Functions and Synthesis of Abscisic Acid (ABA) in Humans—Insights from Computational Approaches
Abstract
1. Introduction
2. The Search for Candidate ABA-Binding Proteins in the Human Proteome
3. Computational Approaches to the Elucidation of ABA Synthesis in Humans
4. Identification and Characterisation of Transcription Factors Regulating the Identified ABA Synthesis Genes
5. ABA-Binding Candidates as Key to Inferring Novel ABA Functions in Humans
6. ABA Synthesis in H. sapiens—An Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| FPP | farnesyl pyrophosphate |
| PPARGA | peroxisome-proliferator-activated receptor gamma |
| RAXRA | retinoic acid receptor |
| CYP | cytochrome P450 |
| GORK | outward-rectifying potassium channel |
References
- Kende, H.; Zeevaart, J.A.D. The Five “Classical” Plant Hormones. Plant Cell 1997, 9, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Nakashima, K.; Miyakawa, T.; Kuromori, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular Basis of the Core Regulatory Network in ABA Responses: Sensing, Signaling and Transport. Plant Cell Physiol. 2010, 51, 1821–1839. [Google Scholar] [CrossRef]
- Hauser, F.; Waadt, R.; Schroeder, J.I. Evolution of Abscisic Acid Synthesis and Signaling Mechanisms. Curr. Biol. 2011, 21, R346–R355. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA Signaling in Stress-Response and Seed Development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Lievens, L.; Pollier, J.; Goossens, A.; Beyaert, R.; Staal, J. Abscisic Acid as Pathogen Effector and Immune Regulator. Front. Plant Sci. 2017, 8, 587. [Google Scholar] [CrossRef]
- Takahashi, Y.; Zhang, J.; Hsu, P.K.; Ceciliato, P.H.O.; Zhang, L.; Dubeaux, G.; Munemasa, S.; Ge, C.; Zhao, Y.; Hauser, F.; et al. MAP3Kinase-Dependent SnRK2-Kinase Activation Is Required for Abscisic Acid Signal Transduction and Rapid Osmotic Stress Response. Nat. Commun. 2020, 11, 12. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, Y.; Wang, Y.; Liu, X.; Ma, L.; Zhang, Z.; Mu, C.; Zhang, Y.; Peng, L.; Xie, S.; et al. Initiation and Amplification of SnRK2 Activation in Abscisic Acid Signaling. Nat. Commun. 2021, 12, 2456. [Google Scholar] [CrossRef]
- Wong, A.; Bi, C.; Pasqualini, S.; Gehring, C. Abscisic Acid (ABA) Signaling: Finding Novel Components off the Beaten Track. Plant Growth Regul. 2022, 97, 585–592. [Google Scholar] [CrossRef]
- Le Page-Degivry, M.T.; Bidard, J.N.; Rouvier, E.; Bulard, C.; Lazdunski, M. Presence of Abscisic Acid, a Phytohormone, in the Mammalian Brain. Proc. Natl. Acad. Sci. USA 1986, 83, 1155–1158. [Google Scholar] [CrossRef]
- Takezawa, D.; Komatsu, K.; Sakata, Y. ABA in Bryophytes: How a Universal Growth Regulator in Life Became a Plant Hormone? J. Plant Res. 2011, 124, 437–453. [Google Scholar] [CrossRef]
- Magnone, M.; Sturla, L.; Guida, L.; Spinelli, S.; Begani, G.; Bruzzone, S.; Fresia, C.; Zocchi, E. Abscisic Acid: A Conserved Hormone in Plants and Humans and a Promising Aid to Combat Prediabetes and the Metabolic Syndrome. Nutrients 2020, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Puce, S.; Basile, G.; Bavestrello, G.; Bruzzone, S.; Cerrano, C.; Giovine, M.; Arillo, A.; Zocchi, E. Abscisic Acid Signaling through Cyclic ADP-Ribose in Hydroid Regeneration. J. Biol. Chem. 2004, 279, 39783–39788. [Google Scholar] [CrossRef]
- Wang, Y.; Weiss, L.M.; Orlofsky, A. Host Cell Autophagy Is Induced by Toxoplasma Gondii and Contributes to Parasite Growth. J. Biol. Chem. 2009, 284, 1694–1701. [Google Scholar] [CrossRef]
- Hirai, N.; Yoshida, R.; Todoroki, Y.; Ohigashi, H. Biosynthesis of Abscisic Acid by the Non-Mevalonate Pathway in Plants, and by the Mevalonate Pathway in Fungi. Biosci. Biotechnol. Biochem. 2000, 64, 1448–1458. [Google Scholar] [CrossRef]
- Sakthivel, P.; Sharma, N.; Klahn, P.; Gereke, M.; Bruder, D. Abscisic Acid: A Phytohormone and Mammalian Cytokine as Novel Pharmacon with Potential for Future Development into Clinical Applications. Curr. Med. Chem. 2016, 23, 1549–1570. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Meier, S.; Tzfadia, O.; Vallabhaneni, R.; Gehring, C.; Wurtzel, E.T. A Transcriptional Analysis of Carotenoid, Chlorophyll and Plastidial Isoprenoid Biosynthesis Genes during Development and Osmotic Stress Responses in Arabidopsis Thaliana. BMC Syst. Biol. 2011, 5, 77. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic Acid Synthesis and Response. Arab. B. 2013, 11, e0166. [Google Scholar] [CrossRef]
- Izquierdo-Bueno, I.; González-Rodríguez, V.E.; Simon, A.; Dalmais, B.; Pradier, J.M.; Le Pêcheur, P.; Mercier, A.; Walker, A.S.; Garrido, C.; Collado, I.G.; et al. Biosynthesis of Abscisic Acid in Fungi: Identification of a Sesquiterpene Cyclase as the Key Enzyme in Botrytis Cinerea. Environ. Microbiol. 2018, 20, 2469–2482. [Google Scholar] [CrossRef] [PubMed]
- Olds, C.L.; Glennon, E.K.K.; Luckhart, S. Abscisic Acid: New Perspectives on an Ancient Universal Stress Signaling Molecule. Microbes Infect. 2018, 20, 484–492. [Google Scholar] [CrossRef]
- Gharib, A.; Marquez, C.; Meseguer-Beltran, M.; Sanchez-Sarasua, S.; Sanchez-Perez, A.M. Abscisic Acid, an Evolutionary Conserved Hormone: Biosynthesis, Therapeutic and Diagnostic Applications in Mammals. Biochem. Pharmacol. 2024, 229, 116521. [Google Scholar] [CrossRef]
- Zhou, N.; Yao, Y.; Ye, H.; Zhu, W.; Chen, L.; Mao, Y. Abscisic-Acid-Induced Cellular Apoptosis and Differentiation in Glioma via the Retinoid Acid Signaling Pathway. Int. J. Cancer 2016, 138, 1947–1958. [Google Scholar] [CrossRef]
- Guri, A.J.; Hontecillas, R.; Ferrer, G.; Casagran, O.; Wankhade, U.; Noble, A.M.; Eizirik, D.L.; Ortis, F.; Cnop, M.; Liu, D.; et al. Loss of PPAR Gamma in Immune Cells Impairs the Ability of Abscisic Acid to Improve Insulin Sensitivity by Suppressing Monocyte Chemoattractant Protein-1 Expression and Macrophage Infiltration into White Adipose Tissue. J. Nutr. Biochem. 2008, 19, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Ameri, P.; Bruzzone, S.; Mannino, E.; Sociali, G.; Andraghetti, G.; Salis, A.; Ponta, M.L.; Briatore, L.; Adami, G.F.; Ferraiolo, A.; et al. Impaired Increase of Plasma Abscisic Acid in Response to Oral Glucose Load in Type 2 Diabetes and in Gestational Diabetes. PLoS ONE 2015, 10, e0115992. [Google Scholar] [CrossRef] [PubMed]
- Scarano, N.; Di Palma, F.; Origlia, N.; Musumeci, F.; Schenone, S.; Spinelli, S.; Passalacqua, M.; Zocchi, E.; Sturla, L.; Cichero, E.; et al. New Insights into the LANCL2-ABA Binding Mode towards the Evaluation of New LANCL Agonists. Pharmaceutics 2023, 15, 2754. [Google Scholar] [CrossRef]
- Magnone, M.; Ameri, P.; Salis, A.; Andraghetti, G.; Emionite, L.; Murialdo, G.; De Flora, A.; Zocchi, E. Microgram Amounts of Abscisic Acid in Fruit Extracts Improve Glucose Tolerance and Reduce Insulinemia in Rats and in Humans. FASEB J. 2015, 29, 4783–4793. [Google Scholar] [CrossRef]
- Iranmanesh, Z.; Dehestani, M.; Esmaeili-Mahani, S. Discovering Novel Targets of Abscisic Acid Using Computational Approaches. Comput. Biol. Chem. 2024, 112, 108157. [Google Scholar] [CrossRef]
- Zocchi, E.; Carpaneto, A.; Cerrano, C.; Bavestrello, G.; Giovine, M.; Bruzzone, S.; Guida, L.; Franco, L.; Usai, C. The Temperature-Signaling Cascade in Sponges Involves a Heat-Gated Cation Channel, Abscisic Acid, and Cyclic ADP-Ribose. Proc. Natl. Acad. Sci. USA 2001, 98, 14859–14864. [Google Scholar] [CrossRef]
- Sturla, L.; Fresia, C.; Guida, L.; Bruzzone, S.; Scarfi, S.; Usai, C.; Fruscione, F.; Magnone, M.; Millo, E.; Basile, G.; et al. LANCL2 Is Necessary for Abscisic Acid Binding and Signaling in Human Granulocytes and in Rat Insulinoma Cells. J. Biol. Chem. 2009, 284, 28045–28057. [Google Scholar] [CrossRef]
- Bruzzone, S.; Ameri, P.; Briatore, L.; Mannino, E.; Basile, G.; Andraghetti, G.; Grozio, A.; Magnone, M.; Guida, L.; Scarfì, S.; et al. The Plant Hormone Abscisic Acid Increases in Human Plasma after Hyperglycemia and Stimulates Glucose Consumption by Adipocytes and Myoblasts. FASEB J. 2012, 26, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, E.; Hontecillas, R.; Leber, A.; Einerhand, A.; Carbo, A.; Bruzzone, S.; Tubau-Juni, N.; Philipson, N.; Zoccoli-Rodriguez, V.; Sturla, L.; et al. Abscisic Acid: A Novel Nutraceutical for Glycemic Control. Front. Nutr. 2017, 4, 24. [Google Scholar] [CrossRef]
- Ooi, A.; Lemtiri-Chlieh, F.; Wong, A.; Gehring, C. Direct Modulation of the Guard Cell Outward-Rectifying Potassium Channel (GORK) by Abscisic Acid. Mol. Plant 2017, 10, 1469–1472. [Google Scholar] [CrossRef]
- Melcher, K.; Ng, L.-M.; Zhou, X.E.; Soon, F.-F.; Xu, Y.; Suino-Powell, K.M.; Park, S.-Y.; Weiner, J.J.; Fujii, H.; Chinnusamy, V.; et al. A Gate–Latch–Lock Mechanism for Hormone Signalling by Abscisic Acid Receptors. Nature 2009, 462, 602–608. [Google Scholar] [CrossRef]
- Wong, A.; Gehring, C.; Irving, H.R. Conserved Functional Motifs and Homology Modeling to Predict Hidden Moonlighting Functional Sites. Front. Bioeng. Biotechnol. 2015, 3, 82. [Google Scholar] [CrossRef]
- Zhou, W.; Chi, W.; Shen, W.; Dou, W.; Wang, J.; Tian, X.; Gehring, C.; Wong, A. Computational Identification of Functional Centers in Complex Proteins: A Step-by-Step Guide With Examples. Front. Bioinforma. 2021, 1, 652286. [Google Scholar] [CrossRef]
- Wong, A.; Bi, C.; Chi, W.; Hu, N.; Gehring, C. Amino Acid Motifs for the Identification of Novel Protein Interactants. Comput. Struct. Biotechnol. J. 2023, 21, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Tian, X.; Gehring, C.; Marondedze, C. Discovery of Novel Functional Centers With Rationally Designed Amino Acid Motifs. Comput. Struct. Biotechnol. J. 2018, 16, 70–76. [Google Scholar] [CrossRef]
- Cichero, E.; Fresia, C.; Guida, L.; Booz, V.; Millo, E.; Scotti, C.; Iamele, L.; de Jonge, H.; Galante, D.; De Flora, A.; et al. Identification of a High Affinity Binding Site for Abscisic Acid on Human Lanthionine Synthetase Component C-like Protein 2. Int. J. Biochem. Cell Biol. 2018, 97, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Gehring, C. Adenyl Cyclases and CAMP in Plant Signaling—Past and Present. Cell Commun. Signal. 2010, 8, 15. [Google Scholar] [CrossRef]
- Gehring, C.; Turek, I.S. Cyclic Nucleotide Monophosphates and Their Cyclases in Plant Signaling. Front. Plant Sci. 2017, 8, 1704. [Google Scholar] [CrossRef]
- Al-Younis, I.; Moosa, B.; Kwiatkowski, M.; Jaworski, K.; Wong, A.; Gehring, C. Functional Crypto-Adenylate Cyclases Operate in Complex Plant Proteins. Front. Plant Sci. 2021, 12, 711749. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Zhang, J.; Zhou, W.; Gehring, C.; Wong, A. Cyclic Nucleotides—the Rise of a Family. Trends Plant Sci. 2024, 29, 915–924. [Google Scholar] [CrossRef]
- Turek, I.; Wong, A.; Domingo, G.; Vannini, C.; Bracale, M.; Irving, H.; Gehring, C. Moonlighting Crypto-Enzymes and Domains as Ancient and Versatile Signaling Devices. Int. J. Mol. Sci. 2024, 25, 9535. [Google Scholar] [CrossRef]
- Fan, S.; Kang, B.; Li, S.; Li, W.; Chen, C.; Chen, J.; Deng, L.; Chen, D.; Zhou, J. Exploring the Multifaceted Role of RASGRP1 in Disease: Immune, Neural, Metabolic, and Oncogenic Perspectives. Cell Cycle 2024, 23, 722–746. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.A.; Anderson, D.; Moran, M.F.; Ellis, C.; Pawson, T. SH2 and SH3 Domains: Elements That Control Interactions of Cytoplasmic Signaling Proteins. Science 1991, 252, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Dionne, U.; Percival, L.J.; Chartier, F.J.M.; Landry, C.R.; Bisson, N. SRC Homology 3 Domains: Multifaceted Binding Modules. Trends Biochem. Sci. 2022, 47, 772–784. [Google Scholar] [CrossRef]
- Schlessinger, J. SH2/SH3 Signaling Proteins. Curr. Opin. Genet. Dev. 1994, 4, 25–30. [Google Scholar] [CrossRef]
- Bae, S.J.; Ni, L.; Osinski, A.; Tomchick, D.R.; Brautigam, C.A.; Luo, X. SAV1 Promotes Hippo Kinase Activation through Antagonizing the PP2A Phosphatase STRIPAK. Elife 2017, 6, e30278. [Google Scholar] [CrossRef]
- Gupta, M.K.; Lenka, S.K.; Gupta, S.; Rawal, R.K. Agonist, Antagonist and Signaling Modulators of ABA Receptor for Agronomic and Post-Harvest Management. Plant Physiol. Biochem. 2020, 148, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Krisans, S.K.; Ericsson, J.; Edwards, P.A.; Keller, G.A. Farnesyl-Diphosphate Synthase Is Localized in Peroxisomes. J. Biol. Chem. 1994, 269, 14165–14169. [Google Scholar] [CrossRef] [PubMed]
- Tzfadia, O.; Diels, T.; De Meyer, S.; Vandepoele, K.; Aharoni, A.; Van De Peer, Y. CoExpNetViz: Comparative Co-Expression Networks Construction and Visualization Tool. Front. Plant Sci. 2016, 6, 1194. [Google Scholar] [CrossRef]
- Zhang, J.; Singh, R. Investigating the Complexity of Gene Co-Expression Estimation for Single-Cell Data. J. Mach. Learn. Model. Comput. 2023, 4, 37–82. [Google Scholar] [CrossRef]
- Hou, J.; Ye, X.; Feng, W.; Zhang, Q.; Han, Y.; Liu, Y.; Li, Y.; Wei, Y. Distance Correlation Application to Gene Co-Expression Network Analysis. BMC Bioinform. 2022, 23, 81. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Guri, A.J.; Lu, P.; Climent, M.; Carbo, A.; Sobral, B.W.; Horne, W.T.; Lewis, S.N.; Bevan, D.R.; Hontecillas, R. Abscisic Acid Regulates Inflammation via Ligand-Binding Domain-Independent Activation of Peroxisome Proliferator-Activated Receptor γ. J. Biol. Chem. 2011, 286, 2504–2516. [Google Scholar] [CrossRef]
- Zhao, C.-C.; Xu, J.; Xie, Q.-M.; Zhang, H.-Y.; Fei, G.-H.; Wu, H.-M. Abscisic acid suppresses the activation of NLRP3 inflammasome and oxidative stress in murine allergic airway inflammation. Phytother. Res. 2021, 35, 3298–3309. [Google Scholar] [CrossRef]
- Oladimeji, P.O.; Chen, T. PXR: More than Just a Master Xenobiotic Receptor. Mol. Pharmacol. 2018, 93, 119–127. [Google Scholar] [CrossRef]
- Sterrenberg, J.N.; Blatch, G.L.; Edkins, A.L. Human DNAJ in Cancer and Stem Cells. Cancer Lett. 2011, 312, 129–142. [Google Scholar] [CrossRef]
- Hennessy, F.; Boshoff, A.; Blatch, G.L. Rational Mutagenesis of a 40 KDa Heat Shock Protein from Agrobacterium Tumefaciens Identifies Amino Acid Residues Critical to Its in vivo Function. Int. J. Biochem. Cell Biol. 2005, 37, 177–191. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, V.; Jayaraj, G.G.; Maiti, S.; Mapa, K. Unique Structural Modulation of a Non-Native Substrate by Cochaperone DnaJ. Biochemistry 2013, 52, 1011–1018. [Google Scholar] [CrossRef]
- Ajit Tamadaddi, C.; Sahi, C. J Domain Independent Functions of J Proteins. Cell Stress Chaperones 2016, 21, 563–570. [Google Scholar] [CrossRef]
- Gotoh, T.; Terada, K.; Mori, M. Hsp70-DnaJ Chaperone Pairs Prevent Nitric Oxide-Mediated Apoptosis in RAW 264.7 Macrophages. Cell Death Differ. 2001, 8, 357–366. [Google Scholar] [CrossRef]
- Gotoh, T.; Terada, K.; Oyadomari, S.; Mori, M. Hsp70-DnaJ Chaperone Pair Prevents Nitric Oxide- and CHOP-Induced Apoptosis by Inhibiting Translocation of Bax to Mitochondria. Cell Death Differ. 2004, 11, 390–402. [Google Scholar] [CrossRef]
- Sohn, S.-Y.; Kim, S.-B.; Kim, J.; Ahn, B.-Y. Negative Regulation of Hepatitis B Virus Replication by Cellular Hsp40/DnaJ Proteins through Destabilization of Viral Core and X Proteins. J. Gen. Virol. 2006, 87, 1883–1891. [Google Scholar] [CrossRef]
- Batra, J.; Tripathi, S.; Kumar, A.; Katz, J.M.; Cox, N.J.; Lal, R.B.; Sambhara, S.; Lal, S.K. Human Heat Shock Protein 40 (Hsp40/DnaJB1) Promotes Influenza A Virus Replication by Assisting Nuclear Import of Viral Ribonucleoproteins. Sci. Rep. 2016, 6, 19063. [Google Scholar] [CrossRef]
- Kumar, M.; Mitra, D. Heat Shock Protein 40 Is Necessary for Human Immunodeficiency Virus-1 Nef-Mediated Enhancement of Viral Gene Expression and Replication. J. Biol. Chem. 2005, 280, 40041–40050. [Google Scholar] [CrossRef]
- Pei, Y.; Fu, W.; Yang, E.; Shen, A.; Chen, Y.C.; Gong, H.; Chen, J.; Huang, J.; Xiao, G.; Liu, F. A Hsp40 Chaperone Protein Interacts with and Modulates the Cellular Distribution of the Primase Protein of Human Cytomegalovirus. PLoS Pathog. 2012, 8, e1002968. [Google Scholar] [CrossRef]
- Zarouchlioti, C.; Parfitt, D.A.; Li, W.; Gittings, L.M.; Cheetham, M.E. DNAJ Proteins in Neurodegeneration: Essential and Protective Factors. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160534. [Google Scholar] [CrossRef]
- Diane, A.; Abunada, H.; Khattab, N.; Moin, A.S.M.; Butler, A.E.; Dehbi, M. Role of the DNAJ/HSP40 Family in the Pathogenesis of Insulin Resistance and Type 2 Diabetes. Ageing Res. Rev. 2021, 67, 101313. [Google Scholar] [CrossRef]
- Kharenko, O.A.; Polichuk, D.; Nelson, K.M.; Abrams, S.R.; Loewen, M.C. Identification and Characterization of Interactions between Abscisic Acid and Human Heat Shock Protein 70 Family Members. J. Biochem. 2013, 154, 383–391. [Google Scholar] [CrossRef]
- Fatima, S.; Pandey, P.; Sharma, S.K.; Priya, S. Structural-Functional Relevance of DNAJBs in Protein Aggregation and Associated Neurodegenerative Diseases. Biochim. Biophys. Acta-Proteins Proteom. 2025, 1873, 141074. [Google Scholar] [CrossRef]
- Scior, A.; Buntru, A.; Arnsburg, K.; Ast, A.; Iburg, M.; Juenemann, K.; Pigazzini, M.L.; Mlody, B.; Puchkov, D.; Priller, J.; et al. Complete Suppression of Htt Fibrilization and Disaggregation of Htt Fibrils by a Trimeric Chaperone Complex. EMBO J. 2018, 37, 282–299. [Google Scholar] [CrossRef]
- Ayala Mariscal, S.M.; Pigazzini, M.L.; Richter, Y.; Özel, M.; Grothaus, I.L.; Protze, J.; Ziege, K.; Kulke, M.; ElBediwi, M.; Vermaas, J.V.; et al. Identification of a HTT-Specific Binding Motif in DNAJB1 Essential for Suppression and Disaggregation of HTT. Nat. Commun. 2022, 13, 4692. [Google Scholar] [CrossRef]
- McLean, P.J.; Kawamata, H.; Shariff, S.; Hewett, J.; Sharma, N.; Ueda, K.; Breakefield, X.O.; Hyman, B.T. TorsinA and Heat Shock Proteins Act as Molecular Chaperones: Suppression of α-Synuclein Aggregation. J. Neurochem. 2002, 83, 846–854. [Google Scholar] [CrossRef]
- Faust, O.; Abayev-Avraham, M.; Wentink, A.S.; Maurer, M.; Nillegoda, N.B.; London, N.; Bukau, B.; Rosenzweig, R. HSP40 Proteins Use Class-Specific Regulation to Drive HSP70 Functional Diversity. Nature 2020, 587, 489–494. [Google Scholar] [CrossRef]
- Irwin, R.; Faust, O.; Petrovic, I.; Wolf, S.G.; Hofmann, H.; Rosenzweig, R. Hsp40s Play Complementary Roles in the Prevention of Tau Amyloid Formation. Elife 2021, 10, e69601. [Google Scholar] [CrossRef]
- Rozales, K.; Younis, A.; Saida, N.; Meller, A.; Goldman, H.; Kellerman, L.; Heinrich, R.; Berlin, S.; Shalgi, R. Differential Roles for DNAJ Isoforms in HTT-PolyQ and FUS Aggregation Modulation Revealed by Chaperone Screens. Nat. Commun. 2022, 13, 516. [Google Scholar] [CrossRef]
- Alves-Borba, L.; Espinosa-Fernández, V.; Canseco-Rodríguez, A.; Sánchez-Pérez, A.M. ABA Supplementation Rescues IRS2 and BDNF MRNA Levels in a Triple-Transgenic Mice Model of Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2023, 7, 1007–1013. [Google Scholar] [CrossRef]
- Espinosa-Fernández, V.; Mañas-Ojeda, A.; Pacheco-Herrero, M.; Castro-Salazar, E.; Ros-Bernal, F.; Sánchez-Pérez, A.M. Early Intervention with ABA Prevents Neuroinflammation and Memory Impairment in a Triple Transgenic Mice Model of Alzheimer’s Disease. Behav. Brain Res. 2019, 374, 112106. [Google Scholar] [CrossRef]
- Maixner, D.W.; Christy, D.; Kong, L.; Viatchenko-Karpinski, V.; Horner, K.A.; Hooks, S.B.; Weng, H.R. Phytohormone Abscisic Acid Ameliorates Neuropathic Pain via Regulating LANCL2 Protein Abundance and Glial Activation at the Spinal Cord. Mol. Pain 2022, 18, 17448069221107781. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, N.; Ju, Y.J.; Gee, M.S.; Lee, D.; Lee, J.K. Phytohormone Abscisic Acid Improves Memory Impairment and Reduces Neuroinflammation in 5XFAD Mice by Upregulation of Lanc-like Protein 2. Int. J. Mol. Sci. 2020, 21, 8425. [Google Scholar] [CrossRef]
- Shabani, M.; Soti, M.; Ranjbar, H.; Naderi, R. Abscisic Acid Ameliorates Motor Disabilities in 6-OHDA-Induced Mice Model of Parkinson’s Disease. Heliyon 2023, 9, e18473. [Google Scholar] [CrossRef]
- Meseguer-Beltrán, M.; Sánchez-Sarasúa, S.; Landry, M.; Kerekes, N.; Sánchez-Pérez, A.M. Targeting Neuroinflammation with Abscisic Acid Reduces Pain Sensitivity in Females and Hyperactivity in Males of an ADHD Mice Model. Cells 2023, 12, 465. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, J.; Ma, T.; Chen, D.; Lu, D. MiR-1205/DNAJB1 Reverses Docetaxel Chemoresistance in Human Triple Negative Breast Carcinoma Cells via Regulation of Mutp53/TAp63 Signaling. Acta Biochim. Biophys. Sin. 2022, 54, 37–46. [Google Scholar] [CrossRef]
- Ren, H.; Luo, M.; Chen, J.; Zhou, Y.; Li, X.; Zhan, Y.; Shen, D.; Chen, B. Identification of TPD52 and DNAJB1 as Two Novel Bile Biomarkers for Cholangiocarcinoma by ITRAQ-based Quantitative Proteomics Analysis. Oncol. Rep. 2019, 42, 2622–2634. [Google Scholar] [CrossRef]
- Kong, L.; Liu, P.; Fei, X.; Wu, T.; Wang, Z.; Zhang, B.; Li, J.; Tan, X. A Prognostic Prediction Model Developed Based on Four CpG Sites and Weighted Correlation Network Analysis Identified DNAJB1 as a Novel Biomarker for Pancreatic Cancer. Front. Oncol. 2020, 10, 1716. [Google Scholar] [CrossRef]
- Moses, M.A.; Kim, Y.S.; Rivera-Marquez, G.M.; Oshima, N.; Watson, M.J.; Beebe, K.E.; Wells, C.; Lee, S.; Zuehlke, A.D.; Shao, H.; et al. Targeting the Hsp40/Hsp70 Chaperone Axis as a Novel Strategy to Treat Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 4022–4035. [Google Scholar] [CrossRef]
- Piette, B.L.; Alerasool, N.; Lin, Z.Y.; Lacoste, J.; Lam, M.H.Y.; Qian, W.W.; Tran, S.; Larsen, B.; Campos, E.; Peng, J.; et al. Comprehensive Interactome Profiling of the Human Hsp70 Network Highlights Functional Differentiation of J Domains. Mol. Cell 2021, 81, 2549–2565.e8. [Google Scholar] [CrossRef]
- De Amorim, Í.S.S.; de Sousa Rodrigues, M.M.; Mencalha, A.L. The Tumor Suppressor Role of Salvador Family WW Domain-Containing Protein 1 (SAV1): One of the Key Pieces of the Tumor Puzzle. J. Cancer Res. Clin. Oncol. 2021, 147, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- El Khouri, E.; Thomas, L.; Jeanson, L.; Bequignon, E.; Vallette, B.; Duquesnoy, P.; Montantin, G.; Copin, B.; Dastot-Le Moal, F.; Blanchon, S.; et al. Mutations in DNAJB13, Encoding an HSP40 Family Member, Cause Primary Ciliary Dyskinesia and Male Infertility. Am. J. Hum. Genet. 2016, 99, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Ni, L.; Luo, X. STK25 Suppresses Hippo Signaling by Regulating SAV1-STRIPAK Antagonism. Elife 2020, 9, e54863. [Google Scholar] [CrossRef]
- Luo, X.; Li, Z.; Yan, Q.; Li, X.; Tao, D.; Wang, J.; Leng, Y.; Gardner, K.; Judge, S.I.V.; Li, Q.Q.; et al. The Human WW45 Protein Enhances MST1-Mediated Apoptosis in vivo. Int. J. Mol. Med. 2009, 23, 357–362. [Google Scholar] [CrossRef]
- Paul, A.; Annunziato, S.; Lu, B.; Sun, T.; Evrova, O.; Planas-Paz, L.; Orsini, V.; Terracciano, L.M.; Charlat, O.; Loureiro, Z.Y.; et al. Cell Adhesion Molecule KIRREL1 Is a Feedback Regulator of Hippo Signaling Recruiting SAV1 to Cell-Cell Contact Sites. Nat. Commun. 2022, 13, 930. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, Z.; Yang, Z.; Nakamura, F. Identification of Filamin A Mechanobinding Partner III: SAV1 Specifically Interacts with Filamin A Mechanosensitive Domain 21. Biochemistry 2023, 62, 1197–1208. [Google Scholar] [CrossRef]
- Lu, L.; Tian, L. Postmenopausal Osteoporosis Coexisting with Sarcopenia: The Role and Mechanisms of Estrogen. J. Endocrinol. 2023, 259, e230116. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Kurzer, M.S. Estrogen Metabolism and Breast Cancer. Cancer Lett. 2015, 356, 231–243. [Google Scholar] [CrossRef]
- Qureshi, R.; Picon-Ruiz, M.; Aurrekoetxea-Rodriguez, I.; Nunes de Paiva, V.; D’Amico, M.; Yoon, H.; Radhakrishnan, R.; Morata-Tarifa, C.; Ince, T.; Lippman, M.E.; et al. The Major Pre- and Postmenopausal Estrogens Play Opposing Roles in Obesity-Driven Mammary Inflammation and Breast Cancer Development. Cell Metab. 2020, 31, 1154–1172.e9. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A Pathology Atlas of the Human Cancer Transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, C.; Zwahlen, M.; von Feilitzen, K.; Karlsson, M.; Shi, M.; Yuan, M.; Song, X.; Li, X.; Yang, H.; et al. Systematic Transcriptional Analysis of Human Cell Lines for Gene Expression Landscape and Tumor Representation. Nat. Commun. 2023, 14, 5417. [Google Scholar] [CrossRef]
- Theodosiou, M.; Laudet, V.; Schubert, M. From Carrot to Clinic: An Overview of the Retinoic Acid Signaling Pathway. Cell. Mol. Life Sci. 2010, 67, 1423–1445. [Google Scholar] [CrossRef]
- Siewers, V.; Kokkelink, L.; Smedsgaard, J.; Tudzynski, P. Identification of an Abscisic Acid Gene Cluster in the Grey Mold Botrytis Cinerea. Appl. Environ. Microbiol. 2006, 72, 4619–4626. [Google Scholar] [CrossRef]
- Li, H.H.; Hao, R.L.; Wu, S.S.; Guo, P.C.; Chen, C.J.; Pan, L.P.; Ni, H. Occurrence, Function and Potential Medicinal Applications of the Phytohormone Abscisic Acid in Animals and Humans. Biochem. Pharmacol. 2011, 82, 701–712. [Google Scholar] [CrossRef] [PubMed]

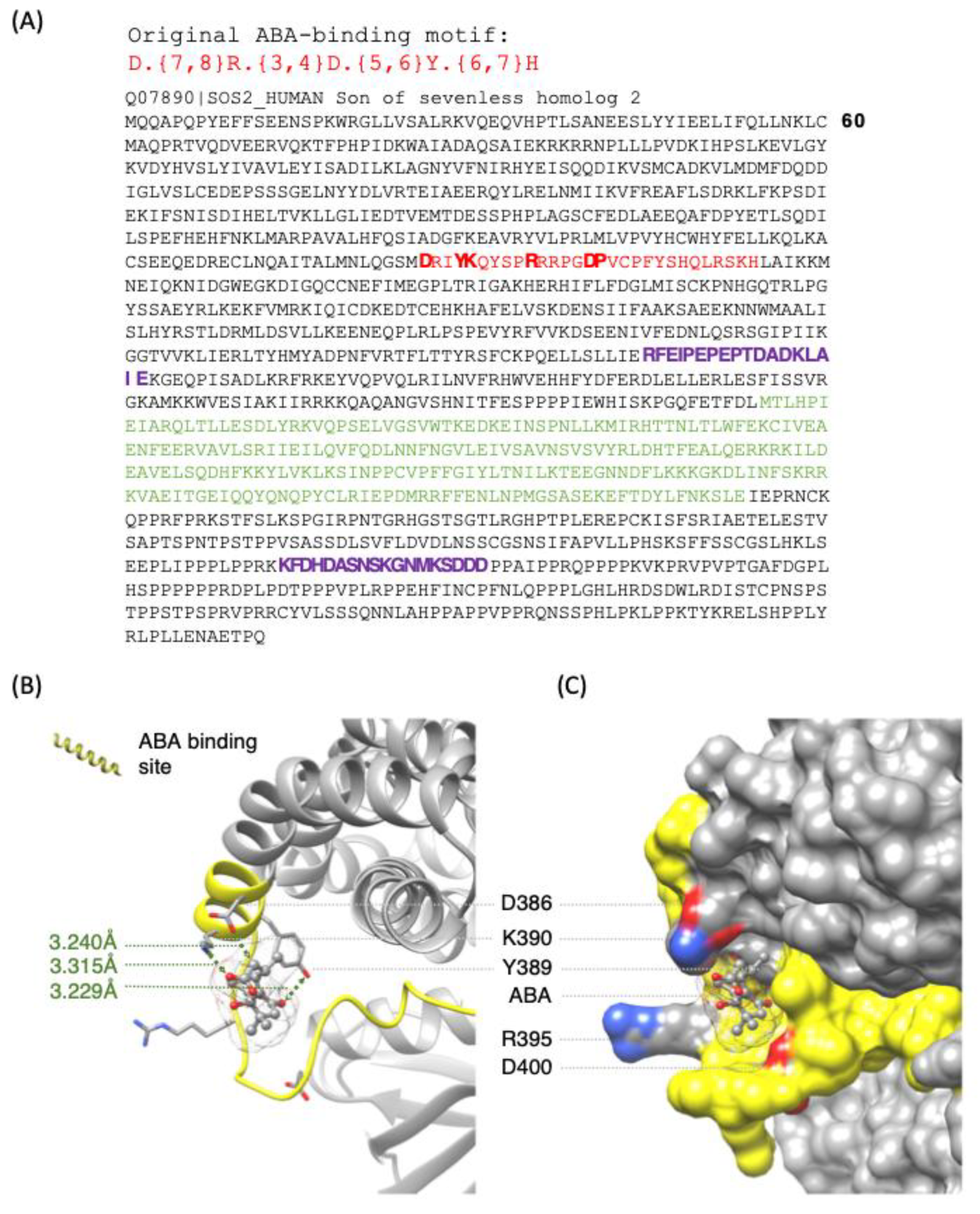
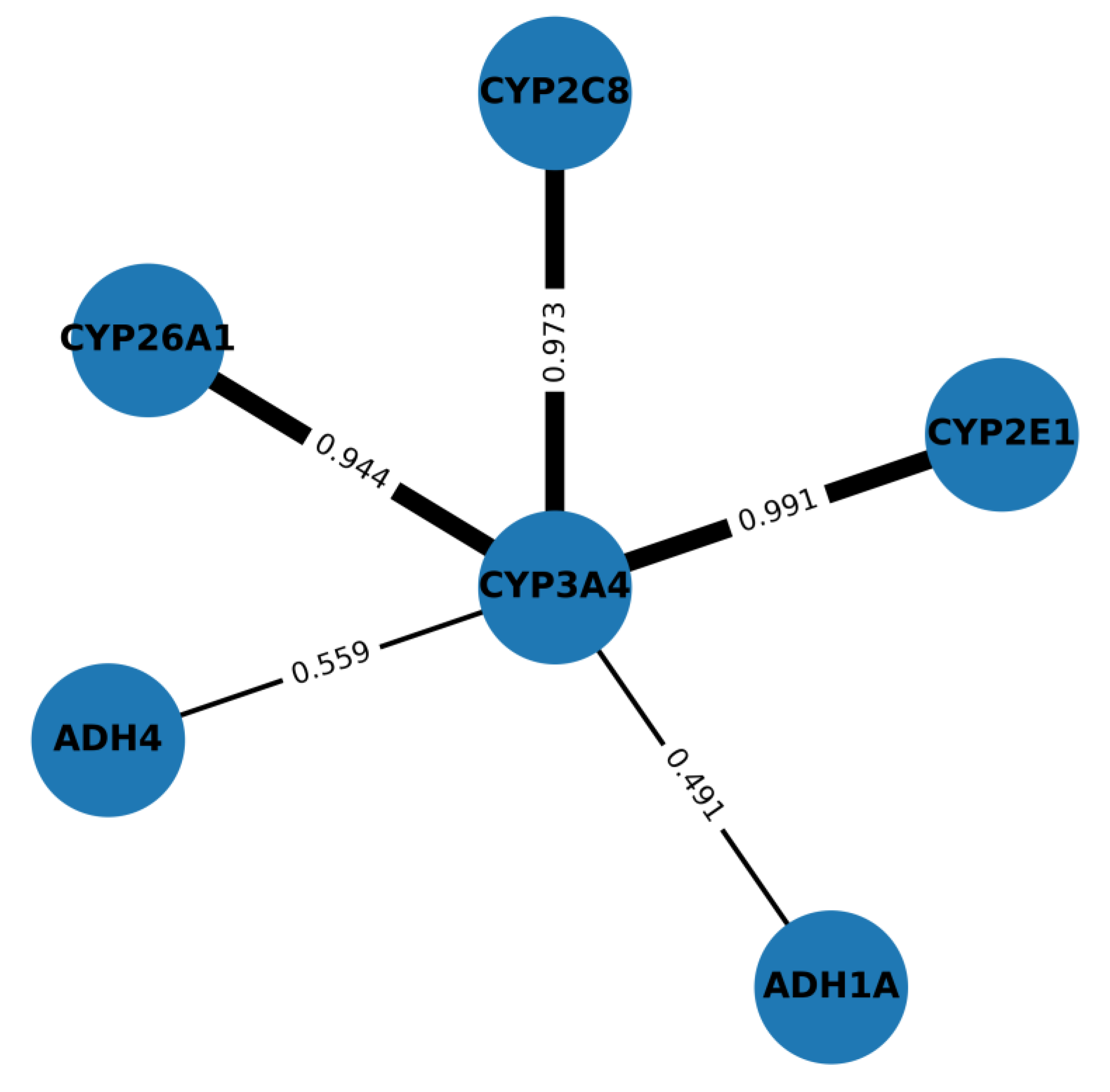
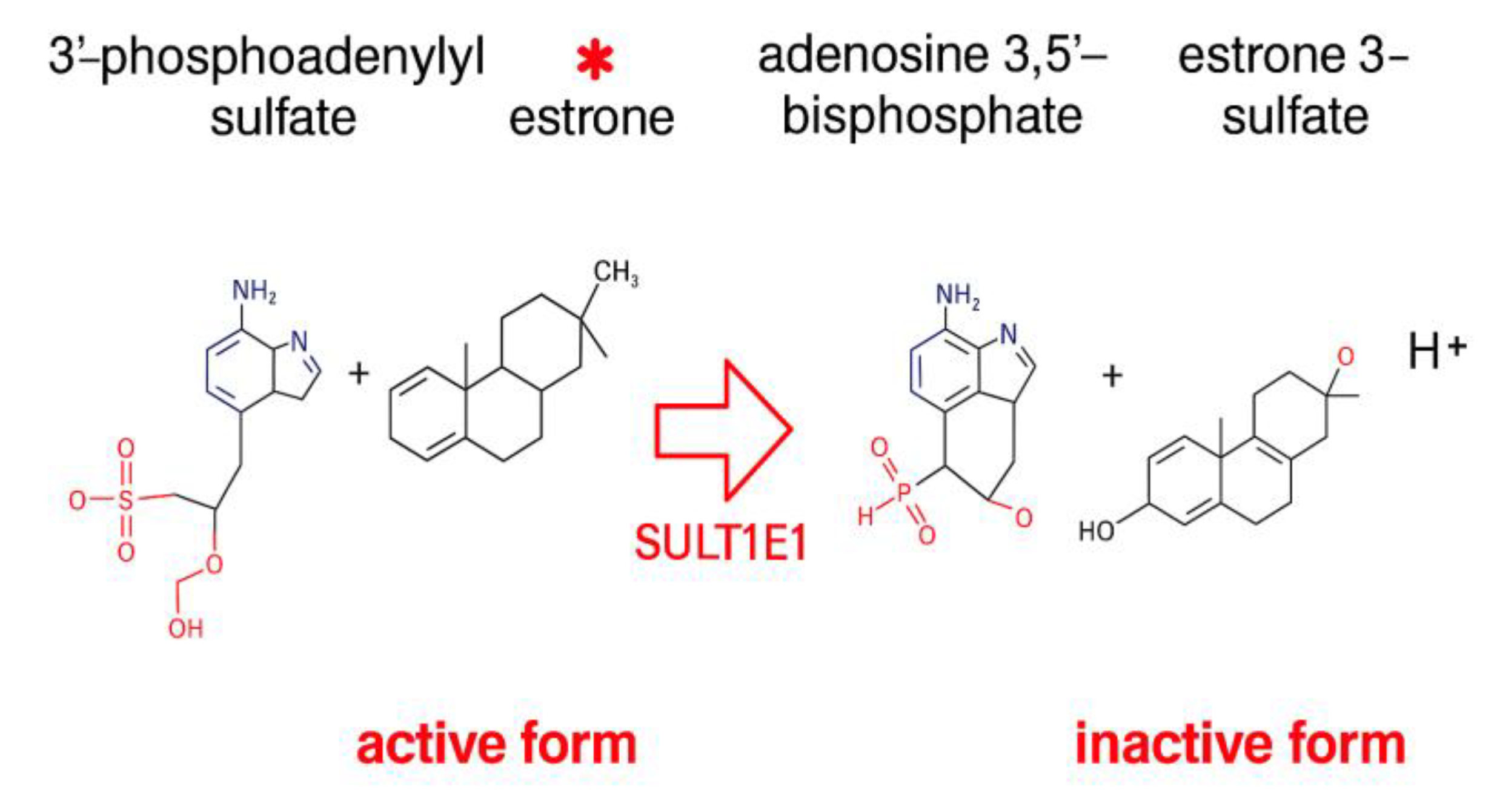
| UniProt ID | Name of the Protein |
|---|---|
| O60504 | Vinexin (SH3 domain cont. annotated) |
| O60583 | Cyclin-T2 |
| O94875 & | Sorbin (SH3 domain cont. annotated) |
| P25685 * | DNAJ homolog subfamily B member 1 (DNAJB1) |
| P31995 ** | Low aff. IG γ Fc region receptor II-c (FCGR2C) |
| P49888 | Sulfotransferase 1E1 (SULT1E1) |
| P59910 * | DNAJ homolog subfamily B member 13 (DNAJB13) |
| Q07890 **,#,& | Son of sevenless (SOS) homolog 2 (SOS2) |
| Q14C86 | GTPase-activating protein & VPS9 domain |
| Q16394 & | Exostosin-1 |
| Q7RTN6 | STE20-related kinase adapter protein |
| Q8N5S9 & | CaM-dependent protein kinase 1 |
| Q8WXX5 * | DNAJ homolog subfamily C member 9 (DNAJC9) |
| Q92839 | Hyaluronan synthase |
| Q9H4B6 & | Protein salvador homolog 1 (SAV1, hWW45) |
| Q9HAC8 & | Ubiquitin domain-containing protein |
| Q9NXW2 * | DNAJ homolog subfamily B member 12 (DNAJB12) |
| Q9NYQ8 & | Protocadherin Fat 2 |
| Q9UKJ3 & | G patch domain-containing protein 8 |
| Q9Y2K2 & | Serine/threonine-protein kinase SIK3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Maslahi, H.; Turek, I.; Bi, C.; Wong, A.; Tzfadia, O.; Irving, H.; Gehring, C. Functions and Synthesis of Abscisic Acid (ABA) in Humans—Insights from Computational Approaches. Int. J. Mol. Sci. 2025, 26, 11115. https://doi.org/10.3390/ijms262211115
El-Maslahi H, Turek I, Bi C, Wong A, Tzfadia O, Irving H, Gehring C. Functions and Synthesis of Abscisic Acid (ABA) in Humans—Insights from Computational Approaches. International Journal of Molecular Sciences. 2025; 26(22):11115. https://doi.org/10.3390/ijms262211115
Chicago/Turabian StyleEl-Maslahi, Houda, Ilona Turek, Chuyun Bi, Aloysius Wong, Oren Tzfadia, Helen Irving, and Chris Gehring. 2025. "Functions and Synthesis of Abscisic Acid (ABA) in Humans—Insights from Computational Approaches" International Journal of Molecular Sciences 26, no. 22: 11115. https://doi.org/10.3390/ijms262211115
APA StyleEl-Maslahi, H., Turek, I., Bi, C., Wong, A., Tzfadia, O., Irving, H., & Gehring, C. (2025). Functions and Synthesis of Abscisic Acid (ABA) in Humans—Insights from Computational Approaches. International Journal of Molecular Sciences, 26(22), 11115. https://doi.org/10.3390/ijms262211115








