Synergistic Effect of Poly(ethylenephosphoric Acid) and Cerium in Bone Substitute Composites on Tissue Response and Bone Remodeling
Abstract
1. Introduction
2. Results and Discussion
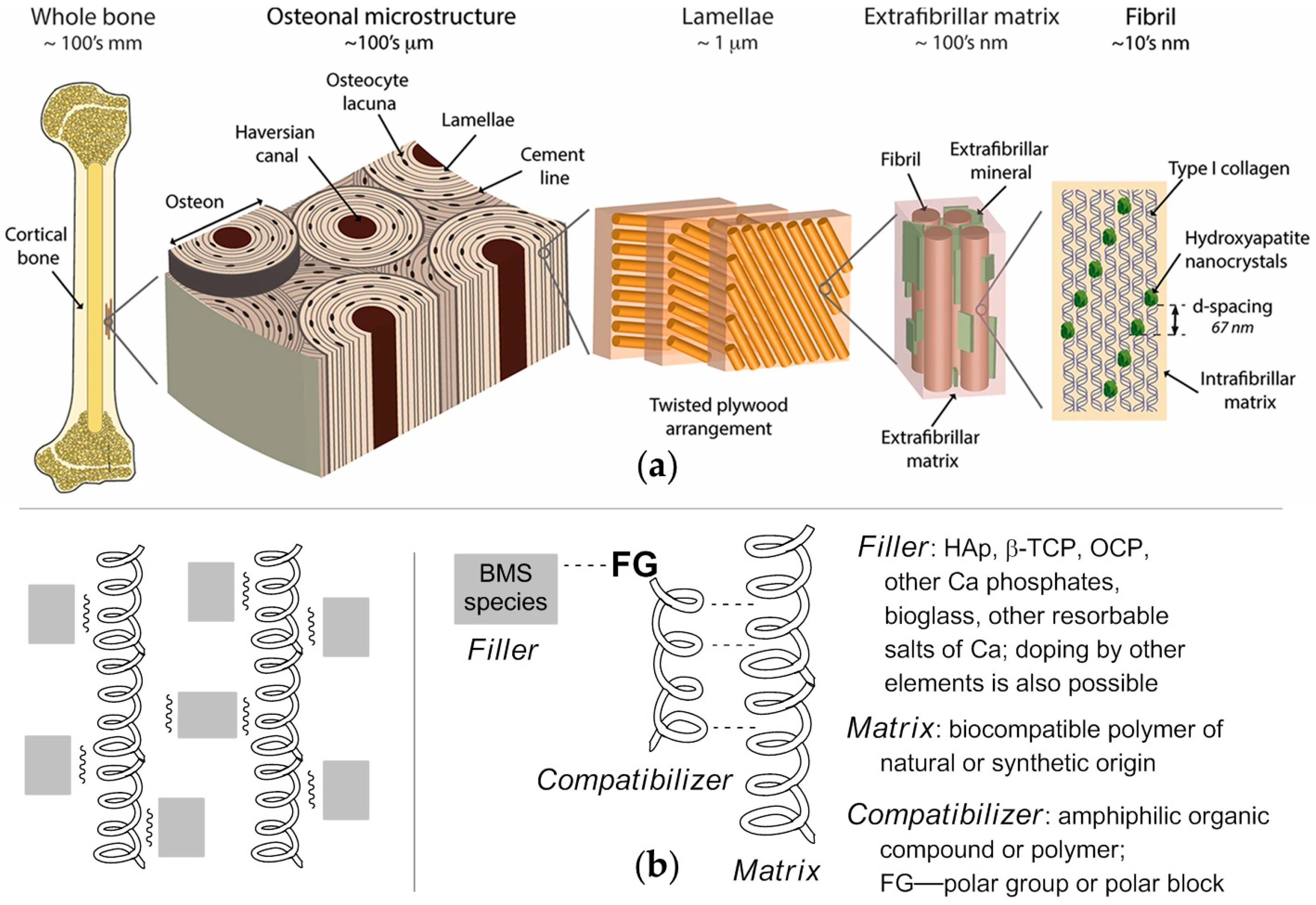
2.1. Inorganic Filler
2.1.1. Rationale for the Selection of the Filler
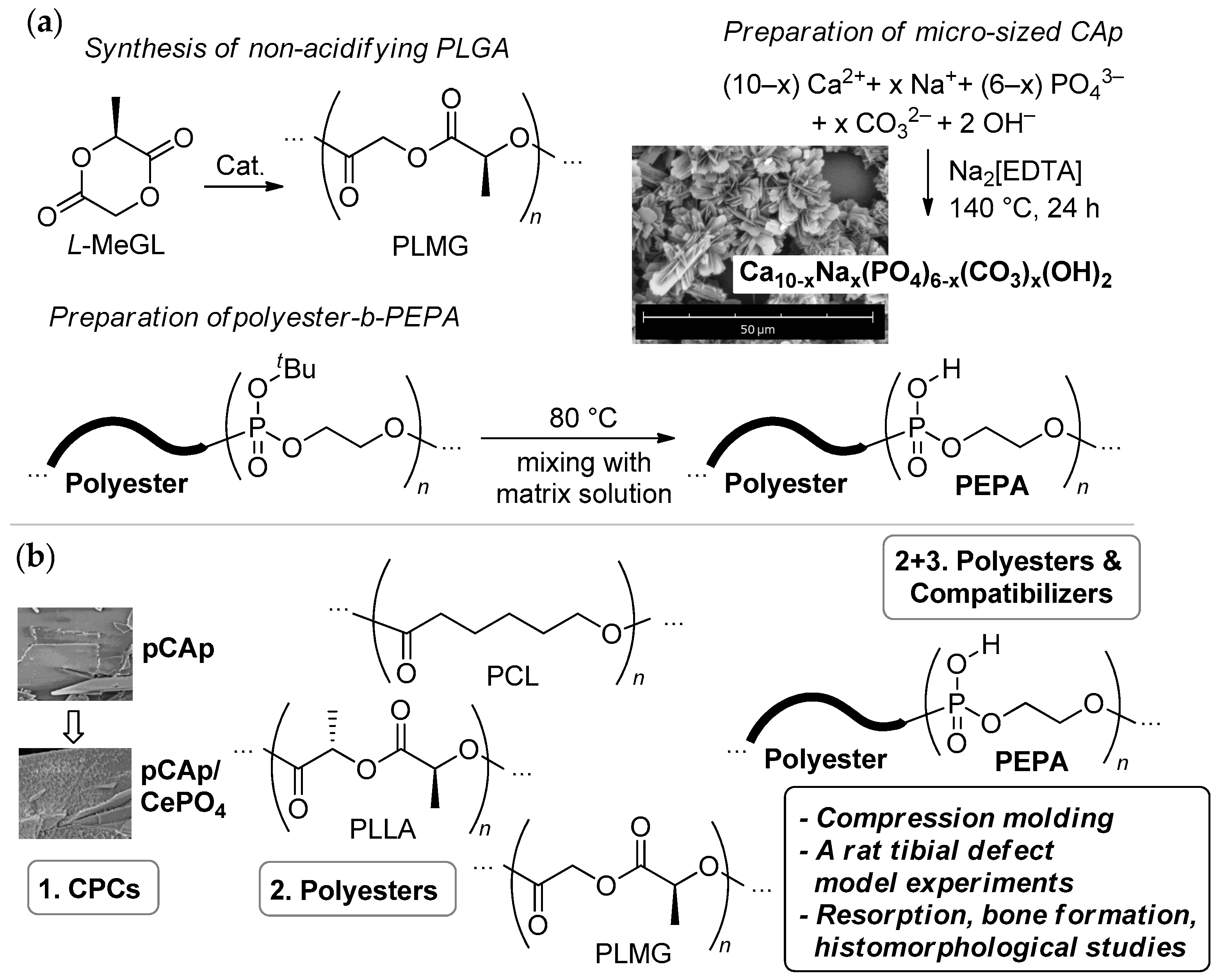
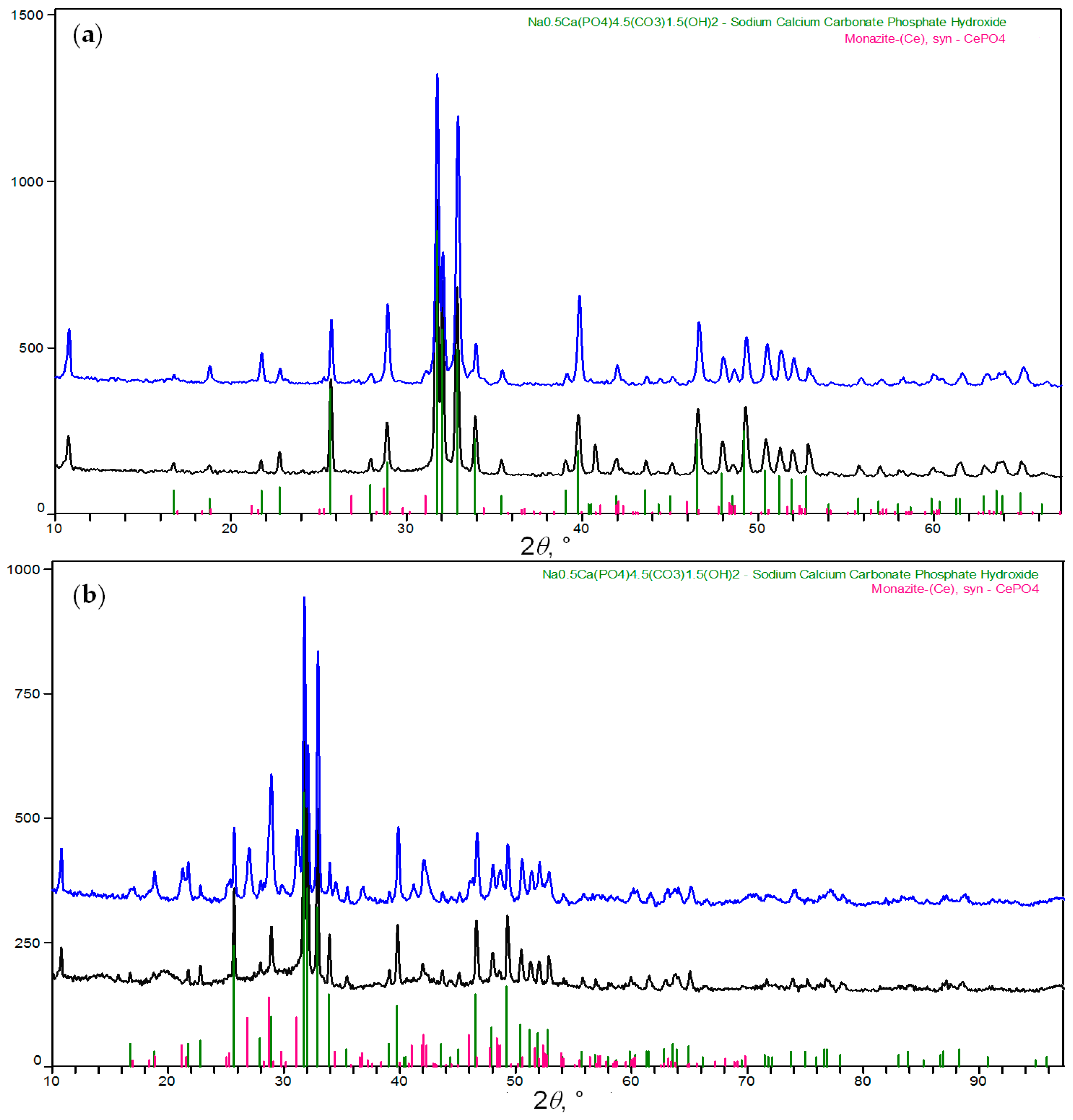
2.1.2. Synthesis and Characterization of pCAp and Ce-Containing pCAp
2.2. Polymer Matrices and Compatibilizers
2.2.1. Polymer Matrixes
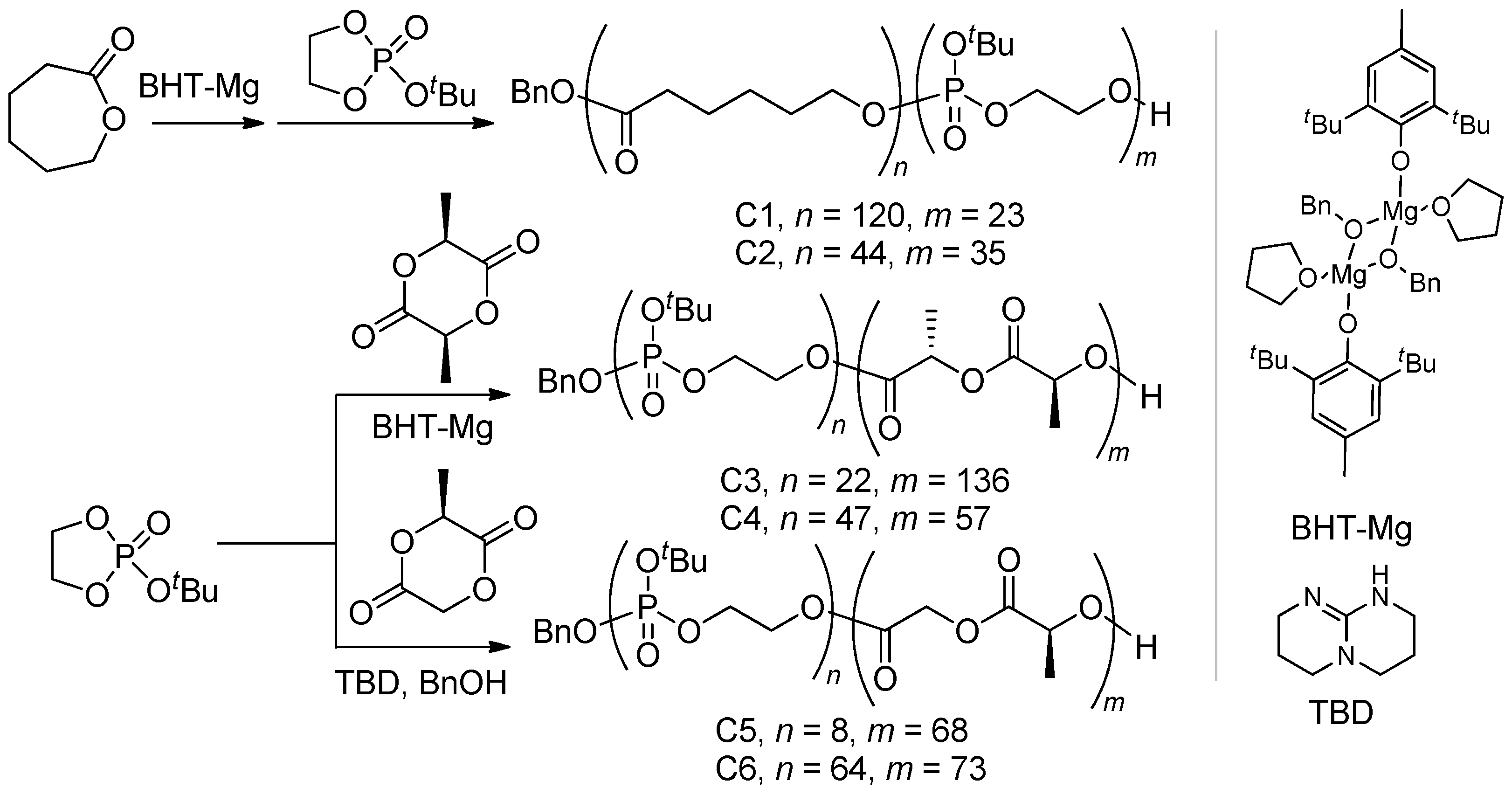
2.2.2. Block Copolymers of tBuOEP
2.3. Preparation of Composite Samples
2.3.1. Preparation of the Composites
2.3.2. Composite Formulations in Light of the Objectives of the Study
- 1.
- For two-component pCAp/polyester formulations, which of the three types of polyesters provides better biocompatibility and higher pCAp resorption?
- 2.
- For three-component pCAp/polyester-b-PEPA formulations,
- -
- Which of the three types of polyesters provides better biocompatibility, higher pCAp resorption, and more expressed new bone formation?
- -
- How does PEPA content in the copolymer influence these processes?
- 3.
- How does the presence of CePO4in pCAp influence composite resorption and bone remodeling?
2.4. Bone Substitution and Regeneration Experiments
2.4.1. Methodology and Description of the Experiments
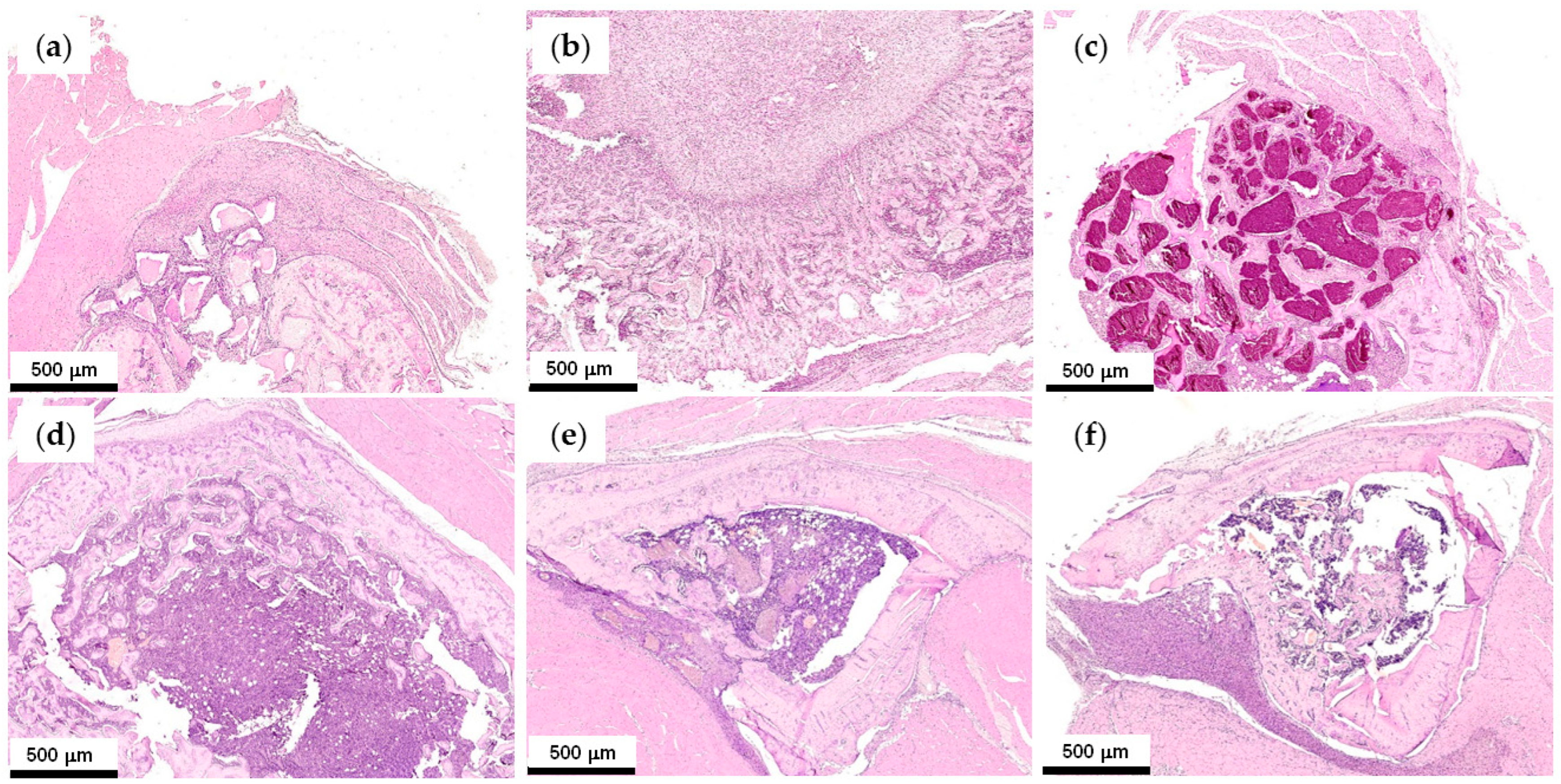
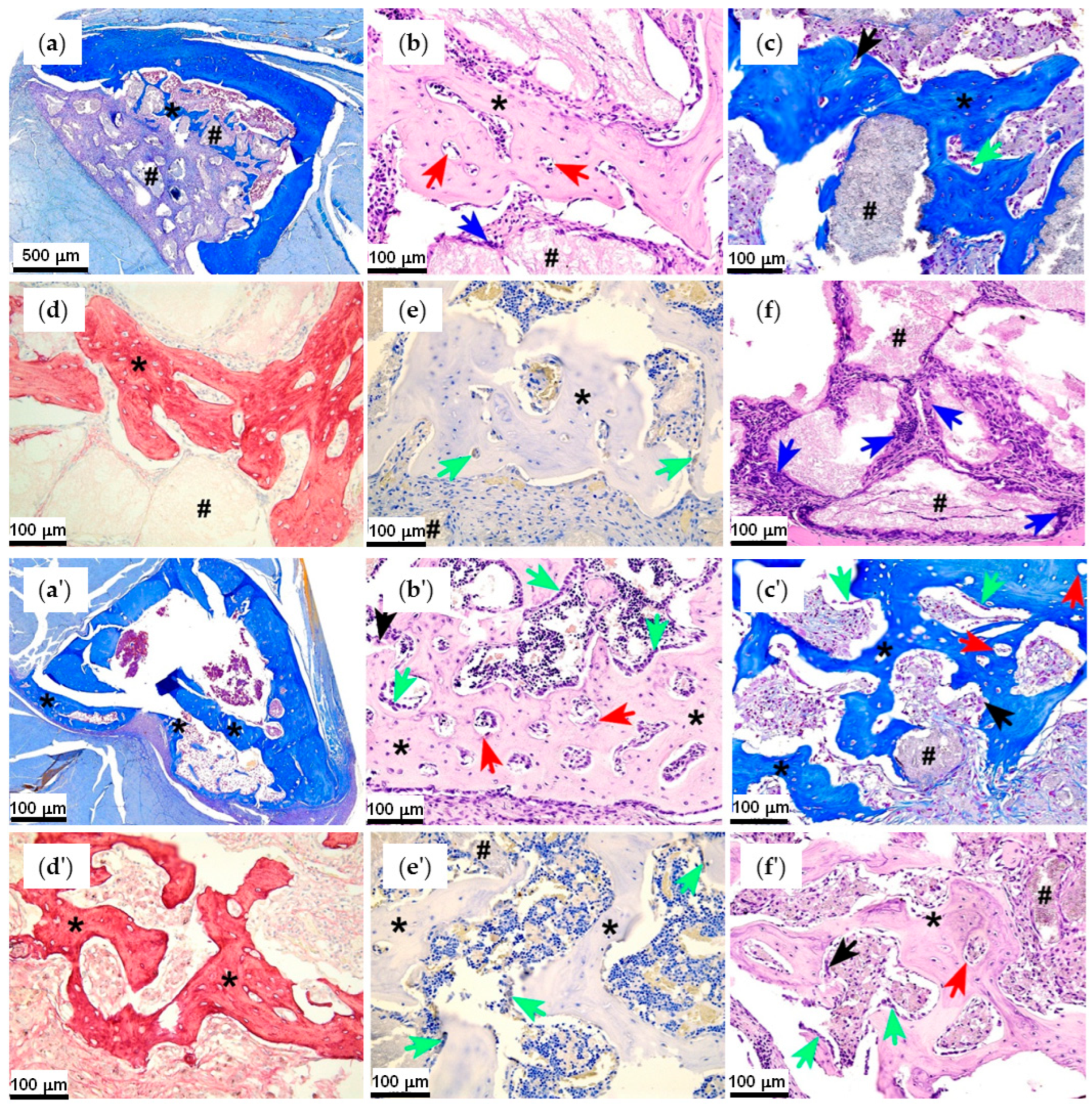
2.4.2. First Series of the Experiments: Effect of Polyester Type and PEPA
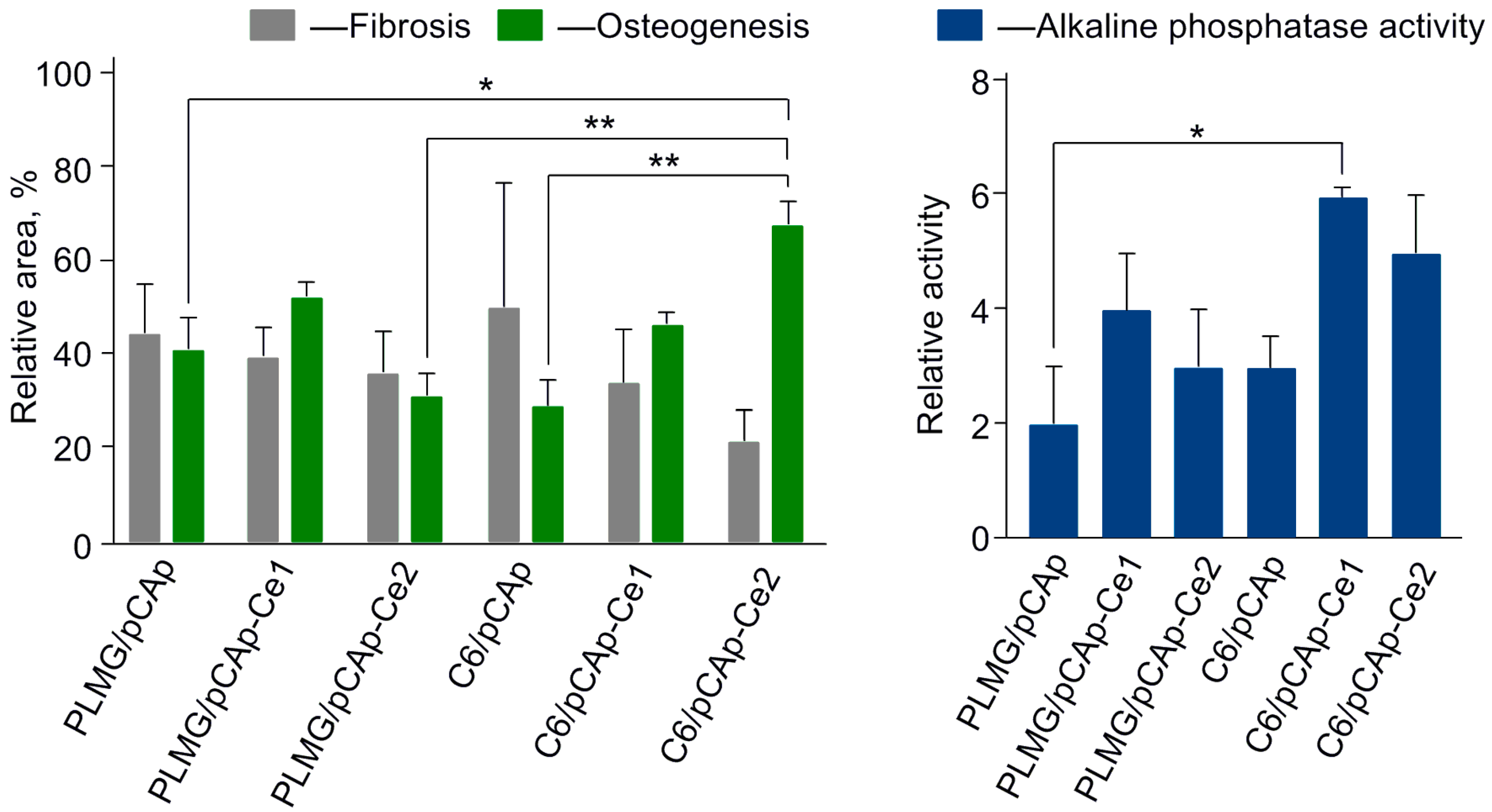
2.4.3. Second Series of the Experiments: Effect of Cerium
3. Materials and Methods
3.1. Solvents and Reagents
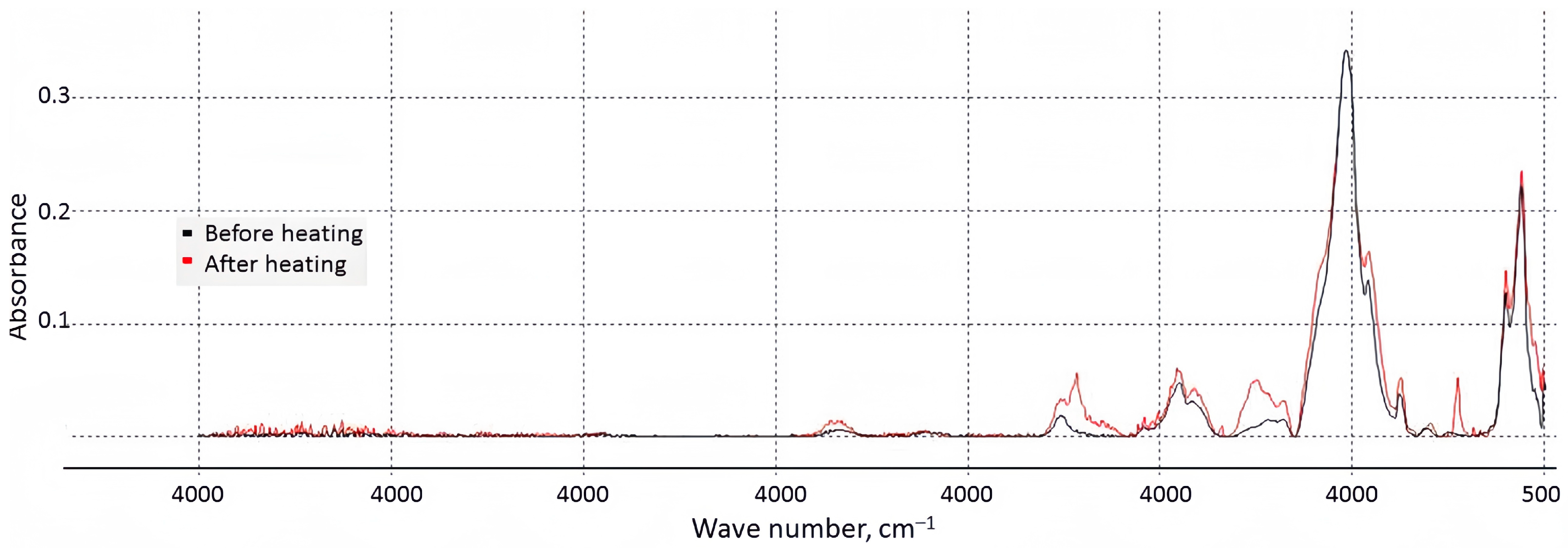
3.2. Physico-Chemical Characterization
3.3. Synthesis of Fillers
3.3.1. Synthesis of pCAp
3.3.2. Synthesis of Cerium-Doped pCAp
3.4. Synthesis of (Co)polymers
3.4.1. PLMG
3.4.2. εCL Copolymers C1 and C2
3.4.3. L-LA Copolymers C3 and C4
3.4.4. L-MeGL Copolymers C5 and C6
3.5. Preparation of the Composite Samples
3.5.1. PCL-, PLLA-,and PLMG-Based Composites
3.5.2. C5- and C6-Based Composites
3.6. In Vivo Experiments
3.6.1. Animals
3.6.2. Bone Repairing Studies
3.6.3. Morphological Studies
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BA | Bone apatite |
| BHT | Butylated hydroxytoluene, 2,6-di-tert-butyl-4-methylphenol |
| BMS | Bone mineral substitute |
| BS | Bone substitute |
| CPCs | Calcium phosphate ceramics |
| CT | Computed tomography |
| DPn | Degree of polymerization |
| EDTA | Ethylenediamine tetraacetate |
| HAp | Hydroxyapatite |
| HFIP | Hexafluoroisopropanol |
| L-LA | L-lactide |
| L-MeGL | L-methylglycolide |
| MNGCs | Multinuclear giant cells |
| pCAp | Plate-like carbonated apatite |
| PCL | Poly(ε-caprolactone) |
| PEPA | Poly(ethylenephosphoric acid) |
| PLLA | Poly(L-lactide) |
| PLMG | Poly(L-methylglycolide) |
| SEC | Size exclusion chromatography |
| SEM/EDX | Scanning electron microscopy/energy-dispersive X-ray spectroscopy |
| βTCP | β-tricalcium phosphate |
| THF | Tetrahydrofuran |
| XRD | X-ray diffraction |
References
- Santoro, A.; Voto, A.; Fortino, L.; Guida, R.; Laudisio, C.; Cillo, M.; D’Ursi, A.M. Bone Defect Treatment in Regenerative Medicine: Exploring Natural and Synthetic Bone Substitutes. Int. J. Mol. Sci. 2025, 26, 3085. [Google Scholar] [CrossRef] [PubMed]
- de Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Faour, O.; Dimitriou, R.; Cousins, C.A.; Giannoudis, P.V. The use of bone graft substitutes in large cancellous voids: Any specific needs? Injury 2011, 42, S87–S90. [Google Scholar] [CrossRef]
- Schlickewei, W.; Schlickewei, C. The Use of Bone Substitutes in the Treatment of Bone Defects–the Clinical View and History. Macromol. Symp. 2007, 253, 10–23. [Google Scholar] [CrossRef]
- Ishikawa, K. Carbonate apatite bone replacement: Learn from the bone. J. Ceramic Soc. Jpn. 2019, 127, 595–601. [Google Scholar] [CrossRef]
- Zimmermann, E.A.; Schaible, E.; Gludovatz, B.; Schmidt, F.N.; Riedel, C.; Krause, M.; Vettorazzi, E.; Acevedo, C.; Hahn, M.; Püschel, K.; et al. Intrinsic mechanical behavior of femoral cortical bone in young, osteoporotic and bisphosphonate-treated individuals in low- and high energy fracture conditions. Sci. Rep. 2016, 6, 21072. [Google Scholar] [CrossRef]
- Su, F.Y.; Pang, S.; Ling, Y.T.T.; Shyu, P.; Novitskaya, E.; Seo, K.; Lambert, S.; Zarate, K.; Graeve, O.A.; Jasiuk, I.; et al. Deproteinization of Cortical Bone: Effects of Different Treatments. Calcif. Tissue Int. 2018, 103, 554–566. [Google Scholar] [CrossRef]
- Pang, S.; Schwarcz, H.P.; Jasiuk, I. Interfacial bonding between mineral platelets in bone and its effect on mechanical properties of bone. J. Mechan. Behav. Biomed. Mater. 2021, 113, 104132. [Google Scholar] [CrossRef]
- Siddiqui, H.A.; Pickering, K.L.; Mucalo, M.R. A Review on the Use of Hydroxyapatite-Carbonaceous Structure Composites in Bone Replacement Materials for Strengthening Purposes. Materials 2018, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Nayak, C.; Singh, I.; Balani, K. Multifunctional Hydroxyapatite Composites for Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2022, 8, 3162–3186. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef] [PubMed]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Fu, Z.; Cui, J.; Zhao, B.; Shen, S.G.; Lin, K. An overview of polyester/hydroxyapatite composites for bone tissue repairing. J. Orthop. Transl. 2021, 28, 118–130. [Google Scholar] [CrossRef]
- Kuczumow, A.; Gorzelak, M.; Kosiński, J.; Lasota, A.; Blicharski, T.; Gągała, J.; Nowak, J.; Jarzębski, M.; Jabłoński, M. Hierarchy of Bioapatites. Int. J. Mol. Sci. 2022, 23, 9537. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, J.; Shen, Z.; Dong, D.; Su, H. Integrated processing of Al2O3/ZrO2 eutectic implants with bioactive Ca-P coatings by laser cladding. J. Mater. Res. Technol. 2022, 18, 2842–2852. [Google Scholar] [CrossRef]
- Bohner, M.; Le Gars Santoni, B.; Döbelin, N. β-Tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Kovrlija, I.; Locs, J.; Loca, D. Octacalcium phosphate: Innovative vehicle for the local biologically active substance delivery in bone regeneration. Acta Biomater. 2021, 135, 27–47. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Dong, D.; Su, H.; Li, X.; Liu, Y.; Shen, Z.; Zhao, D.; Guo, Y.; Zhang, Z.; Ren, W. Enhanced mechanical properties and biological responses of SLA 3D printed biphasic calcium phosphate bioceramics by doping bioactive metal elements. J. Eur. Ceram. Soc. 2023, 43, 4167–4178. [Google Scholar] [CrossRef]
- Dong, D.; Su, H.; Li, X.; Fan, G.; Zhao, D.; Shen, Z.; Liu, Y.; Guo, Y.; Yang, C.; Liu, L.; et al. Microstructures and mechanical properties of biphasic calcium phosphate bioceramics fabricated by SLA 3D printing. J. Manuf. Process. 2022, 81, 433–443. [Google Scholar] [CrossRef]
- Krishnan, L.; Chakrabarty, P.; Govarthanan, K.; Rao, S.; Santra, T.S. Bioglass and nano bioglass: A next-generation biomaterial for therapeutic and regenerative medicine applications. Int. J. Biol. Macronol. 2024, 277, 133073. [Google Scholar] [CrossRef]
- Ishikawa, K.; Hayashi, K. Carbonate apatite artificial bone. Sci. Technol. Adv. Mater. 2021, 22, 683–694. [Google Scholar] [CrossRef]
- Kono, T.; Sakae, T.; Nakada, H.; Kaneda, T.; Okada, H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals 2022, 12, 170. [Google Scholar] [CrossRef]
- Nagai, H.; Kobayashi-Fujioka, M.; Fujisawa, K.; Ohe, G.; Takamaru, N.; Hara, K.; Uchida, D.; Tamatani, T.; Ishikawa, K.; Miyamoto, Y. Effects of low crystalline carbonate apatite on proliferation and osteoblastic differentiation of human bone marrow cells. J. Mater. Sci. Mater. Med. 2015, 26, 99. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Miyamoto, Y.; Tsuchiya, A.; Hayashi, K.; Tsuru, K.; Ohe, G. Physical and Histological Comparison of Hydroxyapatite, Carbonate Apatite, and β-Tricalcium Phosphate Bone Substitutes. Materials 2018, 11, 1993. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Granular Honeycombs Composed of Carbonate Apatite, Hydroxyapatite, and β-Tricalcium Phosphate as Bone Graft Substitutes: Effects of Composition on Bone Formation and Maturation. ACS Appl. Bio Mater. 2020, 3, 1787–1795. [Google Scholar] [CrossRef]
- Mano, T.; Akita, K.; Fukuda, N.; Kamada, K.; Kurio, N.; Ishikawa, K.; Miyamoto, Y. Histological comparison of three apatitic bone substitutes with different carbonate contents in alveolar bone defects in a beagle mandible with simultaneous implant installation. J. Biomed. Mater. Res. 2020, 108, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Nomura, S.; Tsuchiya, A.; Takahashi, I.; Ishikawa, K. Effects of the carbonate content in carbonate apatite on bone replacement. J. Tissue Eng. Regener. Med. 2022, 16, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Miyamoto, Y.; Ishikawa, K.; Satomi, T.; Schaller, B. Osteoclast behaviors on the surface of deproteinized bovine bone mineral and carbonate apatite substitutes in vitro. J. Biomed. Mater. Res. 2022, 110, 1524–1532. [Google Scholar] [CrossRef]
- Zhang, C.; Hayashi, K.; Ishikawa, K. Osseointegration enhancement by controlling dispersion state of carbonate apatite in polylactic acid implant. Coll. Surf. B Biointerfaces 2023, 232, 113588. [Google Scholar] [CrossRef]
- Hayashi, K.; Zhang, C.; Alashkar, A.N.T.; Ishikawa, K. Carbonate Apatite Honeycomb Scaffold-Based Drug Delivery System for Repairing Osteoporotic Bone Defects. ACS Appl. Mater. Interfaces 2024, 16, 45956–45968. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimabukuro, M.; Zhang, C.; Alashkar, A.N.T.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Silver phosphate-modified carbonate apatite honeycomb scaffolds for anti-infective and pigmentation-free bone tissue engineering. Mater. Today Bio 2024, 27, 101161. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Hayashi, K.; Tsuchiya, A.; Kishida, R.; Ishikawa, K. In vivo trial of bioresorbable mesh cages contained bone graft granules in rabbit femoral bone defects. Sci. Rep. 2024, 14, 12449. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Transformable Carbonate Apatite Chains as a Novel Type of Bone Graft. Adv. Healthc. Mater. 2024, 13, 2303245. [Google Scholar] [CrossRef]
- Shibahara, K.; Hayashi, K.; Nakashima, Y.; Ishikawa, K. Controlling the pore size of carbonate apatite honeycomb scaffolds enhances orientation and strength of regenerated bone. Biomater. Adv. 2025, 166, 214026. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Tavtorkin, A.N.; Legkov, S.A.; Korchagina, S.A.; Shandryuk, G.A.; Kretov, E.A.; Dmitrienko, A.O.; Ivchenko, P.V. Hydrothermal synthesis of perfectly shaped micro- and nanosized carbonated apatite. Inorg. Chem. Front. 2021, 8, 4976–4989. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Freitas, P.P.; Nagashima, N.; Ishikawa, K. Influence of pH and ion components in the liquid phase on the setting reaction of carbonate apatite granules. Dent. Mater. J. 2022, 41, 209–213. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Tavtorkin, A.N.; Ryndyk, M.P.; Gavrilov, D.E.; Lukina, Y.S.; Bionyshev-Abramov, L.L.; Serejnikova, N.B.; Smolentsev, D.V.; Ivchenko, P.V. Crystalline Micro-Sized Carbonated Apatites: Chemical Anisotropy of the Crystallite Surfaces, Biocompatibility, Osteoconductivity, and Osteoinductive Effect Enhanced by Poly(ethylene phosphoric acid). ACS Appl. Bio Mater. 2023, 6, 5067–5077. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 8, 346–360. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Tavtorkin, A.N.; Shlyakhtin, A.V.; Ivchenko, P.V. Chemical features of the synthesis, degradation, molding and performance of poly(lactic-co-glycolic) acid (PLGA) and PLGA-based articles. Eur. Polym. J. 2024, 215, 113250. [Google Scholar] [CrossRef]
- Burkhart, S.S. The evolution of clinical applications of biodegradable implants in arthroscopic surgery. Biomaterials 2000, 21, 2631–2634. [Google Scholar] [CrossRef] [PubMed]
- Washington, M.A.; Balmert, S.C.; Fedorchak, M.V.; Little, S.R.; Watkins, S.C.; Meyer, T.Y. Monomer sequence in PLGA microparticles: Effects on acidic microclimates and in vivo inflammatory response. Acta Biomater. 2018, 65, 259–271. [Google Scholar] [CrossRef]
- Shlyakhtin, A.V.; Ryabova, A.V.; Kretov, E.A.; Koroleva, E.A.; Tavtorkin, A.N.; Nifant’ev, I.E.; Bagrov, V.V.; Ivchenko, P.V. Highly statistical PLGAs based on L-methylglycolide: The impact of copolymer microstructure on hydrolytic degradation monitored by pH fluorescent sensor. Eur. Polym. J. 2025, 239, 114309. [Google Scholar] [CrossRef]
- Tavtorkin, A.N.; Kretov, E.A.; Ryndyk, M.P.; Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Vinogradov, A.A.; Ivchenko, P.V. Synthesis, melt molding and hydrolytic degradation of poly(L-lactide-co-L-methylglycolide) and its composites with carbonated apatite. Polym. Degrad. Stab. 2024, 227, 110903. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Ivchenko, P.V. Design, Synthesis and Actual Applications of the Polymers Containing Acidic P–OH Fragments: Part 2—Sidechain Phosphorus-Containing Polyacids. Int. J. Mol. Sci. 2023, 24, 1613. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.; Gavrilov, D.; Tavtorkin, A.; Chinova, M.; Besprozvannykh, V.; Komarov, P.; Zaitsev, V.; Podoprigora, I.; Ivchenko, P. Antibacterial Poly(ε-CL)/Hydroxyapatite Electrospun Fibers Reinforced by Poly(ε-CL)-b-poly(ethylene phosphoric acid). Int. J. Mol. Sci. 2021, 22, 7690. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Tavtorkin, A.; Komarov, P.; Kretov, E.; Korchagina, S.; Chinova, M.; Gavrilov, D.; Ivchenko, P. Dispersant and Protective Roles of Amphiphilic Poly(ethylene phosphate) Block Copolymers in Polyester/Bone Mineral Composites. Int. J. Mol. Sci. 2023, 24, 11175. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Kosarev, M.A.; Tavtorkin, A.N.; Minyaev, M.E.; Roznyatovsky, V.A.; Ivchenko, P.V. Controlled ring-opening polymerisation of cyclic phosphates, phosphonates and phosphoramidatescatalysed by hereroleptic BHT-alkoxy magnesium complexes. Polym. Chem. 2017, 8, 6806–6816. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Tavtorkin, A.N.; Minyaev, M.E.; Kosarev, M.A.; Ivchenko, P.V. Synthesis in aqueous media of poly(ethylene phosphoric acids) by mild thermolysis of homopolymers and block copolymers based on tert-butyl ethylene phosphate. Eur. Polym. J. 2018, 106, 249–256. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Siniavin, A.; Karamov, E.; Kosarev, M.; Kovalchuk, S.; Turgiev, A.; Nametkin, S.; Bagrov, V.; Tavtorkin, A.; Ivchenko, P. A New Approach to Developing Long-Acting Injectable Formulations of Anti-HIV Drugs: Poly(Ethylene Phosphoric Acid) Block Copolymers Increase the Efficiency of Tenofovir against HIV-1 in MT-4 Cells. Int. J. Mol. Sci. 2021, 22, 340. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Bukharova, T.; Dyakonov, A.; Goldshtein, D.; Galitsyna, E.; Kosarev, M.; Shlyakhtin, A.; Gavrilov, D.; Ivchenko, P. Osteogenic Differentiation of Human Adipose Tissue-Derived MSCs by Non-Toxic Calcium Poly(ethylene phosphate)s. Int. J. Mol. Sci. 2019, 20, 6242. [Google Scholar] [CrossRef]
- Otaka, A.; Kiyono, K.; Iwasaki, Y. Enhancement of osteoblast differentiation using poly(ethylene sodium phosphate). Materialia 2021, 15, 100977. [Google Scholar] [CrossRef]
- Noree, S.; Iwasaki, Y. Thermally Assisted Generation of Protein–Poly(ethylene sodium phosphate) Conjugates with High Mineral Affinity. ACS Omega 2019, 4, 3398–3404. [Google Scholar] [CrossRef]
- Iwasaki, Y. Bone Mineral Affinity of Polyphosphodiesters. Molecules 2020, 25, 758. [Google Scholar] [CrossRef]
- Otaka, A.; Iwasaki, Y. Endocytosis of poly(ethylene sodium phosphate) by macrophages and the effect of polymer length on cellular uptake. J. Ind. Eng. Chem. 2019, 75, 115–122. [Google Scholar] [CrossRef]
- Kiyono, K.; Mabuchi, S.; Otaka, A.; Iwasaki, Y. Bone-targeting polyphosphodiesters that promote osteoblastic differentiation. J. Biomed. Mater. Res. A 2023, 111, 714–724. [Google Scholar] [CrossRef]
- Besprozvannykh, V.K.; Nifant’ev, I.E.; Tavtorkin, A.N.; Ivchenko, P.V. Cerium-doped calcium phosphate materials for bone tissue engineering. Russ. Chem. Bull. 2025, 74, 1505–1540. [Google Scholar] [CrossRef]
- Zambon, A.; Malavasi, G.; Pallini, A.; Fraulini, F.; Lusvardi, G. Cerium Containing Bioactive Glasses: A Review. ACS Biomater. Sci. Eng. 2021, 7, 4388–4401. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hoseini, S.J.; Hamzehlou, S.; Darroudi, M.; Verdi, J.; Hasanzadeh, L.; Kim, H.W.; Mozafari, M. Biomedical Applications of Nanoceria: New Roles for an Old Player. Nanomedicine 2018, 13, 3051–3069. [Google Scholar] [CrossRef]
- Gao, J.; Feng, L.; Chen, B.; Fu, B.; Zhu, M. The role of rare earth elements in bone tissue engineering scaffolds—A review. Compos. B Eng. 2022, 235, 109758. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, X.; Mo, M.; Hu, X.; Liu, J.; Chen, L. Systematic review of the osteogenic effect of rare earth nanomaterials and the underlying mechanisms. J. Nanobiotechnol. 2024, 22, 185. [Google Scholar] [CrossRef] [PubMed]
- Pastero, L.; Bruno, M.; Aquilano, D. Habit Change of Monoclinic Hydroxyapatite Crystals Growing from Aqueous Solution in the Presence of Citrate Ions: The Role of 2D Epitaxy. Crystals 2018, 8, 308. [Google Scholar] [CrossRef]
- Feng, Z.; Liao, Y.; Ye, M. Synthesis and structure of cerium-substituted hydroxyapatite. J. Mater. Sci. Mater. Med. 2005, 16, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Guo, D.G.; Zhao, W.A.; Wang, L.Y.; Xu, K.W. Influences of reaction parameters and Ce contents on structure and properties of nano-scale Ce-HA powders. J. Mater. Sci. Technol. 2014, 30, 776–781. [Google Scholar] [CrossRef]
- Petrakova, N.V.; Nikitina, Y.O.; Egorov, A.A.; Titov, D.D.; Baranov, O.V.; Barinov, S.M.; Komlev, V.S. Phases formation in cerium-doped hydroxyapatite. J. Phys. Conf. Ser. 2021, 1942, 012036. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, G.; Lu, Y. One-step synthesis of CePO4 nanoparticle-decorated cerium-doped ultralong flexible hydroxyapatite nanofibers. CrystEngComm 2023, 25, 4411–4416. [Google Scholar] [CrossRef]
- Drouet, C. Apatite Formation: Why It May Not Work as Planned, and How to Conclusively Identify Apatite Compounds. BioMed Res. Int. 2013, 2013, 490946. [Google Scholar] [CrossRef]
- Pellegrino, E.D.; Biltz, R.M. Bone Carbonate and the Ca to P Molar Ratio. Nature 1968, 219, 1261–1262. [Google Scholar] [CrossRef]
- Pusztai, P.; Simon, T.; Kukovecz, Á.; Kónya, Z. Structural stability test of hexagonal CePO4 nanowires synthesized at ambient temperature. J. Mol. Struct. 2013, 1044, 94–98. [Google Scholar] [CrossRef]
- Kabekkodu, S.; Dosen, A.; Blanton, T. PDF-5+: A comprehensive Powder Diffraction File™ for materials characterization. Powder Diffr. 2024, 39, 47–59. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Table of Elements. Cerium. Cerium X-ray Photoelectron Spectra, Cerium Electron Configuration, and Other Elemental Information. Available online: https://www.thermofisher.com/ru/ru/home/materials-science/learning-center/periodic-table/lanthanide-rare-earth/cerium.html (accessed on 5 November 2025).
- Lu, Y.; Swisher, J.H.; Meyer, T.Y.; Coates, G.W. Chirality-Directed Regioselectivity: An Approach for the Synthesis of Alternating Poly(Lactic-co-Glycolic Acid). J. Am. Chem. Soc. 2021, 143, 4119–4124. [Google Scholar] [CrossRef] [PubMed]
- Hadjichristidis, N.; Alagi, P.; Nikam, S.B.; Gopalsamy, K.; Bashihab, L.; Szekely, G. Controlled Ring-Opening Polymerization of Methyl Glycolide with Bifunctional Organocatalyst. Angew. Chem. Int. Ed. 2024, 63, e202411809. [Google Scholar] [CrossRef]
- Guo, X.; Ahmed, H.; Xu, G.; Wang, Q. Catalyst Improved Stereoselectivity and Regioselectivity Control to Access Completely Alternating Poly(lactic-co-glycolic acid) with Enhanced Properties. Angew. Chem. Int. Ed. 2025, 64, e202417075. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Tavtorkin, A.N.; Komarov, P.D.; Churakov, A.V.; Ivchenko, P.V. Substituted glycolides from natural sources: Preparation, alcoholysis and polymerization. Polym. Chem. 2020, 11, 6890–6902. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Minyaev, M.E.; Churakov, A.V.; Karchevsky, S.G.; Birin, K.P.; Ivchenko, P.V. Mono-BHT heteroleptic magnesium complexes: Synthesis, molecular structure and catalytic behavior in the ring-opening polymerization of cyclic esters. Dalton Trans. 2017, 46, 12132–12146. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Karchevsky, S.; Ivchenko, P. Mechanistic Insights of BHT-Mg-Catalyzed Ethylene Phosphate’s Coordination Ring-Opening Polymerization: DFT Modeling and Experimental Data. Polymers 2018, 10, 1105. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Gavrilov, D.; Karchevsky, S.; Ivchenko, P. DFT Visualization and Experimental Evidence of BHT-Mg-Catalyzed Copolymerization of Lactides, Lactones and Ethylene Phosphates. Polymers 2019, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Prieto, E.M.; Talley, A.D.; Gould, N.R.; Zienkiewicz, K.J.; Drapeau, S.J.; Kalpakci, K.N.; Guelcher, S.A. Effects of particle size and porosity on in vivo remodeling of settable allograft bone/polymer composites. J. Biomed. Mater. Res. 2015, 103, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xi, Y.; Teng, F.; Wang, Z.; Yang, G. Comparison of Two Different Sizes of Deproteinized Bovine Bone Mineral Particles in Lateral Sinus Floor Elevation With Simultaneous Implant Placement: A Radiographic Study. Clin. Oral Implants Res. 2025, 36, 736–747. [Google Scholar] [CrossRef]
- Coathup, M.J.; Cai, Q.; Campion, C.; Buckland, T.; Blunn, G.W. The effect of particle size on the osteointegration of injectable silicate-substituted calcium phosphate bone substitute materials. J. Biomed. Mater. Res. 2013, 101, 902–910. [Google Scholar] [CrossRef]
- Abels, M.; Alkildani, S.; Pröhl, A.; Xiong, X.; Krastev, R.; Korzinskas, T.; Stojanovic, S.; Jung, O.; Najman, S.; Barbeck, M. The Granule Size Mediates the In Vivo Foreign Body Response and the Integration Behavior of Bone Substitutes. Materials 2021, 14, 7372. [Google Scholar] [CrossRef]
- Vianna, T.S.W.; Sartoretto, S.C.; Alves, A.T.N.N.; Resende, R.F.B.; Mourão, C.F.A.B.; Calasans-Maia, J.A.; Martinez-Zelaya, V.R.; Ross, A.M.; Granjeiro, J.M.; Calasans-Maia, M.D.; et al. Nanostructured Carbonated Hydroxyapatite Associated to rhBMP-2 Improves Bone Repair in Rat Calvaria. J. Funct. Biomater. 2020, 11, 87. [Google Scholar] [CrossRef]
- Leiblein, M.; Koch, E.; Winkenbach, A.; Schaible, A.; Nau, C.; Büchner, H.; Schröder, K.; Marzi, I.; Henrich, D. Size matters: Effect of granule size of the bone graft substitute (Herafill®) on bone healing using Masquelet’s induced membrane in a critical size defect model in the rat’s femur. J. Biomed. Mater. Res. 2020, 108, 1469–1482. [Google Scholar] [CrossRef]
- Müller, W.E.L.G. Morphogenetically Active Amorphous Calcium Polyphosphate Nanoparticles for Therapeutic Applications. U.S. Patent 1,030,735,0B2, 4 June 2019. [Google Scholar]
- Lozhkin, B.; Shlyakhtin, A.; Bagrov, V.; Ivchenko, P.; Nifant’ev, I. Effective stereoselective approach to substituted 1,4-dioxan-2,5-diones as prospective substrates for ring-opening polymerization. Mendeleev Commun. 2018, 28, 61–63. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Agrawal, Y.K.; Dubey, R.K. Trace analysis of cerium in phosphorites. Chem. Geol. 1977, 20, 93–97. [Google Scholar] [CrossRef]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; pp. 1–219. [Google Scholar]
- Dapson, R.W.; Horobin, R.W. Dyes from a twenty-first century perspective. Biotech. Hystochem. 2009, 84, 135–137. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Theory and Practice of Histological Techniques, 6th ed.; Bancroft, J.D., Gamble, M., Eds.; Elsevier Health Sciences: Philadelphia, PA, USA, 2008; pp. 1–694. [Google Scholar]
- Mao, D.; Scutt, A. Histochemical Localization of Alkaline Phosphatase Activity in Decalcified Bone and Cartilage. J. Histochem. Citochem. 2002, 50, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, X.; Xu, Y.; Shi, L.; Zhang, M.; Nie, M.; Liu, X. Significance and considerations of establishing standardized critical values for critical size defects in animal models of bone tissue regeneration. Heliyon 2024, 10, e33768. [Google Scholar] [CrossRef] [PubMed]
- Vajgel, A.; Mardas, N.; Farias, B.C.; Petrie, A.; Cimões, R.; Donos, N. A systematic review on the critical size defect model. Clin. Oral Implants Res. 2014, 25, 879–893. [Google Scholar] [CrossRef]




| Composite | (Co)polymer, wt.% | PEPA, mol.% in Copolymer | Animals Series 1 |
|---|---|---|---|
| First series of experiments (3 months) | |||
| PCL/pCAp | 15 | 0 | A, B |
| C1/pCAp | 16 | 16 | A |
| C2/pCAp | 15 | 44 | B |
| PLLA/pCAp | 15 | 0 | C |
| C3/pCAp | 16 | 14 | C, D |
| C4/pCAp | 16 | 45 | D |
| PLMG/pCAp | 15 | 0 | E, F |
| C5/pCAp | 16 | 11 | E |
| C6/pCAp | 15 | 47 | F |
| Second series of experiments (1 month) | |||
| pCAp | 0 | 0 | G |
| pCAp-Ce2 | 0 | 0 | G |
| PLMG/pCAp | 15 | 0 | H |
| C5/pCAp | 16 | 11 | H |
| C6/pCAp | 15 | 47 | I |
| PLMG/pCAp-Ce1 | 15 | 0 | I |
| PLMG/pCAp-Ce2 | 15 | 0 | J |
| C6/pCAp-Ce1 | 16 | 47 | K |
| C6/pCAp-Ce2 | 16 | 47 | J, K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besprozvannykh, V.; Ryndyk, M.; Nifant’ev, I.; Tavtorkin, A.; Gavrilov, D.; Lukina, Y.; Bionyshev-Abramov, L.; Serejnikova, N.; Smolentsev, D.; Ivchenko, P. Synergistic Effect of Poly(ethylenephosphoric Acid) and Cerium in Bone Substitute Composites on Tissue Response and Bone Remodeling. Int. J. Mol. Sci. 2025, 26, 11113. https://doi.org/10.3390/ijms262211113
Besprozvannykh V, Ryndyk M, Nifant’ev I, Tavtorkin A, Gavrilov D, Lukina Y, Bionyshev-Abramov L, Serejnikova N, Smolentsev D, Ivchenko P. Synergistic Effect of Poly(ethylenephosphoric Acid) and Cerium in Bone Substitute Composites on Tissue Response and Bone Remodeling. International Journal of Molecular Sciences. 2025; 26(22):11113. https://doi.org/10.3390/ijms262211113
Chicago/Turabian StyleBesprozvannykh, Victoria, Maria Ryndyk, Ilya Nifant’ev, Alexander Tavtorkin, Dmitry Gavrilov, Yulia Lukina, Leonid Bionyshev-Abramov, Natalya Serejnikova, Dmitriiy Smolentsev, and Pavel Ivchenko. 2025. "Synergistic Effect of Poly(ethylenephosphoric Acid) and Cerium in Bone Substitute Composites on Tissue Response and Bone Remodeling" International Journal of Molecular Sciences 26, no. 22: 11113. https://doi.org/10.3390/ijms262211113
APA StyleBesprozvannykh, V., Ryndyk, M., Nifant’ev, I., Tavtorkin, A., Gavrilov, D., Lukina, Y., Bionyshev-Abramov, L., Serejnikova, N., Smolentsev, D., & Ivchenko, P. (2025). Synergistic Effect of Poly(ethylenephosphoric Acid) and Cerium in Bone Substitute Composites on Tissue Response and Bone Remodeling. International Journal of Molecular Sciences, 26(22), 11113. https://doi.org/10.3390/ijms262211113









