Abstract
Elderly individuals are more vulnerable to disease due to their increased frailty. Emerging evidence highlights the potential of probiotics as geroprotective agents by maintaining gut health and modulating key physiological processes involved in aging, such as inflammation, cognitive functions, and metabolism. Here, we investigated the geroprotective potential of four probiotic strains (Lacticaseibacillus paracasei LPC1114, Limosilactobacillus reuteri PBS072, Bifidobacterium breve BB077, and Bifidobacterium animalis subsp. lactis BL050) using Caenorhabditis elegans as an aging model. Mid-life healthspan parameters were assessed, including lifespan, motility, ROS levels, lipofuscin accumulation, and cognitive capabilities. The probiotics exhibited strain-specific effects. L. reuteri PBS072 and B. lactis BL050 significantly increased locomotion by 20% and decreased ROS levels by 70% and 30% respectively, suggesting enhanced oxidative stress response and neuromuscular maintenance. B. breve BB077, L. paracasei LPC1114, and L. reuteri PBS072 enhanced associative learning performance, whereas B. lactis BL050 improved chemotactic response. Notably, only L. paracasei LPC1114 and L. reuteri PBS072 extended the maximum lifespan by 4 and 5 days, respectively, an effect mediated by the longevity-related genes skn1, sir2.1, and daf16. Our findings highlight the multifaceted, strain-specific geroprotective properties of probiotics and support their potential as microbiome-based interventions to promote healthy aging.
1. Introduction
During the aging process, many physiological changes occur, including increased oxidative stress and declines in metabolic, immunological, and cognitive functions. As a result, the prevalence of frailty and chronic diseases is increasing in elderly individuals [1,2,3,4]. Moreover, the 2024 WHO report shows that the number of people aged 60 and over is expected to double in the next 30 years [5] and, therefore, a growing field in the biology of healthy aging is focused on counteracting aging-related changes using geroprotector agents [6,7]. Geroprotectors are emerging agents with the capability of slowing down the aging process by minimizing cellular physiological perturbations, aiming to improve quality of life and to prevent age-related disorders [8,9,10,11,12]. In this context, increasing evidence over the past decade suggests that significant modifications of gut microbiota during aging are correlated with the development of aging-related conditions [9,13]. In particular, dysbiosis (i.e., alteration in gut microbiota composition and function) has been associated with several pathological conditions, including obesity, type 2 diabetes, inflammation, cardiovascular, and neurodegenerative diseases [14]. Therefore, maintaining a homeostasis in host-microbiota interaction is crucial to counteract inflammation, preserve intestinal permeability, and ameliorate both cognitive performance and muscle function [15,16,17,18]. The gut microbiota can be effectively and stably modulated through dietary interventions using different health-promoting components. Among these, probiotics exert multiple beneficial effects on host health, including age-related processes. In fact, the probiotic modulation of the gut microbiota is a valuable approach for the prevention and potential mitigation of age-related disorders [19]. In the FAO/WHO report on food and nutrition [20], probiotics are defined as “live microorganisms which, when consumed in adequate amounts, confer a health effect on the host” [21]. The potential role of probiotics in promoting longevity was first proposed in the early 20th century by Elie Metchnikoff, who observed a positive correlation between the consumption of Lactobacillus bulgaricus, contained in yogurt, and increased longevity in the Bulgarian population [22]. Historically, this hypothesis was further supported by the identification of good health-associated bacteria species in centenarians aged 105–109 years [23]. Finally, recent studies have demonstrated that gut microbiota modulation through probiotics in the elderly can improve immune response, cognitive function, and reduce body fat, metabolic syndromes, cardiovascular risk factors, and insulin resistance markers [24,25].
Lactic acid bacteria (LAB), especially Lactobacillus and Bifidobacterium, are two genera present in human microbiota and represent the most studied probiotics for their species- and strain-specific beneficial effects [26,27] and geroprotective properties [9,24,28]. Their benefits on health were evaluated and confirmed using different preclinical models, including mice, flies, and nematodes [29,30,31,32,33,34,35]. Among these models, Caenorhabditis elegans, a small nematode, provides several advantages for the study of age and host-microbiota interactions, including high experimental reproducibility, low maintenance costs, large size, short lifespan (~2–3 weeks), and no ethical constraints. Furthermore, C. elegans is a bacterivore organism normally maintained in axenic laboratory conditions using Escherichia coli OP50 as a food source, which prevents the formation of a complex intestinal microbiota [36]. This unique feature allows precise control of its gut microbiota through dietary manipulation and facilitates the evaluation of host-probiotic interactions and their physiological effects. The gnotobiotic nature of C. elegans makes it suitable for high-throughput screenings in a whole-organism, enabling the assessment of physiological responses across multiple tissues [37]. By monitoring key phenotypic traits such as lifespan, locomotion, reactive oxygen species (ROS) production, and cognitive-like functions, it is possible to evaluate the potential anti-aging impact of specific probiotic strains both in a controlled and efficient manner.
Taking advantage of the useful features of this model, we assessed the geroprotective effects of two LAB (Lacticaseibacillus paracasei LPC1114 and Limosilactobacillus reuteri PBS072) and two Bifidobacteria (Bifidobacterium breve BB077 and Bifidobacterium animalis subsp. lactis BL050) strains. These selected strains have been previously characterized for their probiotic activity [38,39,40,41], and their anti-inflammatory properties have been demonstrated both in vitro and in clinical studies. In particular, they have been shown to reduce proinflammatory cytokine levels and to alleviate symptoms associated with the cold, allergic, and urogenital diseases [39,40,42,43,44]. Additionally, L. reuteri PBS072 and B. breve BB077 have been shown to enhance serotonin production and to improve cognitive function, sleep quality, and mood under stress conditions, as demonstrated in three different clinical trials [45,46,47].
By assessing key healthspan and cognitive parameters, we aimed to uncover strain-specific effects on C. elegans aging-related traits. Our findings reveal that these probiotics exert distinct and multifaceted effects on neuromuscular function, oxidative stress response, cognitive performance, and, in some cases, lifespan extension. These effects seem to involve conserved signaling pathways related to immunity, stress resistance, and metabolism. Overall, our results support the potential of microbiome-based strategies to promote healthy aging and underline the importance of mechanistic insights into strain-specific probiotic actions.
2. Results
2.1. The Administration of Lactobacillus and Bifidobacterium spp. Ameliorates Phenotypic Aging Parameters in a Strain-Specific Manner
To evaluate the geroprotective effects of probiotics on healthspan parameters, synchronized C. elegans N2 nematodes were grown on E. coli OP50 until day-1 adulthood, then washed and transferred to NGM plates seeded with living L. paracasei LPC1114, L. reuteri PBS072, B. breve BB077, and B. lactis BL050 probiotic strains. Healthspan parameters, including lifespan, locomotion, total ROS levels, and lipofuscin accumulation data, were assessed on day 11th of adulthood and compared to control worms continuously fed with E. coli OP50.
As shown in Figure 1A,B and Table 1, the survival curves of C. elegans grown on the different probiotic strains showed that the probiotic diets did not alter the median lifespan compared to the control. However, a significant increase in maximum lifespan was observed in worms fed with LAB strains, suggesting a targeted effect in aged worms. Specifically, populations fed with L. paracasei LPC1114 and L. reuteri PBS072 lived 4 and 5 days longer than the control group, respectively.
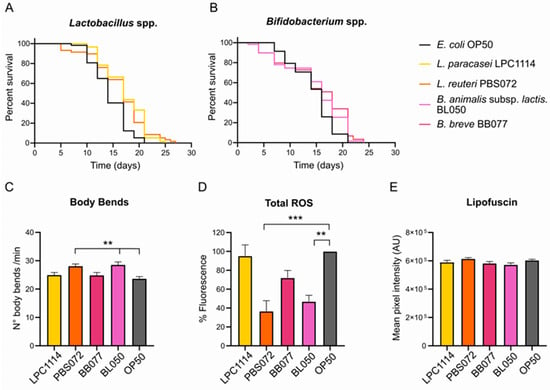
Figure 1.
Effects of probiotics treatment on C. elegans healthspan parameters. (A,B). Lifespan assay of C. elegans population treated with LAB (A) and Bifidobacteria (B) strains. Probiotics were administered to a population of 60 1-day adult nematodes every two days until the whole population died. The representative Kaplan–Meier graphs with log-rank (Mantel–Cox) test were reported and represent the survival of the populations. (C–E) Healthspan parameters on day 11 of adulthood of C. elegans populations fed with probiotic strains. (C) Body bends in one minute. Data reported represents mean ± s.e.m. of 60 nematodes from three independent experiments. (D) Total ROS in C. elegans whole organisms. ROS signals were obtained from 20 living nematodes using the fluorescent probe DCF-DA and expressed as a percentage with respect to the control. Data reported represent mean ± s.e.m. of three independent experiments. (E) Lipofuscin content. Lipofuscin was measured by assessing autofluorescence by confocal analysis. Data reported represent mean ± s.e.m. of 30 nematodes that are derived from three independent biological experiments. An ordinary one-way ANOVA with Dunnet’s multiple comparison test was performed for the statistical analysis. ** p < 0.01, *** p < 0.001.

Table 1.
Lifespan parameters of C. elegans treated with LAB and Bifidobacteria probiotic strains. The table reports the median and maximum lifespan values obtained from the Kaplan–Meier graph (Figure 1) and expressed as the mean ± standard deviation from three independent experiments.
Additional healthspan parameters were assessed on day 11 of adulthood, including locomotion, total intracellular ROS levels, and lipofuscin accumulation. Among all probiotic treatments, only L. reuteri PBS072 and B. lactis BL050 induced statistically significant changes. Administration of these strains led to an approximately 20% increase in body bends (Figure 1C) and a significant reduction in total ROS levels of about 70% and 30%, respectively (Figure 1D). In contrast, none of the probiotic strains, including L. reuteri PBS072 and B. lactis BL050, produced significant changes in lipofuscin accumulation compared to the control (Figure 1E and Figure S1).
To confirm the presence of the administered probiotics in the gut, colony-forming units (CFUs) were quantified in aged nematodes (11th day of adulthood). Although E. coli OP50 exhibited a CFU count up to 105-fold higher, the probiotic strains showed comparable levels of gut colonization (Table 2). This indicates that the observed beneficial effects are not attributable to differences in bacterial load within the gut.

Table 2.
Colonization ability of probiotic strains in the nematode intestine at day 11 of adulthood. Colony Forming Unit (CFU) per mL was obtained from nematode lysate and mean ± s.d., from three replicates was reported.
These findings suggest that the administration of LAB and Bifidobacteria spp. improves phenotypic aging markers in a strain-specific manner.
2.2. Probiotic Diets Do Not Affect Neuromuscular Trasmission but Enhance Cognitive Functions in Aged C. elegans
The age-related decline in motility observed in C. elegans is partly attributed to the deterioration of motor neurons [48]. To determine whether the observed increase in motility was associated with enhanced synaptic transmission in motor neurons, nematodes fed with probiotic strains were subjected to Aldicarb sensitivity assay on day 11 of adulthood. Aldicarb acts by inhibiting the acetylcholinesterase enzyme, leading to the accumulation of acetylcholine at the neuromuscular junction and ultimately causing paralysis [49]. Differences in the time to paralysis can thus indicate alterations in neuromuscular signaling. Following administration of 1 mM Aldicarb, no significant differences in paralysis onset were observed among the groups (Figure 2A), indicating that probiotic diets do not affect neuromuscular transmission.
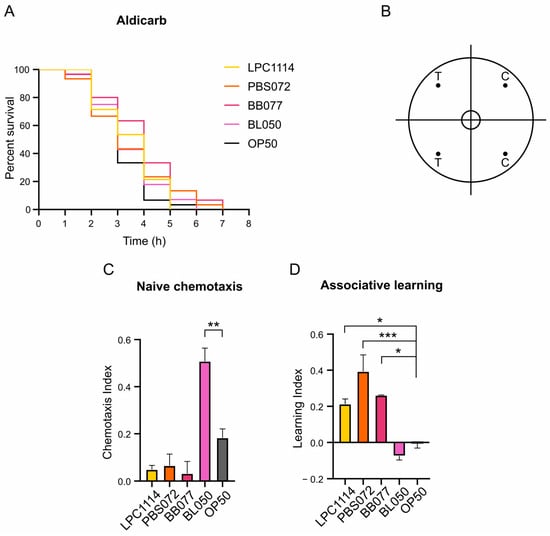
Figure 2.
Effects of probiotics treatment on C. elegans neuronal functions at the 11th day of adulthood treated with LAB and Bifidobacteria probiotic strains. (A) Synaptic impairment was assessed through Aldicarb assay. On day 11 of lifespan, 30 nematodes were treated with 1 mM of Aldicarb. Representative Kaplan–Meier graphs with log-rank (Mantel–Cox) test of the survival of the populations were reported. No significant differences were observed. (B) Representative chemotaxis test plate. T = test quadrants with 10% butanone; C = control quadrants with EtOH. Dots represent the point in which butanone or EtOH were dropped. (C,D). Naïve chemotaxis and associative learning graphs. Chemotaxis and learning indices were calculated as described in Materials and Methods section. The experiment was performed using at least 100 nematodes. Data represents the mean ± s.e.m. of three independent experiments. Ordinary one-way ANOVA with Dunnet’s multiple comparison test was performed for statistical analysis. * p < 0.05, ** p < 0.01, *** p < 0.001.
Beyond motor function, aging also impairs sensory perception due to progressive neuronal functions decline. In C. elegans, odor sensitivity decreased with age as a consequence of sensory neuronal circuits deterioration [50]. The nematode can distinguish between a plethora of attractive and repellent odorants through its 24 chemosensory neurons [51,52]. To evaluate whether probiotics supplementation supports sensory neuronal function during aging, chemotaxis assays were performed. Butanone, recognized by C. elegans as an attractive stimulus, and ethanol, used as a neutral control, were spotted on opposite sides of the chemotaxis plate (Figure 2B). On the 11th day of adulthood, nematodes were extensively washed and dropped in the center of a plate. After one hour, the naïve chemotaxis index (Cinaïve) was calculated, and the results showed that nematodes fed with B. lactis BL050 exhibited a two-fold increase in attraction to butanone compared to controls fed with E. coli OP50 (Figure 2C). In contrast, worms treated with the L. paracasei LPC1114, L. reuteri PBS072, or B. breve BB077 showed a slight but non-significant reduction in Cinaïve (Figure 2C).
Finally, the ability of probiotic strains to enhance learning was evaluated in the same population. C. elegans can perform associative learning by linking an odor with food presence [53]. After washing with M9 buffer and one hour of starvation, worms were exposed to butanone to induce conditioning. Following an additional wash, chemotaxis index (Ci) after conditioning was measured (Table 3) and compared with the Cinaïve to calculate the learning index (Li; see Materials and Methods section for formula details). Otherwise to the Cinaïve results, L. paracasei LPC1114, L. reuteri PBS072, and B. breve BB077 treatment led to a significant increase in Li compared to the control (Figure 2D and Table 3), whereas B. lactis BL050 showed no improvement in Li.

Table 3.
Chemotaxis index after butanone enhancement. The table presents the mean ± s.e.m. of three independent experiments.
These findings demonstrate that L. paracasei LPC1114, L. reuteri PBS072, B. breve BB077, and B. lactis BL050 can ameliorate neuronal plasticity in aging nematodes, although different mechanisms, supporting their strain-specific geroprotective effects on the nervous system.
2.3. Probiotic Diets Differently Increase Progeny Production
In C. elegans, the fertile period is about five days starting from the first day of adulthood, with a peak in egg-laying on day one followed by a rapid decline [36]. Aging reduces offspring production from the second fertility day, but longevity-promoting agents can influence both total progeny and the egg-laying deposition [54]. To assess the impact of probiotic supplementation on fertility, the daily progeny deposited by a single nematode was quantified over five days (Figure 3). The results showed that the administration of the strains L. paracasei LPC1114, B. breve BB077, and B. lactis BL050 led to an increase in total progeny of 18%, 39%, and 42%, respectively; conversely, L. reuteri PBS072 showed no significant differences compared to the control. Notably, L. paracasei LPC1114 and B. lactis BL050 stimulated egg deposition primarily on day one. Interestingly, B. breve BB077 significantly altered the deposition curve, with a marked increase in egg-laying on days two and three, 5-fold and 17-fold higher compared to the control, respectively. This clearly demonstrates that B. breve BB077 is the only strain capable of exerting an anti-senescence effect on the laying curve by extending fertility beyond the initial reproductive burst.
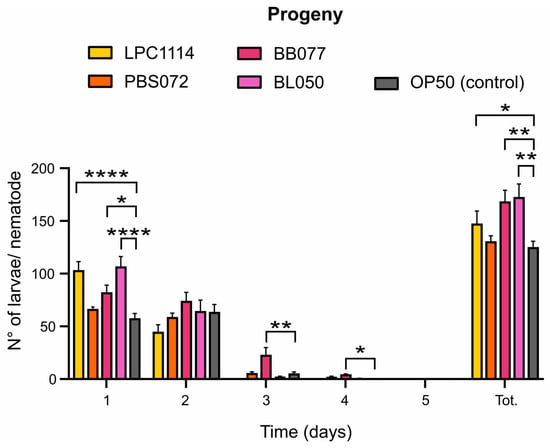
Figure 3.
Effects of probiotics on C. elegans progeny. The mean ± s.e.m. of progeny from 10 nematodes was reported. Ordinary one-way ANOVA with Dunnet’s multiple comparison test was performed for statistical analysis. * p < 0.05, ** p < 0.01, **** p < 0.0001.
2.4. Identification of Genes Involved in Lifespan Extension
As mentioned in the introduction, the lifespan extension and geroprotective effects of probiotics may involve the regulation of evolutionarily conserved pathways related to innate immunity, energy metabolism, and cellular stress response. These pathways are functionally interconnected and converge on two key transcriptional factors: DAF16 (FOXO human ortholog) and SKN1 (Nrf2 human ortholog) [9,37]. To identify which pathways are involved in the lifespan extension observed with LAB strains (Figure 1A and Table 1), lifespan assays were performed using the mutant strains GR1307 (Δdaf16) and EU1 (Δskn1) fed with L. paracasei LPC1114, L. reuteri PBS072, or E. coli OP50 (as a control) since the first day of adulthood. Worms fed with L. paracasei LPC1114 showed no significant differences in survival in both mutants (Figure 4A,B and Table 4) with respect to the control, indicating that both DAF16 and SKN1 are required for its pro-longevity effect. In contrast, worms fed with L. reuteri PBS072 showed an extended lifespan in the DAF16 mutant but not in the SKN1 mutant, suggesting that only SKN1 is involved in mediating its probiotic effect (Figure 4D,E and Table 4).
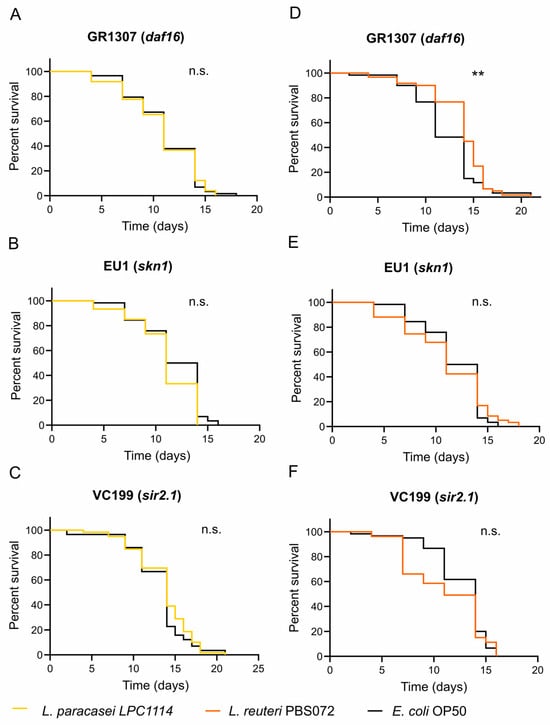
Figure 4.
Lifespan assay of C. elegans mutants treated with LAB strains. L. paracasei LPC1114 (A–C) and L. reuteri PBS072 (D–F) were administered to a 1-day adult C. elegans GR1307 (Δdaf16), EU1 (Δskn1), and VC199 (Δsir-2.1) mutants every two days until the whole population died. The representative Kaplan–Meier graphs with log-rank (Mantel–Cox) test were reported and represent the survival of the populations. ** p < 0.01. n.s. not significant.

Table 4.
Lifespan parameters of C. elegans treated with LAB. The table presents the median and maximum days of the lifespan reported from the Kaplan–Meier graph (Figure 4) and expressed as the mean ± s.d. from three independent experiments.
To further explore the molecular basis of these differential responses, we considered the role of SIR2.1, a NAD+-dependent deacetylase implicated in longevity and activated by different signals (such as dietary restriction) [55,56,57]. In our assays (Figure 4C,F and Table 4), neither LAB strains extended the lifespan in the VC199 strain (Δsir2.1),indicating that SIR2.1 is essential for their pro-longevity activity, likely through distinct signaling mechanisms. Collectively, these data suggest that L. paracasei LPC1114 effects require the coordinated activity of DAF16, SKN1, and SIR2.1, whereas L. reuteri PBS072 acts primarily through SKN1 and SIR2.1.
3. Discussion
Aging is a multifactorial, physiological process characterized by the gradual deterioration of cellular functions, which increases the risk of pathological diseases in the elderly [1]. Although aging is a natural and inevitable aspect of life, its progression and impact on health can vary significantly among individuals, suggesting the involvement of other factors beyond genetics. Among these factors, the gut microbiota composition has emerged as a key determinant of healthy aging and is now widely recognized as one of the main modulators of age-related physiological decline [58,59,60,61]. In fact, it has been reported that microbiota in centenarians contains specific beneficial bacterial species that are positively correlated with healthy aging [62,63]. These findings prompted scientists to further investigate the mechanisms underlying the beneficial probiotics’ effects on aging, using model organisms. Several findings indicate that probiotics can ameliorate healthspan and, in some cases, extend the lifespan by modulating specific biological pathways [9,37].
In this work, we used the well-characterized C. elegans model organism for studying the geroprotective effects of four probiotic strains: L. paracasei LPC1114, L. reuteri PBS072, B. breve BB077, and B. lactis BL050. C. elegans is a well-established model organism for aging research due to its several advantageous features, including short lifespan (2–3 weeks), fully sequenced genome, and highly conserved aging-related pathways [64,65,66,67]. We exploited these characteristics to evaluate multiple phenotypic parameters in nematode populations fed with a single living probiotic strain until mid-life (11th day of adulthood, except for lifespan assay). This time point corresponds to a stage when age-related physiological decline becomes evident, allowing for a comparison of aging-associated traits [68,69]. Starting with the general healthspan parameters, i.e., lifespan, locomotion, total ROS levels, and lipofuscin accumulation, we observed strain-specific improvements in the phenotypic traits during aging. In particular, L. reuteri PBS072 and B. lactis BL050 administration ameliorated movement and reduced total ROS content, but only L. reuteri PBS072 significantly extended lifespan. Otherwise, L. paracasei LPC1114 increased maximum lifespan, but did not ameliorate the other healthspan parameters, while B. breve BB077 showed no beneficial effects on any of the assessed parameters. Notably, the observed increase in the body bends following probiotics administration does not appear to be associated with enhanced synaptic transmission. This is supported by the lack of differences in survival curves after Aldicarb treatment, suggesting that L. reuteri PBS072 and B. lactis BL050 likely promote locomotion through enhanced muscle function rather than neuronal modulation. The lack of effect of these probiotic strains on Aldicarb-induced paralysis, with respect to E. coli OP50 used as a control, may reflect their limited influence on acetylcholine signaling at the neuromuscular junction. Aldicarb induces paralysis by inhibiting acetylcholinesterase, leading to acetylcholine accumulation and muscle hypercontraction. Probiotics that primarily modulate oxidative stress or overall healthspan may not significantly affect this pathway. Furthermore, strain-specific differences in metabolite production might be insufficient to alter Aldicarb sensitivity.
On the other hand, we observed improvements in cognitive functions, indicating that the nervous system may also benefit from specific probiotic geroprotection. Specifically, we evaluated both the naïve chemotaxis (Cinaïve) and associative learning (Li) indexes using butanone 10%. The treatment with both LAB and B. breve BB077 did not alter the Cinaïve, while B. lactis BL050 administration led to a two-fold increase in attraction to butanone compared to control nematodes fed with E. coli OP50. A similar probiotic-enhanced chemotaxis activity was previously reported in aged C. elegans fed with Limosilactobacillus reuteri (formerly Lactobacillus reuteri), where an increased naïve chemotaxis response to diacetyl was observed in comparison with E. coli-fed nematodes. This enhancement was attributed to upregulated expression of the diacetyl receptor ODR-10, which appears to be regulated by DAF-16 transcriptional factor [70]. Butanone is one of the five attractive volatile odorants sensed by the paired Amphid wing “C” (AWC) neurons [71], and its recognition is specifically dependent on the transmembrane guanylyl cyclase ODR-1 localized in the sensory cilia of AWC [72]. Although its regulation is not yet fully understood, it is possible to hypothesize that B. lactis BL050 administration may enhance odr-1 expression, as observed for ODR-10 receptor.
Moreover, previous studies have shown that the pre-exposure to butanone in combination with food (a process known as conditioning) induced a specific form of behavioral plasticity, called butanone enhancement, characterized by increased chemotaxis toward butanone following conditioning [53]. This represents an example of sensory integration, in which the combination of two stimuli, odor and food, triggers a plastic behavioral response like associative learning. In this context, our results showed that feeding C. elegans with L. paracasei LPC1114, L. reuteri PBS072, or B. breve BB077, but not with B. lactis BL050, enhances the associative learning index (Li) after conditioning in the presence of food with respect to the worms fed with E. coli OP50. It is important to note, however, that B. lactis BL050 Ci after conditioning has the highest index, resulting in a comparatively lower Li. These findings support the earlier hypothesis that the ODR-1 expression may also be upregulated in B. lactis BL050-fed nematodes, leading to a stronger attraction to butanone and thus a reduced capacity for further associative learning. Further investigations are needed to elucidate the underlying mechanism of this effect during C. elegans aging. Overall, our results highlight the strain-specific effects of tested probiotics on cognitive functions in C. elegans aging.
Then, we investigated the effects of the different probiotic strains on reproductive aging. Both Bifidobacteria strains and L. paracasei LPC1114 increased the total number of larvae produced; however, only B. breve BB077 significantly extended the egg-laying curve, resulting in a prolonged reproductive span. Aging in C. elegans is known to affect reproduction through various mechanisms [54], and serotonin plays a fundamental role in regulating egg-laying and oocyte quality [73]. The extended reproductive span observed with B. breve BB077 could be attributed to enhanced function of aged serotonin-producing neuron function and/or increased serotonin levels. This hypothesis is also supported by a previous work in which B. breve BB077 has been shown to increase serotonin production in in vitro cell lines [45].
Finally, we used C. elegans Δdaf16, Δskn1, and Δsir-2.1 mutants to identify the molecular pathways involved in lifespan extension induced by L. paracasei LPC1114 and L. reuteri PBS072 strains. These genes play crucial roles in the signaling pathways that mediate lifespan extension induced by probiotic bacteria [9]. In C. elegans, DAF16 and SKN1 are homologous to mammalian FOXO and Nrf2 transcriptional factors, respectively, and both contribute to promoting C. elegans lifespan and healthspan through the insulin/IGF-1 (insulin-like growth factor) signaling (IIS) pathway [9,36]. Inhibition of the insulin/IGF-1 receptor activity leads to the nuclear accumulation of both SKN1 and DAF16 in intestinal cells, thereby activating downstream target genes involved in stress resistance and longevity [74]. L. paracasei LPC1114 failed to extend lifespan in both Δdaf16 and Δskn1 mutants, indicating that these two key genes are required for its pro-longevity effect via the modulation of the IIS pathway. In addition, L. paracasei LPC1114 enhances nervous system plasticity, with no other major healthspan improvements observed. These two phenotypical outcomes may be functionally related, as gustatory and olfactory neurons contribute to lifespan extension through the IIS pathway [75]. In particular, increased neuronal DAF-16 activity modestly induced the expression of its target genes in other tissues (i.e., muscle and epidermis), resulting in a 5–20% increase in lifespan, even though mid-life motility remains unchanged [76,77].
On the other hand, L. reuteri PBS072 lifespan extension seems to be dependent on SKN1, but not on DAF16. SKN1 is known to mediate oxidative and xenobiotic stress response, regulate proteasome activity under stress conditions, and maintain cellular homeostasis under non-stress conditions [74]. Besides its regulation via the IIS pathway, SKN1 can be activated by phosphorylation through the p38 kinase PMK1 (the C. elegans homolog of human p38-MAP kinase) in response to inflammation and immune modulation, two hallmarks of immunosenescence [78,79]. We speculate that this mechanism could contribute to SKN1 activation by L. reuteri PBS072. SKN1 downstream gene targets regulate Phase I, II, and III detoxification pathways, contributing to both acute defense mechanisms and cellular homeostasis maintenance [74,80]. This dual function likely explains the observed reduction in ROS levels and the improvement in locomotion, consistent with studies linking reduced oxidative stress to enhanced motility in C. elegans [81,82,83].
SIR2.1, a member of the NAD+-dependent SIR2 deacetylases family, is a well-known regulator of lifespan in various organisms, including C. elegans, Drosophila melanogaster, and mammals [55]. SIR-2.1 can mediate lifespan extension through two main mechanisms. In the first, SIR2.1 promotes DAF16 nuclear translocation via interaction with the conserved acidic protein 14-3-3 under stress conditions [56], acting in parallel with the IIS pathway, both converging on the DAF16 transcriptional factor. This mechanism appears to be involved in L. paracasei LPC1114-mediated lifespan extension, as it fails to extend lifespan in SIR2.1, DAF16, and SKN1 C. elegans mutants. The second mechanism by which SIR2.1 can mediate lifespan extension is DAF16-independent [57,84]. It has been shown that resveratrol extends lifespan by mimicking dietary restriction in C. elegans through the involvement of both SIR2.1 and the energy-sensing AMP-activated protein kinase (AMPK), but not DAF-16 [57]. This mechanism seems to work in parallel with the SKN1 pathway to promote the lifespan extension observed with L. reuteri PBS072, as Δsir2.1 C. elegans mutant hasn’t extended lifespan after L. reuteri PBS072 supplementation. While our lifespan assays in DAF-16, SKN-1, and SIR-2.1 mutants suggest a potential involvement of these key regulators in mediating the observed effects, we emphasize that these results are preliminary. Direct molecular evidence—such as qPCR analysis of downstream target genes or reporter assays—will be necessary to definitively clarify their involvement.
In conclusion, our results demonstrate that the probiotic strains L. paracasei LPC1114, L. reuteri PBS072, B. breve BB077, and B. lactis BL050 exert strain-specific geroprotective effects in the C. elegans model. While L. paracasei LPC1114 and L. reuteri PBS072 showed both healthspan and lifespan enhancement, B. breve BB077 and B. lactis. BL050 significantly improved healthspan parameters without extending lifespan. These findings underline the importance of distinguishing between healthspan and lifespan in the evaluation of probiotic efficacy. The observed difference in the outcomes could be attributed to the distinct bioactive compounds and metabolites produced by each strain, which may act as signaling molecules, influencing host physiology through specific molecular pathways. Further metabolomic and transcriptomic analyses will be essential to elucidate these mechanisms and identify the involved compounds. Importantly, although certain strains did not promote longevity under basal conditions, their positive impact on health-related functions suggests a potential geroprotective role under stress conditions, such as oxidative stress. Future investigations will be necessary to clarify this observation.
C. elegans proves to be a robust and versatile model for the screening of probiotic geroprotective properties. Its bacterivorous nature allows for direct dietary modulation of the gut microbiota, enabling the evaluation of individual probiotic strains in vivo, yet within the complexity of a multicellular organism. This aspect makes C. elegans a useful organism to evaluate tissue-specific responses and the modulation of the gut-brain axis upon probiotic administration, both of which are crucial aspects in the development of effective probiotic-based interventions aimed at promoting healthy aging.
Overall, our findings contribute to the growing evidence supporting the use of specific probiotics as modulators of host aging and healthspan and lay the basis for future studies aiming to translate these effects into higher organisms and, eventually, clinical applications.
4. Materials and Methods
4.1. Caenorhabditis elegans Strains and Maintenance
The C. elegans wild-type N2 (Bristol), GR1307 (Δdaf16), EU1 (Δskn1), and VC199 (Δsir-2.1) strains used in this work were procured from the Caenorhabditis Genetics Center, University of Minnesota (CGC, University of Minnesota, Minneapolis, MN, USA). All the strains were maintained on Nematode Growth Medium (NGM; 50 mM NaCl, 2.5 g/L peptone, 17 g/L agar; 1 mM CaCl2, 1 mM MgSO4, 5 μg/mL cholesterol in ethanol) plates, at 20 °C and seeded with live E. coli OP50 at OD600 = 1 as a food source. Synchronized adult populations were obtained by placing gravid worms on NGM plates seeded with E. coli OP50 and allowed to lay eggs for 16 h at 20 °C. Then, adult worms were sacrificed, and newly laid eggs were grown at 20 °C until they reached the 1-day adult stage. The synchronized adult populations obtained were then used to perform experiments.
4.2. Bacterial Strains and Culture Preparations
Lacticaseibacillus paracasei LPC1114 (DSM 34559), Limosilactobacillus reuteri PBS072 (DSM 25175), Bifidobacterium breve BB077 (LMG P-30157), and Bifidobacterium animalis subsp. lactis BL050 (DSM 25566) were provided by SynBalance S.r.l. (Origgio (VA), Italy) and cultured overnight in static condition, at 37 °C using the De Man, Rogosa, and Sharpe (MRS) broth, with the supplementation of 0.05% cysteine chlorohydrate for Bifidobacteria strains. The E. coli OP50 was grown in Luria Bertani broth overnight, at 37 °C with shaking. Then, strains were collected and washed with M9 buffer (22 mM KH2PO4, 42 mM Na2HPO4, 86 mM NaCl, 1 mM MgSO4) and resuspended at final OD600 = 1.
4.3. Lifespan Assay
Sixty synchronized nematodes were moved on NGM plates seeded with 200 μL of living bacterial strains at OD600 = 1. Every two days, living nematodes were scored and moved to new, fresh plates. Nematodes were considered dead if they did not respond to a gentle stimulation with the transfer pick. 5-Fluoro-2′-deoxyuridine (FUDR) 0.04 μM was added only in the first week to prevent eggs from hatching. The experiment was replicated at least three times, and median and maximum values were reported in the correlated table as mean ± s.d. Log-rank Mantel–Cox test of Kaplan-Meier survival curves was performed for the statistical analysis.
All subsequent experiments were conducted with animals at day 11 of the lifespan.
4.4. Colony-Forming Unit (CFU) Count
Probiotics CFU in aged worms was performed following the protocol described by Palominos et al., 2020 [85]. Briefly, 30 nematodes grown on probiotics strains or E. coli OP50 were picked up on day 11 of adulthood and washed at least three times with 1 mL of PBST. Then nematodes were resuspended in a final volume of 100 μL and disrupted mechanically using a micropestel. Nematode lysates were serially diluted and plated on MRS agar. For Bifidobacteria strains, MRS plates were supplemented with 0.05% cysteine chlorohydrate. Plates were incubated for 72 h at 37 °C in anaerobic condition and then the colonies were counted. The experiment was replicated at least three times, and mean ± s.d was reported.
4.5. Body Bends Assay
The motility of twenty nematodes was evaluated by counting the body bend numbers using the SteREO Discovery V12 microscope (Carl Zeiss Microscopy GmbH, Munich, Germany), as previously described [86]. The experiment was replicated three times and ordinary one-way ANOVA with Dunnett’s multiple comparison test was used for the statistical analysis.
4.6. ROS Mesurements
Total ROS content can be quantified in living nematodes using the 2,7-dichlorofluorescein diacetate (H2DCFH-DA; Sigma-Aldrich Co., St. Louis, MO, USA) permeable probe. Briefly, H2DCFH-DA is a probe that can easily permeate into the cells, and rapidly oxidize by ROS, producing the highly fluorescent DCF form [87]. Twenty nematodes were extensively washed with PBS to remove bacteria and moved to a black 96 well plate. Then, 50 µM of H2DCFH-DA probe was added and the fluorescence (485 nm excitation, 530 nm emission) was measured at 37 °C for 6 h using a multi-well fluorophotometer (Victor 3, PerkinElmer, Waltham, MA, USA). The experiment was replicated three times and the data were normalized with respect to E. coli OP50, used as a control, and reported as mean ± s.e.m. Ordinary one-way ANOVA with Dunnett’s multiple comparison test was performed.
4.7. Lipofuscin Accumulation
Thirty nematodes were assessed for lipofuscin accumulation. Briefly, nematodes were washed with PBS and immobilized using a solution of 5% glycerol and 1% NaN3 in PBS. Images were obtained using the Nikon confocal microscope system A1 (Nikon Europe B.V., Amstelveen, The Netherlands) and processed with Nikon NIS-Element AR 6.20.02 software. Lipofuscin quantification was performed using FITC (gain 500 ms) filterset (Nikon Europe B.V., Amstelveen, The Netherlands) and the mean pixel intensity (AU) was measured with Fiji (ImageJ 1.54g) software. Data reported represent mean ± s.e.m. of 30 nematodes that were derived from three independent biological experiments. An ordinary one-way ANOVA with Dunnett’s multiple comparison test was performed for the statistical analysis.
4.8. Aldicarb Assay
Thirty nematodes were transferred onto NGM plates supplemented with 1 mM Aldicarb, in the absence of bacteria, following the protocol described before [49]. Aldicarb acts by inhibiting the acetylcholinesterase enzyme, leading to the accumulation of acetylcholine at the neuromuscular junction and ultimately causing paralysis. Briefly, the nematode response to gentle stimulation with a worm picker was assessed every hour, until all worms were paralyzed. The experiment was repeated three times and statistical analysis was performed using the log-rank Mantel–Cox test on Kaplan–Meier survival curves.
4.9. Cognitive Tests
At least one hundred nematodes were assayed for cognitive tests, including naïve chemotaxis (Cinaïve) and associative learning (Li), following the protocol described by Heydarian et al., 2024 [88]. Briefly, after extensive washing with M9 buffer, nematodes were placed at the center of the chemotaxis plates (Figure 2B), where a drop of 1.5 μL of either 10% butanone (attractant) or 95% EtOH (control) solution was applied to the four corners of the plates. After 1 h, nematodes present in the two poles were counted to determine the Cinaïve, following the above formula.
For the associative learning assay, after washing, nematodes were starved for 1 h in M9 buffer and then placed on a plate seeded with OP50 bacteria. For the conditioning, 2 μL of 10% butanone was applied to the inner side of the plate’s lid. Then, worms were washed with M9 and placed on the chemotaxis plates as described above. After 1 h of chemotaxis, the number of nematodes at the two poles was counted, and the conditioned chemotaxis index (Ci after conditioning) was calculated. The Li index was determined using the formula:
Data represents the mean ± s.e.m. of three independent experiments and ordinary one-way ANOVA with Dunnett’s multiple comparison test was used for the statistical analysis.
4.10. Fertility
The progeny of 1-day adult nematodes was evaluated by counting the number of larvae released during the first five days of adulthood. A single nematode was moved on an NGM petri dish seeded with living bacteria at OD600 = 1 and allowed to lay eggs. Every day for 5 days, the nematode was transferred to a fresh-seeded NGM plate. Then, the same plate was maintained at 20 °C overnight to get eggs hatching, and then the larvae were counted. The experiment was replicated ten times for each condition and an ordinary one-way ANOVA with Dunnett’s multiple comparison test was performed for the statistical analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262211205/s1.
Author Contributions
Conceptualization, B.S., D.F.S., P.M. and M.E.R.; methodology, B.S. and M.E.R.; software, B.S.; validation, B.S. and M.E.R.; formal analysis, B.S.; investigation, B.S. and M.E.R.; data curation, B.S. and M.E.R.; writing—original draft preparation, B.S.; writing—review and editing, B.S., D.F.S., P.M. and M.E.R.; visualization, B.S. and M.E.R.; supervision, P.M. and M.E.R.; project administration, P.M. and M.E.R.; funding acquisition, M.E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was directly funded by SynBalance S.r.l.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
P.M. and D.F.S. are full-time employees of Synbalance. This does not alter the author’s adherence to all the journal policies on sharing data and materials. The other authors declare no conflicts of interest.
References
- Harman, D. The Aging Process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. CIA 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The Origins of Age-Related Proinflammatory State. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef]
- Bixby, R.L. Impacts of Aging on the Federal Budget and Economy: A Cross-Cutting Challenge. Public Policy Aging Rep. 2020, 30, 46–51. [Google Scholar] [CrossRef]
- WHO Results Report 2024–2025. Available online: https://www.who.int/about/accountability/results/who-results-report-2024-2025 (accessed on 15 July 2025).
- Scott, A.J.; Ellison, M.; Sinclair, D.A. The Economic Value of Targeting Aging. Nat. Aging 2021, 1, 616–623. [Google Scholar] [CrossRef]
- Choudhary, P.; Kathuria, D.; Suri, S.; Bahndral, A.; Kanthi Naveen, A. Probiotics- Its Functions and Influence on the Ageing Process: A Comprehensive Review. Food Biosci. 2023, 52, 102389. [Google Scholar] [CrossRef]
- Bahour, N.; Cortez, B.; Pan, H.; Shah, H.; Doria, A.; Aguayo-Mazzucato, C. Diabetes Mellitus Correlates with Increased Biological Age as Indicated by Clinical Biomarkers. GeroScience 2022, 44, 415–427. [Google Scholar] [CrossRef]
- Miller, B.C.; Mathai, M.; Yadav, H.; Jain, S. Geroprotective Potential of Microbiome Modulators in the Caenorhabditis elegans Model. GeroScience 2024, 46, 129–151. [Google Scholar] [CrossRef]
- Stolbov, L.; Rudik, A.; Lagunin, A.; Druzhilovskiy, D.; Filimonov, D.; Poroikov, V. In Silico Assessment of Potential Geroprotectors: From Separate Endpoints to Complex Pharmacotherapeutic Effects. Int. J. Mol. Sci. 2025, 26, 8858. [Google Scholar] [CrossRef] [PubMed]
- Rayson, A.; Boudiffa, M.; Naveed, M.; Griffin, J.; Dall’Ara, E.; Bellantuono, I. Geroprotectors and Skeletal Health: Beyond the Headlines. Front. Cell Dev. Biol. 2022, 10, 682045. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.; Chernyagina, E.; Kudryavtseva, A.; Shaposhnikov, M. Geroprotectors: A Unified Concept and Screening Approaches. Aging Dis. 2017, 8, 354–363. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut Microbiota and Aging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3509–3534. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667, Correction in PLoS ONE 2010, 8, 5. [Google Scholar] [CrossRef]
- Nicoletti, C. Age-Associated Changes of the Intestinal Epithelial Barrier: Local and Systemic Implications. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Thuret, S. Gut Microbiota: A Modulator of Brain Plasticity and Cognitive Function in Ageing. Healthcare 2015, 3, 898–916. [Google Scholar] [CrossRef]
- Iyer, S.R.; Shah, S.B.; Lovering, R.M. The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease. Int. J. Mol. Sci. 2021, 22, 8058. [Google Scholar] [CrossRef]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Consultation, on. 2001. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/382476b3-4d54-4175-803f-2f26f3526256/content (accessed on 17 November 2025).
- Guarner, F.; Schaafsma, G.J. Probiotics. Int. J. Food Microbiol. 1998, 39, 237–238. [Google Scholar] [CrossRef] [PubMed]
- The Prolongation of Life: Optimistic Studies—Metchnikoff, Elie—Ebook in Inglese—EPUB2 Con Adobe DRM|IBS. Available online: https://www.ibs.it/prolongation-of-life-optimistic-studies-ebook-inglese-elie-metchnikoff/e/4057664619976?srsltid=AfmBOoraVlcYg_gt2lXHFOkSb0BwpN6q4gMbxoFVAihbMxfc1aJwyIQT (accessed on 15 September 2025).
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef]
- Du, Y.; Gao, Y.; Zeng, B.; Fan, X.; Yang, D.; Yang, M. Effects of Anti-Aging Interventions on Intestinal Microbiota. Gut Microbes 2021, 13, 1994835. [Google Scholar] [CrossRef] [PubMed]
- Sandionigi, A.; De Giani, A.; Tursi, F.; Michelotti, A.; Cestone, E.; Giardina, S.; Zampolli, J.; Di Gennaro, P. Effectiveness of Multistrain Probiotic Formulation on Common Infectious Disease Symptoms and Gut Microbiota Modulation in Flu-Vaccinated Healthy Elderly Subjects. Biomed. Res. Int. 2022, 2022, 3860896. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.; Gill, H.; Smart, J.; Gopal, P.K. Selection and Characterisation of Lactobacillus and Bifidobacterium Strains for Use as Probiotics. Int. Dairy J. 1998, 8, 993–1002. [Google Scholar] [CrossRef]
- Ramos, C.L.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-Specific Probiotics Properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis Isolates from Brazilian Food Products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef]
- Sharma, M.; Wasan, A.; Sharma, R.K. Recent Developments in Probiotics: An Emphasis on Bifidobacterium. Food Biosci. 2021, 41, 100993. [Google Scholar] [CrossRef]
- Balaguer, F.; Barrena, M.; Enrique, M.; Maicas, M.; Álvarez, B.; Tortajada, M.; Chenoll, E.; Ramón, D.; Martorell, P. Bifidobacterium Animalis Subsp. Lactis BPL1TM and Its Lipoteichoic Acid Modulate Longevity and Improve Age/Stress-Related Behaviors in Caenorhabditis elegans. Antioxidants 2023, 12, 2107. [Google Scholar] [CrossRef]
- Kumar, A.; Joishy, T.; Das, S.; Kalita, M.C.; Mukherjee, A.K.; Khan, M.R. A Potential Probiotic Lactobacillus Plantarum JBC5 Improves Longevity and Healthy Aging by Modulating Antioxidative, Innate Immunity and Serotonin-Signaling Pathways in Caenorhabditis elegans. Antioxidants 2022, 11, 268. [Google Scholar] [CrossRef]
- Kishimoto, S.; Nono, M.; Makizaki, Y.; Tanaka, Y.; Ohno, H.; Nishida, E.; Uno, M. Lactobacillus paracasei subsp. paracasei 2004 Improves Health and Lifespan in Caenorhabditis elegans. Sci. Rep. 2024, 14, 10453. [Google Scholar] [CrossRef]
- Hu, T.; Chen, R.; Qian, Y.; Ye, K.; Long, X.; Park, K.-Y.; Zhao, X. Antioxidant Effect of Lactobacillus fermentum HFY02-Fermented Soy Milk on D-Galactose-Induced Aging Mouse Model. Food Sci. Hum. Wellness 2022, 11, 1362–1372. [Google Scholar] [CrossRef]
- Kim, H.; Shin, J.; Kim, S.; Kim, S.; Cho, B.; Park, S.; Park, G.; Shin, H.; Park, M.S.; Kim, J. Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI Promotes Neuronal Rejuvenation in Aged Mice. Biochem. Biophys. Res. Commun. 2022, 603, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.-S.; Shin, Y.-J.; Ma, X.; Park, H.-S.; Hwang, Y.-H.; Kim, D.-H. Bifidobacterium bifidum and Lactobacillus paracasei Alleviate Sarcopenia and Cognitive Impairment in Aged Mice by Regulating Gut Microbiota-Mediated AKT, NF-κB, and FOXO3a Signaling Pathways. Immun. Ageing 2023, 20, 56. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Lee, J.-H.; Kim, S.H.; Jo, S.-Y.; Min, K.-J. Probiotic Limosilactobacillus reuteri (Lactobacillus reuteri) Extends the Lifespan of Drosophila Melanogaster Through Insulin/IGF-1 Signaling. Aging Dis. 2023, 14, 1407–1424. [Google Scholar] [CrossRef]
- Girard, L.R.; Fiedler, T.J.; Harris, T.W.; Carvalho, F.; Antoshechkin, I.; Han, M.; Sternberg, P.W.; Stein, L.D.; Chalfie, M. WormBook: The Online Review of Caenorhabditis elegans Biology. Nucleic Acids Res. 2007, 35, D472–D475. [Google Scholar] [CrossRef]
- Poupet, C.; Chassard, C.; Nivoliez, A.; Bornes, S. Caenorhabditis elegans, a Host to Investigate the Probiotic Properties of Beneficial Microorganisms. Front. Nutr. 2020, 7, 135. [Google Scholar] [CrossRef]
- Presti, I.; D’Orazio, G.; Labra, M.; La Ferla, B.; Mezzasalma, V.; Bizzaro, G.; Giardina, S.; Michelotti, A.; Tursi, F.; Vassallo, M.; et al. Evaluation of the Probiotic Properties of New Lactobacillus and Bifidobacterium Strains and Their In Vitro Effect. Appl. Microbiol. Biotechnol. 2015, 99, 5613–5626. [Google Scholar] [CrossRef] [PubMed]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Boccarusso, M.; Di Gennaro, P.; Schiano, I.; Michelotti, A.; Labra, M. Orally Administered Multispecies Probiotic Formulations to Prevent Uro-Genital Infections: A Randomized Placebo-Controlled Pilot Study. Arch. Gynecol. Obstet. 2017, 295, 163–172, Erratum in Arch. Gynecol. Obstet. 2017, 295, 527. [Google Scholar] [CrossRef]
- Malfa, P.; Brambilla, L.; Giardina, S.; Masciarelli, M.; Squarzanti, D.F.; Carlomagno, F.; Meloni, M. Evaluation of Antimicrobial, Antiadhesive and Co-Aggregation Activity of a Multi-Strain Probiotic Composition Against Different Urogenital Pathogens. Int. J. Mol. Sci. 2023, 24, 1323. [Google Scholar] [CrossRef]
- Squarzanti, D.F.; Dell’Atti, F.; Scalia, A.C.; Najmi, Z.; Cochis, A.; Malfa, P. Exploring the In Vitro Antibacterial Potential of Specific Probiotic Strains Against Oral Pathogens. Microorganisms 2024, 12, 441. [Google Scholar] [CrossRef]
- Vicariotto, F.; Malfa, P.; Viciani, E.; Dell’Atti, F.; Squarzanti, D.F.; Marcante, A.; Castagnetti, A.; Ponchia, R.; Governini, L.; De Leo, V. Efficacy of Lactiplantibacillus plantarum PBS067, Bifidobacterium animalis subsp. lactis BL050, and Lacticaseibacillus rhamnosus LRH020 in the Amelioration of Vaginal Microbiota in Post-Menopausal Women: A Prospective Observational Clinical Trial. Nutrients 2024, 16, 402. [Google Scholar] [CrossRef]
- Lungaro, L.; Malfa, P.; Manza, F.; Costanzini, A.; Valentini, G.; Squarzanti, D.F.; Viciani, E.; Velichevskaya, A.; Castagnetti, A.; Barbalinardo, M.; et al. Clinical Efficacy of Probiotics for Allergic Rhinitis: Results of an Exploratory Randomized Controlled Trial. Nutrients 2024, 16, 4173. [Google Scholar] [CrossRef] [PubMed]
- Lungaro, L.; Malfa, P.; Manza, F.; Negrelli, M.; Costanzini, A.; Squarzanti, D.F.; Lo Re, M.; Cariani, A.; Ghisellini, S.; Caputo, F.; et al. Clinical Efficacy of Probiotics for Relieving Cold Symptoms in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2025, 17, 1490. [Google Scholar] [CrossRef]
- Nobile, V.; Giardina, S.; Puoci, F. The Effect of a Probiotic Complex on the Gut-Brain Axis: A Translational Study. Neuropsychobiology 2021, 81, 116–126. [Google Scholar] [CrossRef]
- Nobile, V.; Puoci, F. Effect of a Multi-Strain Probiotic Supplementation to Manage Stress During the COVID-19 Pandemic: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Clinical Trial. Neuropsychobiology 2023, 82, 61–71. [Google Scholar] [CrossRef]
- Vicariotto, F.; Malfa, P.; Torricelli, M.; Lungaro, L.; Caio, G.; De Leo, V. Beneficial Effects of Limosilactobacillus reuteri PBS072 and Bifidobacterium breve BB077 on Mood Imbalance, Self-Confidence, and Breastfeeding in Women During the First Trimester Postpartum. Nutrients 2023, 15, 3513. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Lei, H.; Feng, Z.; Liu, J.; Hsu, A.-L.; Xu, X.Z.S. Functional Aging in the Nervous System Contributes to Age-Dependent Motor Activity Decline in C. elegans. Cell Metab. 2013, 18, 392–402. [Google Scholar] [CrossRef]
- Mahoney, T.R.; Luo, S.; Nonet, M.L. Analysis of Synaptic Transmission in Caenorhabditis elegans Using an Aldicarb-Sensitivity Assay. Nat. Protoc. 2006, 1, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Leinwand, S.G.; Chalasani, S.H. Neuropeptide Signaling Remodels Chemosensory Circuit Composition in Caenorhabditis elegans. Nat. Neurosci. 2013, 16, 1461–1467. [Google Scholar] [CrossRef]
- Leinwand, S.G.; Yang, C.J.; Bazopoulou, D.; Chronis, N.; Srinivasan, J.; Chalasani, S.H. Circuit Mechanisms Encoding Odors and Driving Aging-Associated Behavioral Declines in Caenorhabditis elegans. eLife 2015, 4, e10181. [Google Scholar] [CrossRef]
- Ferkey, D.M.; Sengupta, P.; L’Etoile, N.D. Chemosensory Signal Transduction in Caenorhabditis elegans. Genetics 2021, 217, iyab004, Erratum in Caenorhabditis elegans. Genetics 2022, 220, iyab181. [Google Scholar] [CrossRef] [PubMed]
- Torayama, I.; Ishihara, T.; Katsura, I. Caenorhabditis elegans Integrates the Signals of Butanone and Food to Enhance Chemotaxis to Butanone. J. Neurosci. 2007, 27, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Scharf, A.; Pohl, F.; Egan, B.M.; Kocsisova, Z.; Kornfeld, K. Reproductive Aging in Caenorhabditis elegans: From Molecules to Ecology. Front. Cell Dev. Biol. 2021, 9, 718522. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin Activators Mimic Caloric Restriction and Delay Ageing in Metazoans. Nature 2004, 430, 686–689, Correction in Nature 2004, 430, 107. [Google Scholar] [CrossRef]
- Berdichevsky, A.; Viswanathan, M.; Horvitz, H.R.; Guarente, L.C. elegans SIR-2.1 Interacts with 14-3-3 Proteins to Activate DAF-16 and Extend Life Span. Cell 2006, 125, 1165–1177. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, G.; Park, J.; Kim, J.-K.; Lim, Y.-H. Brief Communication: SIR-2.1-Dependent Lifespan Extension of Caenorhabditis elegans by Oxyresveratrol and Resveratrol. Exp. Biol. Med. 2016, 241, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Feng, Y.; Zhao, J.; Chen, W.; Lu, W. Achieving Healthy Aging through Gut Microbiota-Directed Dietary Intervention: Focusing on Microbial Biomarkers and Host Mechanisms. J. Adv. Res. 2025, 68, 179–200. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M.; et al. Faecal Microbiota Transplant from Aged Donor Mice Affects Spatial Learning and Memory via Modulating Hippocampal Synaptic Plasticity- and Neurotransmission-Related Proteins in Young Recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef]
- Li, Y.; Ning, L.; Yin, Y.; Wang, R.; Zhang, Z.; Hao, L.; Wang, B.; Zhao, X.; Yang, X.; Yin, L.; et al. Age-Related Shifts in Gut Microbiota Contribute to Cognitive Decline in Aged Rats. Aging 2020, 12, 7801–7817. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Ferrini, F.; Gervasi, M.; Barbieri, E.; Bartolacci, A.; Piccoli, G.; Saltarelli, R.; Sestili, P.; Stocchi, V. Interventions on Gut Microbiota for Healthy Aging. Cells 2023, 12, 34. [Google Scholar] [CrossRef]
- Yu, X.; Wu, X.; Qiu, L.; Wang, D.; Gan, M.; Chen, X.; Wei, H.; Xu, F. Analysis of the Intestinal Microbial Community Structure of Healthy and Long-Living Elderly Residents in Gaotian Village of Liuyang City. Appl. Microbiol. Biotechnol. 2015, 99, 9085–9095. [Google Scholar] [CrossRef]
- Kashtanova, D.A.; Klimenko, N.S.; Strazhesko, I.D.; Starikova, E.V.; Glushchenko, O.E.; Gudkov, D.A.; Tkacheva, O.N. A Cross-Sectional Study of the Gut Microbiota Composition in Moscow Long-Livers. Microorganisms 2020, 8, 1162. [Google Scholar] [CrossRef]
- Mytilinaiou, E.; Kitopoulou, K.; Palikaras, K. Caenorhabditis elegans as a Screening Platform for Anti-Aging Compounds. Methods Mol. Biol. 2025, 2906, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.; Vantipalli, M.C.; Lithgow, G.J. Using Caenorhabditis elegans as a Model for Aging and Age-Related Diseases. Ann. N. Y. Acad. Sci. 2006, 1067, 120–128. [Google Scholar] [CrossRef]
- Nigon, V.M.; Félix, M.-A. History of Research on C. elegans and Other Free-Living Nematodes as Model Organisms. WormBook 2017, 2017, 1–84. [Google Scholar] [CrossRef]
- Jeayeng, S.; Thongsroy, J.; Chuaijit, S. Caenorhabditis elegans as a Model to Study Aging and Photoaging. Biomolecules 2024, 14, 1235. [Google Scholar] [CrossRef]
- Oswal, N.; Martin, O.M.F.; Stroustrup, S.; Bruckner, M.A.M.; Stroustrup, N. A Hierarchical Process Model Links Behavioral Aging and Lifespan in C. elegans. PLOS Comput. Biol. 2022, 18, e1010415. [Google Scholar] [CrossRef]
- Newell Stamper, B.L.; Cypser, J.R.; Kechris, K.; Kitzenberg, D.A.; Tedesco, P.M.; Johnson, T.E. Movement Decline Across Lifespan of Caenorhabditis elegans Mutants in the Insulin/Insulin-like Signaling Pathway. Aging Cell 2018, 17, e12704. [Google Scholar] [CrossRef] [PubMed]
- Suryawinata, N.; Yokosawa, R.; Hui, K.; Tan, C.; Lai, A.L.; Sone, R.; Mori, I.; Noma, K. Dietary E. coli Promotes Age-Dependent Chemotaxis Decline in C. elegans. Sci. Rep. 2024, 14, 5529. Available online: https://www.nature.com/articles/s41598-024-52272-4 (accessed on 31 July 2025). [CrossRef]
- Bargmann, C.I.; Hartwieg, E.; Horvitz, H.R. Odorant-Selective Genes and Neurons Mediate Olfaction in C. elegans. Cell 1993, 74, 515–527. [Google Scholar] [CrossRef]
- L’Etoile, N.D.; Bargmann, C.I. Olfaction and Odor Discrimination Are Mediated by the C. elegans Guanylyl Cyclase ODR-1. Neuron 2000, 25, 575–586. [Google Scholar] [CrossRef]
- Aprison, E.Z.; Dzitoyeva, S.; Ruvinsky, I. The Serotonin Circuit That Coordinates Germline Proliferation and Egg Laying with Other Reproductive Functions in Caenorhabditis elegans. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220913. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, Stress Responses, and Aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef]
- Alcedo, J.; Kenyon, C. Regulation of C. elegans Longevity by Specific Gustatory and Olfactory Neurons. Neuron 2004, 41, 45–55. [Google Scholar] [CrossRef]
- Libina, N.; Berman, J.R.; Kenyon, C. Tissue-Specific Activities of C. elegans DAF-16 in the Regulation of Lifespan. Cell 2003, 115, 489–502. [Google Scholar] [CrossRef]
- Roy, C.; Molin, L.; Alcolei, A.; Solyga, M.; Bonneau, B.; Vachon, C.; Bessereau, J.-L.; Solari, F. DAF-2/Insulin IGF-1 Receptor Regulates Motility During Aging by Integrating Opposite Signaling from Muscle and Neuronal Tissues. Aging Cell 2022, 21, e13660. [Google Scholar] [CrossRef]
- Papp, D.; Csermely, P.; Sőti, C. A Role for SKN-1/Nrf in Pathogen Resistance and Immunosenescence in Caenorhabditis elegans. PLOS Pathog. 2012, 8, e1002673. [Google Scholar] [CrossRef]
- Roselli, M.; Schifano, E.; Guantario, B.; Zinno, P.; Uccelletti, D.; Devirgiliis, C. Caenorhabditis elegans and Probiotics Interactions from a Prolongevity Perspective. Int. J. Mol. Sci. 2019, 20, 5020. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.P.; Abate, J.P.; Dilks, K.; Landis, J.; Ashraf, J.; Murphy, C.T.; Blackwell, T.K. Condition-Adapted Stress and Longevity Gene Regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell 2009, 8, 524–541. [Google Scholar] [CrossRef]
- Dou, T.; Chen, J.; Wang, R.; Pu, X.; Wu, H.; Zhao, Y. Complementary Protective Effects of Autophagy and Oxidative Response against Graphene Oxide Toxicity in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2022, 248, 114289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Y.-Y.; Huang, L.; Shi, L.; Zheng, Z.-Y.; Chen, J.-N.; Qu, Y.; Xiao, H.-T.; Luo, H.-R.; Wu, G.-S. Para-Hydroxybenzyl Alcohol Delays the Progression of Neurodegenerative Diseases in Models of Caenorhabditis elegans Through Activating Multiple Cellular Protective Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 8986287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, K.; Zhang, Y.; Ge, S.; Zhang, S. Defense Against Oxidative Stress in Caenorhabditis elegans by Dark Tea. Front. Vet. Sci. 2024, 10, 1342747. [Google Scholar] [CrossRef]
- Greer, E.L.; Brunet, A. Different Dietary Restriction Regimens Extend Lifespan by Both Independent and Overlapping Genetic Pathways in C. elegans. Aging Cell 2009, 8, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Palominos, M.F.; Calixto, A. Quantification of Bacteria Residing in Caenorhabditis elegans Intestine. Bio. Protoc. 2020, 10, e3605. [Google Scholar] [CrossRef] [PubMed]
- Sciandrone, B.; Kentsop, R.A.D.; Pensotti, R.; Ottolina, G.; Mascheretti, I.; Mattana, M.; Regonesi, M.E. Toxicological Analysis of the Arylnaphthalene Lignan Justicidin B Using a Caenorhabditis elegans Model. Molecules 2024, 29, 5516. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, M.-H.; Cha, D.S. Measurement of Intracellular ROS in Caenorhabditis elegans Using 2′,7′-Dichlorodihydrofluorescein Diacetate. Bio. Protoc. 2018, 8, e2774. [Google Scholar] [CrossRef]
- Heydarian, D.; Flavel, M.; Munasinghe, M.; Jois, M.; Thomas, J. Improving Cognitive and Chemosensory Function in Caenorhabditis elegans Through Polyphenol-Rich Sugarcane Extract. Stresses 2024, 4, 816–826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).