kaushal, M.; George, B.; Kanapuru, B.; Theoret, M.R. FDA Approval Summary: Ciltacabtagene Autoleucel for Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2024, 30, 2865–2871. [Google Scholar] [CrossRef]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. ‘Off-the-Shelf’ Allogeneic CAR T Cells: Development and Challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Zhu, Y.; Fang, Y.; Lyu, Z.; Yang, L. Emerging Trends in Clinical Allogeneic CAR Cell Therapy. Med 2025, 6, 100677. [Google Scholar] [CrossRef]
- Daher, M.; Melo Garcia, L.; Li, Y.; Rezvani, K. CAR-NK Cells: The next Wave of Cellular Therapy for Cancer. Clin. Transl. Immunol. 2021, 10, e1274. [Google Scholar] [CrossRef]
- Parikh, R.H.; Lonial, S. Chimeric Antigen Receptor T-cell Therapy in Multiple Myeloma: A Comprehensive Review of Current Data and Implications for Clinical Practice. CA Cancer J. Clin. 2023, 73, 275–285. [Google Scholar] [CrossRef]
- Mansouri, V.; Yazdanpanah, N.; Rezaei, N. The Immunologic Aspects of Cytokine Release Syndrome and Graft versus Host Disease Following CAR T Cell Therapy. Int. Rev. Immunol. 2022, 41, 649–668. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine Release Syndrome and Associated Neurotoxicity in Cancer Immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef]
- Kikuchi, T.; Tsukada, N.; Kunisada, K.; Nomura-Yogo, M.; Ishida, T. Local Cytokine Release Syndrome After Idecabtagene Vicleucel Therapy in Patients With Multiple Myeloma: Two Case Reports. Cureus 2024, 16, e72364. [Google Scholar] [CrossRef]
- Cheng, H.; Shao, L.; Wang, D.; Chen, Y.; Sun, Y.; Chen, Z.; Liu, J.; Wang, X.; Chen, W.; Sang, W.; et al. Comprehensive Characterization of Cytopenia after Chimeric Antigen Receptor-T Cell Infusion in Patients with Relapsed or Refractory Multiple Myeloma. Cytotherapy 2025, 27, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Sperling, A.S. Cytopenias in BCMA CAR T: Unraveling Inflammatory Mechanisms. Blood Adv. 2024, 8, 5527–5528. [Google Scholar] [CrossRef]
- Sanber, K.; Savani, B.; Jain, T. Graft-versus-Host Disease Risk after Chimeric Antigen Receptor T-Cell Therapy: The Diametric Opposition of T Cells. Br. J. Haematol. 2021, 195, 660–668. [Google Scholar] [CrossRef]
- Yang, R.; Yang, Y.; Liu, R.; Wang, Y.; Yang, R.; He, A. Advances in CAR-NK Cell Therapy for Hematological Malignancies. Front. Immunol. 2024, 15, 1414264. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, M.; Zhang, W.; Liu, N.; Wang, D.; Jing, L.; Xu, N.; Yang, N.; Ren, T. Chimeric Antigen Receptor-Based Natural Killer Cell Immunotherapy in Cancer: From Bench to Bedside. Cell Death Dis. 2024, 15, 50. [Google Scholar] [CrossRef]
- Harris, D.T.; Hager, M.V.; Smith, S.N.; Cai, Q.; Stone, J.D.; Kruger, P.; Lever, M.; Dushek, O.; Schmitt, T.M.; Greenberg, P.D.; et al. Comparison of T Cell Activities Mediated by Human TCRs and CARs That Use the Same Recognition Domains. J. Immunol. 2018, 200, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Salter, A.I.; Rajan, A.; Kennedy, J.J.; Ivey, R.G.; Shelby, S.A.; Leung, I.; Templeton, M.L.; Muhunthan, V.; Voillet, V.; Sommermeyer, D.; et al. Comparative Analysis of TCR and CAR Signaling Informs CAR Designs with Superior Antigen Sensitivity and in Vivo Function. Sci. Signal. 2022, 14, eabe2606. [Google Scholar] [CrossRef]
- Goodridge, J.P.; Bjordahl, R.; Mahmood, S.; Reiser, J.; Gaidarova, S.; Blum, R.; Cichocki, F.; Chu, H.; Bonello, G.; Lee, T.; et al. FT576: Multi-Specific Off-the-Shelf CAR-NK Cell Therapy Engineered for Enhanced Persistence, Avoidance of Self-Fratricide and Optimized Mab Combination Therapy to Prevent Antigenic Escape and Elicit a Deep and Durable Response in Multiple Myeloma. Blood 2020, 136 (Suppl. 1), 4–5. [Google Scholar] [CrossRef]
- Ullrich, E.; Salzmann-Manrique, E.; Bakhtiar, S.; Bremm, M.; Gerstner, S.; Herrmann, E.; Bader, P.; Hoffmann, P.; Holler, E.; Edinger, M.; et al. Relation between Acute GVHD and NK Cell Subset Reconstitution Following Allogeneic Stem Cell Transplantation. Front. Immunol. 2016, 7, 595. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, N.; Jain, A.; Campana, D. NK Cells for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Lamb, M.G.; Rangarajan, H.G.; Tullius, B.P.; Lee, D.A. Natural Killer Cell Therapy for Hematologic Malignancies: Successes, Challenges, and the Future. Stem Cell Res. Ther. 2021, 12, 211. [Google Scholar] [CrossRef]
- Li, Y.R.; Fang, Y.; Niu, S.; Chen, Y.; Lyu, Z.; Yang, L. Managing Allorejection in Off-the-Shelf CAR-Engineered Cell Therapies. Mol. Ther. 2025, 33, 2368–2390. [Google Scholar] [CrossRef]
- Liu, F.; Tarannum, M.; Zhao, Y.; Zhang, Y.J.; Ham, J.D.; Lei, K.; Qiang, Y.; Deng, X.; Nguyen, M.; Dinh, K.; et al. Selective HLA Knockdown and PD-L1 Expression Prevent Allogeneic CAR-NK Cell Rejection and Enhance Safety and Anti-Tumor Responses in Xenograft Mice. Nat. Commun. 2025, 16, 8809. [Google Scholar] [CrossRef]
- Hammer, Q.; Perica, K.; Mbofung, R.M.; van Ooijen, H.; Martin, K.E.; Momayyezi, P.; Varady, E.; Pan, Y.; Jelcic, M.; Groff, B.; et al. Genetic Ablation of Adhesion Ligands Mitigates Rejection of Allogeneic Cellular Immunotherapies. Cell Stem Cell 2024, 31, 1376–1386.e8. [Google Scholar] [CrossRef]
- Kim, H. Overcoming Immune Barriers in Allogeneic CAR-NK Therapy: From Multiplex Gene Editing to AI-Driven Precision Design. Biomolecules 2025, 15, 935. [Google Scholar] [CrossRef]
- Ghobadi, A.; Bachanova, V.; Patel, K.; Park, J.H.; Flinn, I.; Riedell, P.A.; Bachier, C.; Diefenbach, S.C.; Wong, C.; Bickers, C.; et al. Induced Pluripotent Stem-Cell-Derived CD19-Directed Chimeric Antigen Receptor Natural Killer Cells in B-Cell Lymphoma: A Phase 1, First-in-Human Trial. Lancet 2025, 405, 127–136. [Google Scholar] [CrossRef]
- Dhakal, B.; Berdeja, J.G.; Gregory, T.; Ly, T.; Bickers, C.; Zong, X.; Wong, L.; Goodridge, J.P.; Cooley, S.; Valamehr, B.; et al. Interim Phase I Clinical Data of FT576 As Monotherapy and in Combination with Daratumumab in Subjects with Relapsed/Refractory Multiple Myeloma. Blood 2022, 140 (Suppl. 1), 4586–4587. [Google Scholar] [CrossRef]
- Marofi, F.; Saleh, M.M.; Rahman, H.S.; Suksatan, W.; Al-Gazally, M.E.; Abdelbasset, W.K.; Thangavelu, L.; Yumashev, A.V.; Hassanzadeh, A.; Yazdanifar, M.; et al. CAR-Engineered NK Cells; a Promising Therapeutic Option for Treatment of Hematological Malignancies. Stem Cell Res. Ther. 2021, 12, 374. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, J.; Cooper, M.L.; Ritchey, J.K.; Gladney, S.; Niswonger, J.; González, L.S.; Street, E.; Haas, G.J.; Carter, A.; Amayta, P.N.; et al. Anti-Myeloma Efficacy of CAR-INKT Is Enhanced with a Long-Acting IL-7, RhIL-7-HyFc. Blood Adv. 2023, 7, 6009–6022. [Google Scholar] [CrossRef]
- Fiorenza, S.; Ritchie, D.S.; Ramsey, S.D.; Turtle, C.J.; Roth, J.A. Value and Affordability of CAR T-Cell Therapy in the United States. Bone Marrow Transplant. 2020, 55, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Joseph, N.; Crivera, C.; Kharat, A.; Jackson, C.C.; Valluri, S.; Cost, P.; Phelps, H.; Slowik, R.; Klein, T.; et al. Component Costs of CAR-T Therapy in Addition to Treatment Acquisition Costs in Patients with Multiple Myeloma. Oncol. Ther. 2023, 11, 263–275. [Google Scholar] [CrossRef]
- Nahi, H.; Chrobok, M.; Meinke, S.; Gran, C.; Marquardt, N.; Afram, G.; Sutlu, T.; Gilljam, M.; Stellan, B.; Wagner, A.K.; et al. Autologous NK Cells as Consolidation Therapy Following Stem Cell Transplantation in Multiple Myeloma. Cell Rep. Med. 2022, 3, 100508. [Google Scholar] [CrossRef]
- D’Souza, C.; Keam, S.P.; Yeang, H.X.A.; Neeson, M.; Richardson, K.; Hsu, A.K.; Canfield, R.; Bezman, N.; Robbins, M.; Quach, H.; et al. Myeloma Natural Killer Cells Are Exhausted and Have Impaired Regulation of Activation. Haematologica 2021, 106, 2522–2526. [Google Scholar] [CrossRef]
- Pazina, T.; MacFarlane, A.W., IV; Bernabei, L.; Dulaimi, E.; Kotcher, R.; Yam, C.; Bezman, N.A.; Robbins, M.D.; Ross, E.A.; Campbell, K.S.; et al. Alterations of Nk Cell Phenotype in the Disease Course of Multiple Myeloma. Cancers 2021, 13, 226. [Google Scholar] [CrossRef]
- Tahri, S.; de Jong, M.M.E.; Fokkema, C.; Papazian, N.; Kellermayer, Z.; Vermeulen, M.; van Duin, M.; van de Woestijne, P.; Nasserinejad, K.; Saraci, E.; et al. Single-Cell Transcriptomic Analysis Reveals Reduction of Cytotoxic NK Cells in a Subset of Newly Diagnosed Multiple Myeloma Patients Impacting Outcome after Daratumumab Therapy. Blood 2022, 140 (Suppl. 1), 9982–9984. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural Killer Cells in Cancer Biology and Therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Lamers-Kok, N.; Panella, D.; Georgoudaki, A.M.; Liu, H.; Özkazanc, D.; Kučerová, L.; Duru, A.D.; Spanholtz, J.; Raimo, M. Natural Killer Cells in Clinical Development as Non-engineered, Engineered, and Combination Therapies. J. Hematol. Oncol. 2022, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Berrien-Elliott, M.M.; Jacobs, M.T.; Fehniger, T.A. Allogeneic Natural Killer Cell Therapy. Blood 2023, 141, 856–868. [Google Scholar] [CrossRef]
- Kennedy, P.R.; Felices, M.; Miller, J.S. Challenges to the Broad Application of Allogeneic Natural Killer Cell Immunotherapy of Cancer. Stem Cell Res. Ther. 2022, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Meng, Y.; Zhang, L.; Han, Z.; Feng, X. High-Efficient Generation of Natural Killer Cells from Peripheral Blood with Preferable Cell Vitality and Enhanced Cytotoxicity by Combination of IL-2, IL-15 and IL-18. Biochem. Biophys. Res. Commun. 2021, 534, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Boje, A.S.; Langner, A.; Gehlert, C.L.; Reitinger, C.; Nimmerjahn, F.; Murga Penas, E.M.; Bendig, S.; Chitadze, G.; Brüggemann, M.; Diemer, K.; et al. A Novel Platform Technology for the Development of NK Cell-Based Cellular Immunotherapies. Blood 2024, 144, 914–915. [Google Scholar] [CrossRef]
- Reina-Ortiz, C.; Constantinides, M.; Fayd-Herbe-de-Maudave, A.; Présumey, J.; Hernandez, J.; Cartron, G.; Giraldos, D.; Díez, R.; Izquierdo, I.; Azaceta, G.; et al. Expanded NK Cells from Umbilical Cord Blood and Adult Peripheral Blood Combined with Daratumumab Are Effective against Tumor Cells from Multiple Myeloma Patients. Oncoimmunology 2021, 10, 1853314. [Google Scholar] [CrossRef]
- Woll, P.S.; Grzywacz, B.; Tian, X.; Marcus, R.K.; Knorr, D.A.; Verneris, M.R.; Kaufman, D.S. Human Embryonic Stem Cells Differentiate into a Homogeneous Population of Natural Killer Cells with Potent in Vivo Antitumor Activity. Blood 2009, 113, 6094–6101. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.A.; Ni, Z.; Hermanson, D.; Hexum, M.K.; Bendzick, L.; Cooper, L.J.N.; Lee, D.A.; Kaufman, D.S. Clinical-Scale Derivation of Natural Killer Cells From Human Pluripotent Stem Cells for Cancer Therapy. Stem Cells Transl. Med. 2013, 2, 274–283. [Google Scholar] [CrossRef]
- Zhu, H.; Kaufman, D.S. An Improved Method to Produce Clinical-Scale Natural Killer Cells from Human Pluripotent Stem Cells. In In Vitro Differentiation of T-Cells: Methods and Protocols; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 2048, pp. 107–119. [Google Scholar]
- Luevano, M.; Madrigal, A.; Saudemont, A. Generation of Natural Killer Cells from Hematopoietic Stem Cells in Vitro for Immunotherapy. Cell. Mol. Immunol. 2012, 9, 310–320. [Google Scholar] [CrossRef]
- Fabian, K.P.; Hodge, J.W. The Emerging Role of Off-the-Shelf Engineered Natural Killer Cells in Targeted Cancer Immunotherapy. Mol. Ther. Oncolytics 2021, 23, 266–276. [Google Scholar] [CrossRef]
- Shereck, E.; Day, N.S.; Awasthi, A.; Ayello, J.; Chu, Y.; McGuinn, C.; van de Ven, C.; Lim, M.S.; Cairo, M.S. Immunophenotypic, Cytotoxic, Proteomic and Genomic Characterization of Human Cord Blood vs. Peripheral Blood CD56Dim NK Cells. Innate Immun. 2019, 25, 294–304. [Google Scholar] [CrossRef]
- Kroll, K.; Reeves, R.K. Protocol for Identification and Computational Analysis of Human Natural Killer Cells Using Flow Cytometry and R. STAR Protoc. 2023, 4, 102044. [Google Scholar] [CrossRef]
- Angelo, L.S.; Banerjee, P.P.; Monaco-Shawver, L.; Rosen, J.B.; Makedonas, G.; Forbes, L.R.; Mace, E.M.; Orange, J.S. Practical NK Cell Phenotyping and Variability in Healthy Adults. Immunol. Res. 2015, 62, 341–356. [Google Scholar] [CrossRef]
- Porrata, L.F. Natural Killer Cells Are Key Host Immune Effector Cells Affecting Survival in Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation. Cells 2022, 11, 3469. [Google Scholar] [CrossRef]
- Dogra, P.; Rancan, C.; Ma, W.; Toth, M.; Senda, T.; Carpenter, D.J.; Kubota, M.; Matsumoto, R.; Thapa, P.; Szabo, P.A.; et al. Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell 2020, 180, 749–763.e13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wallace, D.L.; De Lara, C.M.; Ghattas, H.; Asquith, B.; Worth, A.; Griffin, G.E.; Taylor, G.P.; Tough, D.F.; Beverley, P.C.L.; et al. In Vivo Kinetics of Human Natural Killer Cells: The Effects of Ageing and Acute and Chronic Viral Infection. Immunology 2007, 121, 258–265. [Google Scholar] [CrossRef]
- Caligiuri, M.A. Human Natural Killer Cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Imai, C.; Iwamoto, S.; Campana, D. Genetic Modification of Primary Natural Killer Cells Overcomes Inhibitory Signals and Induces Specific Killing of Leukemic Cells. Blood 2005, 106, 376–383. [Google Scholar] [CrossRef]
- Ng, Y.Y.; Du, Z.; Zhang, X.; Chng, W.J.; Wang, S. CXCR4 and Anti-BCMA CAR Co-Modified Natural Killer Cells Suppress Multiple Myeloma Progression in a Xenograft Mouse Model. Cancer Gene Ther. 2022, 29, 475–483. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, C.; Wang, Y.; Wang, C.; Wang, Q.; Ye, G.; Liu, T.; Wang, Q.; Wang, H.; Gong, Y.; et al. Allogeneic CAR-NK Cell Therapy Targeting Both BCMA and GPRC5D for the Treatment of Multiple Myeloma. Blood 2022, 140 (Suppl. 1), 7378. [Google Scholar] [CrossRef]
- Huang, R.; Wen, Q.; Zhang, X. CAR-NK Cell Therapy for Hematological Malignancies: Recent Updates from ASH 2022. J. Hematol. Oncol. 2023, 16, 35. [Google Scholar] [CrossRef]
- Kotylo, P.K.; Baenzinger, J.C.; Yoder, M.C.; Engle, W.A.; Bolinger, C.D. Rapid Analysis of Lymphocyte Subsets in Cord Blood. Am. J. Clin. Pathol. 1990, 93, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, H.; Marin, D.; Xi, Y.; Miao, Q.; Lv, J.; Banerjee, P.P.; Shaim, H.; Daher, M.; Basar, R.; et al. A Novel Immature Natural Killer Cell Subpopulation Predicts Relapse after Cord Blood Transplantation. Blood Adv. 2019, 3, 4117–4130. [Google Scholar] [CrossRef] [PubMed]
- Luevano, M.; Daryouzeh, M.; Alnabhan, R.; Querol, S.; Khakoo, S.; Madrigal, A.; Saudemont, A. The Unique Profile of Cord Blood Natural Killer Cells Balances Incomplete Maturation and Effective Killing Function upon Activation. Hum. Immunol. 2012, 73, 248–257. [Google Scholar] [CrossRef]
- Alnabhan, R.; Madrigal, A.; Saudemont, A. Differential Activation of Cord Blood and Peripheral Blood Natural Killer Cells by Cytokines. Cytotherapy 2015, 17, 73–85. [Google Scholar] [CrossRef]
- Liu, E.; Ang, S.O.T.; Kerbauy, L.; Basar, R.; Kaur, I.; Kaplan, M.; Li, L.; Tong, Y.; Daher, M.; Ensley, E.L.; et al. GMP-Compliant Universal Antigen Presenting Cells (UAPC) Promote the Metabolic Fitness and Antitumor Activity of Armored Cord Blood CAR-NK Cells. Front. Immunol. 2021, 12, 626098. [Google Scholar] [CrossRef]
- Castellano, E.; García-Ortiz, A.; Ugalde, L.; Maroto Martin, E.; Encinas Mayoral, J.; Oliva, R.; Garcia-Garcia, L.; Peña, I.; Álvarez, N.; Carreño, G.; et al. CRISPR/Cas9 Multi-Editing Enhances CAR NK Cells Therapeutic Potential Against Multiple Myeloma. Blood 2024, 144 (Suppl. 1), 3442. [Google Scholar] [CrossRef]
- Lin, P.; Reyes Silva, F.C.; Lin, P.; Gilbert, A.L.; Acharya, S.; Nunez Cortes, A.K.; Banerjee, P.; Fang, D.; Melo Garcia, L.; Daher, M.; et al. CD70 CAR NK Cells in the Treatment of Multiple Myeloma. Blood 2023, 142 (Suppl. 1), 3463. [Google Scholar] [CrossRef]
- Veluchamy, J. An off the Shelf, GMP Compliant, Fully Closed and Semi-Automated Large-Scale Production System for Allogeneic NK Cells. Cytotherapy 2020, 22, S161–S162. [Google Scholar] [CrossRef]
- Spanholtz, J.; Preijers, F.; Tordoir, M.; Trilsbeek, C.; Paardekooper, J.; de Witte, T.; Schaap, N.; Dolstra, H. Clinical-Grade Generation of Active NK Cells from Cord Blood Hematopoietic Progenitor Cells for Immunotherapy Using a Closed-System Culture Process. PLoS ONE 2011, 6, e20740. [Google Scholar] [CrossRef]
- Somanchi, S.; Guo, X.; He, S.; Mathur, R.; Difiglia, A.; Rana, H.; Ling, W.; Edinger, J.; Kaufmann, G.F.; Zeldis, J.B.; et al. Development of CD38 CAR Engineered Human Placental Hematopoietic Stem Cell Derived Natural Killer Cells (PNK-CAR38) As Allogeneic Cancer Immunotherapy. Blood 2019, 134 (Suppl. 1), 2070. [Google Scholar] [CrossRef]
- Goldenson, B.H.; Zhu, H.; Wang, Y.Z.M.; Heragu, N.; Bernareggi, D.; Ruiz-Cisneros, A.; Bahena, A.; Ask, E.H.; Hoel, H.J.; Malmberg, K.J.; et al. Umbilical Cord Blood and IPSC-Derived Natural Killer Cells Demonstrate Key Differences in Cytotoxic Activity and KIR Profiles. Front. Immunol. 2020, 11, 561553. [Google Scholar] [CrossRef]
- van Hauten, P.M.M.; Hooijmaijers, L.; Vidal-Manrique, M.; van der Waart, A.B.; Hobo, W.; Wu, J.; Blijlevens, N.M.A.; Jansen, J.H.; Walcheck, B.; Schaap, N.P.M.; et al. Engineering of CD34+ Progenitor-Derived Natural Killer Cells with Higher-Affinity CD16a for Enhanced Antibody-Dependent Cellular Cytotoxicity. Cytotherapy 2024, 26, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; Cooley, S.; Hari, P.; Jagannath, S.; Balint, C.R.; van Der Touw, W.; Donato, M.L.; McCarthy, P.L.; Wallace, P.K.; Zhang, X.; et al. Results of a Phase I Study of Pnk-007, Allogeneic, Off the Shelf NK Cell, Post Autologous Transplant in Multiple Myeloma (NCT02955550). Blood 2019, 134 (Suppl. 1), 4451. [Google Scholar] [CrossRef]
- van Der Touw, W.; Kang, L.; Tario, J.D.; Stout, B.; Voskinarian-Berse, V.; Rousseva, V.; Wallace, P.K.; Hariri, R.; Zhang, X.P. Immune Monitoring of CD34+ Placental Cell Derived Natural Killer Cell Therapy (PNK-007) in Phase I Study of Multiple Myeloma. Blood 2019, 134 (Suppl. 1), 4457. [Google Scholar] [CrossRef]
- Ilic, D.; Ogilvie, C. Pluripotent Stem Cells in Clinical Setting-New Developments and Overview of Current Status. Stem Cells 2022, 40, 791–801. [Google Scholar] [CrossRef]
- Karagiannis, P.; Kim, S.I. IPSC-Derived Natural Killer Cells for Cancer Immunotherapy. Mol. Cells 2021, 44, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; van der Stegen, S.J.C.; Miller, J.S. Engineered and Banked IPSCs for Advanced NK- and T-Cell Immunotherapies. Blood 2023, 141, 846–855. [Google Scholar] [CrossRef]
- Bock, A.M.; Knorr, D.; Kaufman, D.S. Development, Expansion, and In Vivo Monitoring of Human NK Cells from Human Embryonic Stem Cells (HESCs) and and Induced Pluripotent Stem Cells (IPSCs). J. Vis. Exp. 2013, 74, e50337. [Google Scholar] [CrossRef]
- Euchner, J.; Sprissler, J.; Cathomen, T.; Fürst, D.; Schrezenmeier, H.; Debatin, K.M.; Schwarz, K.; Felgentreff, K. Natural Killer Cells Generated From Human Induced Pluripotent Stem Cells Mature to CD56brightCD16+NKp80+/− In-Vitro and Express KIR2DL2/DL3 and KIR3DL1. Front. Immunol. 2021, 12, 640672. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Human Induced Pluripotent Stem Cells: From Cell Origin, Genomic Stability, and Epigenetic Memory to Translational Medicine. Stem Cells 2022, 40, 546–555. [Google Scholar] [CrossRef]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.; Ji, H.; Ehrlich, L.; et al. Epigenetic Memory in Induced Pluripotent Stem Cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef]
- Woan, K.V.; Kim, H.; Bjordahl, R.; Davis, Z.B.; Gaidarova, S.; Goulding, J.; Hancock, B.; Mahmood, S.; Abujarour, R.; Wang, H.; et al. Harnessing Features of Adaptive NK Cells to Generate IPSC-Derived NK Cells for Enhanced Immunotherapy. Cell Stem Cell 2021, 28, 2062–2075.e5. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, J.P.; Bjordahl, R.; Mahmood, S.; Reiser, J.; Gaidarova, S.; Blum, R.; Cichocki, F.; Chu, H.; Bonello, G.; Lee, T.; et al. FT576 Path to First-of-Kind Clinical Trial: Translation of a Versatile Multi-Antigen Specific off-the-Shelf NK Cell for Treatment of Multiple Myeloma. Cancer Res. 2021, 81 (Suppl. 13), 1550. [Google Scholar] [CrossRef]
- Cichocki, F.; Bjordahl, R.; Goodridge, J.P.; Mahmood, S.; Gaidarova, S.; Abujarour, R.; Davis, Z.B.; Merino, A.; Tuininga, K.; Wang, H.; et al. Quadruple Gene-Engineered Natural Killer Cells Enable Multi-Antigen Targeting for Durable Antitumor Activity against Multiple Myeloma. Nat. Commun. 2022, 13, 7341. [Google Scholar] [CrossRef]
- ATCC. NK-92®. Available online: https://www.atcc.org/products/crl-2407 (accessed on 15 October 2025).
- Gunesch, J.Y.; Angelo, L.S.; Mahapatra, S.; Deering, R.P.; Kowalko, J.E.; Sleiman, P.; Tobias, J.W.; Monaco-Shawver, L.; Orange, J.S.; Mace, E.M. Genome-Wide Analyses and Functional Profiling of Human NK Cell Lines. Mol. Immunol. 2019, 115, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Klingemann, H. The Natural Killer Cell Line NK-92 and Its Genetic Variants: Impact on NK Cell Research and Cancer Immunotherapy. Cancers 2025, 17, 1968. [Google Scholar] [CrossRef]
- Klingemann, H. The NK-92 Cell Line—30 Years Later: Its Impact on Natural Killer Cell Research and Treatment of Cancer. Cytotherapy 2023, 25, 451–457. [Google Scholar] [CrossRef]
- Snyder, K.M.; Hullsiek, R.; Mishra, H.K.; Mendez, D.C.; Li, Y.; Rogich, A.; Kaufman, D.S.; Wu, J.; Walcheck, B. Expression of a Recombinant High Affinity IgG Fc Receptor by Engineered NK Cells as a Docking Platform for Therapeutic MAbs to Target Cancer Cells. Front. Immunol. 2018, 9, 2873. [Google Scholar] [CrossRef]
- Huang, R.S.; Shih, H.A.; Lai, M.C.; Chang, Y.J.; Lin, S. Enhanced NK-92 Cytotoxicity by CRISPR Genome Engineering Using Cas9 Ribonucleoproteins. Front. Immunol. 2020, 11, 1008. [Google Scholar] [CrossRef]
- Navarrete-Galvan, L.; Guglielmo, M.; Cruz Amaya, J.; Smith-Gagen, J.; Lombardi, V.C.; Merica, R.; Hudig, D. Optimizing NK-92 Serial Killers: Gamma Irradiation, CD95/Fas-Ligation, and NK or LAK Attack Limit Cytotoxic Efficacy. J. Transl. Med. 2022, 20, 151. [Google Scholar] [CrossRef]
- Chu, J.; Deng, Y.; Benson, D.M.; He, S.; Hughes, T.; Zhang, J.; Peng, Y.; Mao, H.; Yi, L.; Ghoshal, K.; et al. CS1-Specific Chimeric Antigen Receptor (CAR)-Engineered Natural Killer Cells Enhance in Vitro and in Vivo Antitumor Activity against Human Multiple Myeloma. Leukemia 2014, 28, 917–927. [Google Scholar] [CrossRef]
- Jiang, H.; Zhana, W.; Shang, P.; Zhana, H.; Fu, W.; Ye, F.; Zeng, T.; Huanga, H.; Zhang, X.; Sun, W.; et al. Transfection of Chimeric Anti-CD138 Gene Enhances Natural Killer Cell Activation and Killing of Multiple Myeloma Cells. Mol. Oncol. 2014, 8, 297–310. [Google Scholar] [CrossRef]
- Maroto-Martín, E.; Encinas, J.; García-Ortiz, A.; Alonso, R.; Leivas, A.; Paciello, M.L.; Garrido, V.; Cedena, T.; Ugalde, L.; Powell, D.J.; et al. PS1209 NKG2D and BCMA-CAR NK cells efficiently eliminate multiple myeloma cells. A comprehensive comparison between two clinically relevant CARs. HemaSphere 2019, 3 (Suppl. 1), 550–551. [Google Scholar] [CrossRef]
- Laurent, S.A.; Hoffmann, F.S.; Kuhn, P.-H.; Cheng, Q.; Chu, Y.; Schmidt-Supprian, M.; Hauck, S.M.; Schuh, E.; Krumbholz, M.; Rubsamen, H.; et al. G-Secretase Directly Sheds the Survival Receptor BCMA from Plasma Cells. Nat. Commun. 2015, 6, 7333. [Google Scholar] [CrossRef]
- Motais, B.; Charvátová, S.; Walek, Z.; Hájek, R.; Bagó, J.R. NK92 Expressing Anti-BCMA CAR and Secreted TRAIL for the Treatment of Multiple Myeloma: Preliminary In Vitro Assessment. Cells 2023, 12, 2748. [Google Scholar] [CrossRef] [PubMed]
- Fate Therapeutics. Fate Therapeutics Reports First Quarter 2024 Financial Results and Business Updates. Available online: https://ir.fatetherapeutics.com/news-releases/news-release-details/fate-therapeutics-reports-first-quarter-2024-financial-results (accessed on 15 October 2025).
- Mikkilineni, L.; Kochenderfer, J.N. Chimeric Antigen Receptor T-Cell Therapies for Multiple Myeloma. Blood 2017, 130, 2594–2602. [Google Scholar] [CrossRef] [PubMed]
- Roex, G.; Campillo-Davo, D.; Flumens, D.; Shaw, P.A.G.; Krekelbergh, L.; De Reu, H.; Berneman, Z.N.; Lion, E.; Anguille, S. Two for One: Targeting BCMA and CD19 in B-Cell Malignancies with off-the-Shelf Dual-CAR NK-92 Cells. J. Transl. Med. 2022, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, Y.; Chen, C.; Li, Z.; Xu, K.; Zhao, K. Targeting GPRC5D for Multiple Myeloma Therapy. J. Hematol. Oncol. 2024, 17, 88. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Liu, T.; Wang, C.; Wang, H.; Wang, Q.; Wang, Q.; Ye, G.; Tang, R.; Cao, Z. Dual-Targeted CAR-NK Cell Therapy: Optimized CAR Design to Prevent Antigen Escape and Elicit a Deep and Durable Response in Multiple Myeloma. Cancer Res. 2023, 83 (Suppl. 7), 4077. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, L.; Zhu, Z.; Yan, Y.; Wu, G.; Wei, M.; Ning, J.; Yang, J. Efficacy of Human IPSC-Derived CAR-NK Cells Targeting Multiple Myeloma Cells. Blood 2023, 142 (Suppl. 1), 4802. [Google Scholar] [CrossRef]
- Leivas, A.; Valeri, A.; Córdoba, L.; García-Ortiz, A.; Ortiz, A.; Sánchez-Vega, L.; Graña-Castro, O.; Fernández, L.; Carreño-Tarragona, G.; Pérez, M.; et al. NKG2D-CAR-Transduced Natural Killer Cells Efficiently Target Multiple Myeloma. Blood Cancer J. 2021, 11, 146. [Google Scholar] [CrossRef]
- Gozzetti, A.; Ciofini, S.; Simoncelli, M.; Santoni, A.; Pacelli, P.; Raspadori, D.; Bocchia, M. Anti CD38 Monoclonal Antibodies for Multiple Myeloma Treatment. Hum. Vaccines Immunother. 2022, 18, 2052658. [Google Scholar] [CrossRef]
- de Acha, O.P.; Reiman, L.; Jayabalan, D.S.; Walker, Z.J.; Bosma, G.; Keller, A.L.; Parzych, S.E.; Abbott, D.; Idler, B.M.; Ribadeneyra, D.; et al. CD38 Antibody Re-Treatment in Daratumumab-Refractory Multiple Myeloma after Time on Other Therapies. Blood Adv. 2023, 7, 6430–6440. [Google Scholar] [CrossRef] [PubMed]
- Karvouni, M.; Vidal-Manrique, M.; Susek, K.H.; Hussain, A.; Gilljam, M.; Zhang, Y.; Gray, J.D.; Lund, J.; Kaufmann, G.; Ljunggren, H.-G.; et al. Challenges in αCD38-Chimeric Antigen Receptor (CAR)-Expressing Natural Killer (NK) Cell-Based Immunotherapy in Multiple Myeloma: Harnessing the CD38dim Phenotype of Cytokine-Stimulated NK Cells as a Strategy to Prevent Fratricide. Cytotherapy 2023, 25, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Ruby Chan, S.; Mathavan, K.; Sillitti, D.; Mottershead, C.; Mattson, B.; Pache, M.; Gutierrez, A.; Scoon, W.; Zhu, Y.; et al. FT555: Off-the-Shelf CAR-NK Cell Therapy Co-Targeting GPRC5D and CD38 for the Treatment of Multiple Myeloma. Blood 2022, 140 (Suppl. 1), 4560–4561. [Google Scholar] [CrossRef]
- Akhmetzyanova, I.; McCarron, M.J.; Parekh, S.; Chesi, M.; Bergsagel, P.L.; Fooksman, D.R. Dynamic CD138 Surface Expression Regulates Switch between Myeloma Growth and Dissemination. Leukemia 2020, 34, 245–256. [Google Scholar] [CrossRef]
- Li, H.; Song, W.; Li, Z.; Zhang, M. Preclinical and Clinical Studies of CAR-NK-Cell Therapies for Malignancies. Front. Immunol. 2022, 13, 992232. [Google Scholar] [CrossRef]
- Jo, D.H.; Kaczmarek, S.; Khan, A.U.H.; Pervin, J.; Clark, D.M.; Gadde, S.; Wang, L.; McComb, S.; Visram, A.; Lee, S.H. Entinostat, a Histone Deacetylase Inhibitor, Enhances CAR-NK Cell Anti-Tumor Activity by Sustaining CAR Expression. Front. Immunol. 2025, 16, 1533044. [Google Scholar] [CrossRef]
- Hsi, E.D.; Steinle, R.; Balasa, B.; Szmania, S.; Draksharapu, A.; Shum, B.P.; Huseni, M.; Powers, D.; Nanisetti, A.; Zhang, Y.; et al. CS1, a Potential New Therapeutic Antibody Target for the Treatment of Multiple Myeloma. Clin. Cancer Res. 2008, 14, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Pfefferle, A.; Contet, J.; Wong, K.; Chen, C.; Verhoeyen, E.; Slichter, C.K.; Schluns, K.S.; Cursons, J.; Berry, R.; Nikolic, I.; et al. Optimisation of a Primary Human CAR-NK Cell Manufacturing Pipeline. Clin. Transl. Immunol. 2024, 13, e1507. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Takeuchi, I.; Yamaguchi, H. Scalable Production Process Development for NK Cells Targeting Large-Scale Expansion. Regen. Ther. 2025, 30, 535–543. [Google Scholar] [CrossRef]
- Food and Drug Administration. Available online: https://www.govinfo.gov/content/pkg/CFR-2011-title21-vol8/pdf/CFR-2011-title21-vol8-part1271.pdf (accessed on 15 October 2025).
- Lowdell, M.W. Considerations for Manufacturing of Cell and Gene Medicines for Clinical Development. Cytotherapy 2025, 27, 874–883. [Google Scholar] [CrossRef]
- Europesn Medicines Agency. Guideline on Quality, Non-Clinical and Clinical Requirements for Investigational Advanced Therapy Medicinal Products in Clinical Trials-Scientific Guideline. Available online: https://www.ema.europa.eu/en/guideline-quality-non-clinical-clinical-requirements-investigational-advanced-therapy-medicinal-products-clinical-trials-scientific-guideline (accessed on 15 October 2025).
- Lu, S.-J.; Feng, F. CAR-NK Cells from Engineered Pluripotent Stem Cells: Off-the-Shelf Therapeutics for All Patients. Stem Cells Transl. Med. 2021, 10, S10–S17. [Google Scholar] [CrossRef]
- Dhaiban, S.; Chandran, S.; Noshi, M.; Sajini, A.A. Clinical Translation of Human IPSC Technologies: Advances, Safety Concerns, and Future Directions. Front. Cell Dev. Biol. 2025, 13, 1627149. [Google Scholar] [CrossRef]
- Madrid, M.; Lakshmipathy, U.; Zhang, X.; Bharti, K.; Wall, D.M.; Sato, Y.; Muschler, G.; Ting, A.; Smith, N.; Deguchi, S.; et al. Considerations for the Development of iPSC-Derived Cell Therapies: A Review of Key Challenges by the JSRM-ISCT iPSC Committee. Cytotherapy 2024, 26, 1382–1399. [Google Scholar] [CrossRef]
- Kwon, D.; Moon, B.K.; Han, M.; Lee, T.; Lee, J.; Kang, K. Genetically Stable Multi-Gene Edited iPSCs-Derived NK Cells for Enhanced Cancer Immunotherapy. Mol. Ther. Oncolytics 2024, 32, 200885. [Google Scholar] [CrossRef] [PubMed]
| NCT | Organization | Phase | Status of Study | Study’s Official Title | NK Cell Source | Target |
|---|---|---|---|---|---|---|
| Ongoing clinical trials | ||||||
| NCT05092451 | M.D. Anderson Cancer Center (Houston, TX, USA) | Phase 1/2 | Recruiting | Phase I/II Study of CAR.70-Engineered IL15-transduced Cord Blood-derived NK Cells in Conjunction With Lymphodepleting Chemotherapy for the Management of Relapse/Refractory Hematological Malignances | Allogenic Cord Blood NK cells | CD70 |
| NCT05182073 | Fate Therapeutics (USA, multi-center trial) | Phase 1 | Active, not recruiting | A Phase I Study of FT576 as Monotherapy and in Combination With Daratumumab in Subjects With Relapsed/Refractory Multiple Myeloma | Allogenic iPSCs | BCMA |
| NCT06594211 | RenJi Hospital (Shanghai, Shanghai Municipality, China) | Not Applicable | Not yet recruiting | A Single-Arm, Open-Label Study of Allogeneic Anti-BCMA/GPRC5D Bispecific CAR-NK Cells (ACT-001) in Patients With Relapsed or Refractory Multiple Myeloma | Allogeneic unknown | BCMA/GPRC5D |
| NCT06045091 | Hrain Biotechnology Co., Ltd. (Shanghai, Shanghai Municipality, China) | Early Phase 1 | Recruiting | An Early Phase 1 Clinical Trial to Evaluate the Safety and Efficacy of Human BCMA Targeted CAR-NK Cells Injection for Subjects With Relapsed/Refractory Multiple Myeloma or Plasma Cell Leukemia | Unknown | BCMA |
| NCT06242249 | Shahid Beheshti University of Medical Sciences (Tehran, Iran) | Phase 1, Phase 2 | Not yet recruiting | Determining Safety and Maximum Tolerated Dose (MTD) of Anti-BCMA CAR-NK Therapy in Relapsed or Refractory Multiple Myeloma | Unknown | BCMA |
| NCT05498545 | Second Affiliated Hospital of Xi’an Jiaotong University (Xi’an, Shaanxi, China) | Phase 1 | Not yet recruiting | Universal BCMA-targeted LUCAR-B68 Cells in Patients With Relapsed/Refractory Multiple Myeloma | Unknown | BCMA |
| NCT06379451 | Changzhou No.2 People’s Hospital (Changzhou, Jiangsu, China) | Early Phase 1 | Not yet recruiting | An Exploratory Clinical Study of the Safety and Efficacy of NKG2D Chimeric Antigen Receptor NK Cell Injections for the Treatment of Refractory Recurrent Multiple Myeloma | Unknown | NKG2D |
| Clinical trials with unknown status | ||||||
| NCT05008536 | Xinqiao Hospital of Chongqing (Chongqing, Chongqing Municipality, China) | Early Phase 1 | Unknown | Phase I Study to Evaluate the Safety and Effectiveness of Anti-BCMA CAR-NK Therapy in Relapsed or Refractory Multiple Myeloma | Allogenic Umbilical and Cord Blood NK Cells | BCMA |
| NCT03940833 | Asclepius Technology Company Group (Suzhou) Co., Ltd. (Wuxi, Jiangsu, China) | Phase 1, Phase 2 | Unknown | Clinical Research of Adoptive BCMA CAR-NK Cells on Relapse/Refractory MM | NK-92 | BCMA |
| NCT05652530 | Shenzhen Pregene Biopharma Co., Ltd. (Zhenghou, Henan, China) | Early Phase 1 | Unknown | Clinical Study of the Safety and Efficacy of Chimeric Antigen Receptor NK Cell Injection Targeting BCMA (BCMA CAR-NK) in Patients With Relapsed/Refractory Multiple Myeloma | Unknown | BCMA |
| Category | Representative Barriers | Translational Impact |
|---|---|---|
| Manufacturing scalability and consistency | Donor-to-donor variability; limited expansion yields of primary NK cells; absence of fully automated, closed-system processes | Batch-to-batch inconsistency and limited large-scale GMP production capacity |
| Logistics and supply-chain constraints | Cryopreservation effects on viability and potency; complex cold-chain distribution; need for rapid, decentralized manufacturing networks | Increased cost and reduced accessibility of therapy |
| Regulatory and quality-control hurdles | Lack of standardized potency and identity assays; variability across manufacturing sites; evolving oversight of multiplex gene-edited NK lines | Delayed clinical translation and higher Chemistry, Manufacturing, and Controls (CMC)/regulatory burden |
| Technological platform limitations | iPSC-derived and immortalized NK sources require master-cell-bank characterization, genomic-stability monitoring, and differentiation control | Added safety and validation demands before “off-the-shelf” application |
| Global standardization gaps | Fragmented regulatory expectations between U.S., EU, and Asia; limited ICH/ISCT harmonization | Increased development cost and longer time to market |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastrich, A.; Vinogradova, K.; Mokrousova, D.; Efremova, A.; Makhnach, O.; Goldshtein, D. State of the Art of CAR-NK Cell Therapy in Multiple Myeloma: A Comprehensive Review of Cell Sources and Target Antigens. Int. J. Mol. Sci. 2025, 26, 11224. https://doi.org/10.3390/ijms262211224
Bastrich A, Vinogradova K, Mokrousova D, Efremova A, Makhnach O, Goldshtein D. State of the Art of CAR-NK Cell Therapy in Multiple Myeloma: A Comprehensive Review of Cell Sources and Target Antigens. International Journal of Molecular Sciences. 2025; 26(22):11224. https://doi.org/10.3390/ijms262211224
Chicago/Turabian StyleBastrich, Asya, Kamilla Vinogradova, Diana Mokrousova, Anna Efremova, Oleg Makhnach, and Dmitry Goldshtein. 2025. "State of the Art of CAR-NK Cell Therapy in Multiple Myeloma: A Comprehensive Review of Cell Sources and Target Antigens" International Journal of Molecular Sciences 26, no. 22: 11224. https://doi.org/10.3390/ijms262211224
APA StyleBastrich, A., Vinogradova, K., Mokrousova, D., Efremova, A., Makhnach, O., & Goldshtein, D. (2025). State of the Art of CAR-NK Cell Therapy in Multiple Myeloma: A Comprehensive Review of Cell Sources and Target Antigens. International Journal of Molecular Sciences, 26(22), 11224. https://doi.org/10.3390/ijms262211224







