Abstract
The limitations of conventional cancer therapies, including toxicity and resistance, underscore the need for safer and more versatile alternatives that can either complement or substitute existing regimens. Garcinol, a polyisoprenylated benzophenone derived primarily from the rind and leaves of Garcinia indica and Garcinia cambogia, has drawn significant interest in recent decades. Although traditionally used to relieve inflammatory disorders, its biomedical relevance expanded considerably after reports in the late 20th century demonstrated antimicrobial and subsequently anti-cancer properties. A growing body of cell-based research, supported by a smaller set of animal experiments, now suggests that garcinol acts as a potent epigenetic modulator. Its activities include inhibition of histone acetyltransferases (HATs), a groundbreaking research followed by regulation of oncogenic microRNAs, and modulation of signaling pathways critical to tumor progression. This review integrates current findings on garcinol’s dual role as a HAT inhibitor and regulator of oncogenic networks with updates on in vitro and in vivo studies with a more focused approach on in vivo animal models, highlighting its potential as an emerging therapeutic against malignancies and inflammatory diseases. Nonetheless, translation into clinical settings remains premature, as robust in vivo evidence is sparse and human trials are lacking. Moving forward, systematic investigations are required to clarify safety profiles, establish effective dosing strategies, and evaluate its efficacy across different cancer types.
1. Introduction
Garcinia cambogia (commonly known as Malabar tamarind) and Garcinia indica (Kokum) are tropical fruits native to India and Southeast Asia, valued for centuries in traditional medicine for their anti-inflammatory properties.
Today, extracts from G. cambogia are widely marketed as dietary supplements, particularly in weight management. Phytochemical analyses reveal that the rinds of these fruits are abundant in organic acids, amino acids, xanthones, benzophenones, and flavonoids. Among these constituents, garcinol—present at approximately 2–3% of rind content—has emerged as a key bioactive molecule, first structurally characterized by Sahu and colleagues [1]. The standard extraction process involves sequential treatments: initial water extraction to remove hydroxycitric acid, followed by exhaustive methanolic extraction of the residue, solvent partitioning, and final purification [2,3].
Structurally, garcinol shares several functional similarities with curcumin. The C-3 ketonic group and hydroxylated phenolic rings participate in redox reactions, while the α,β-unsaturated ketone moiety facilitates apoptosis induction [Figure 1C and Figure 2]. Antioxidant activity is conferred by double bonds within the isoprenyl side chain, whereas the dihydroxy substituents at carbons 13 and 14, together with the C8 side chain, have been implicated in its cytotoxic effects against cancer cells [4,5].
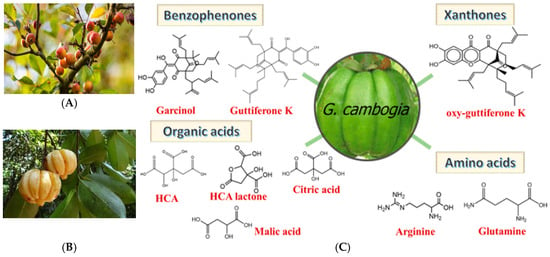
Figure 1.
(A) Garcinia Indica, (B) Garcinia Cambogia, (C) chemical constituents of Garcinia fruit [6].
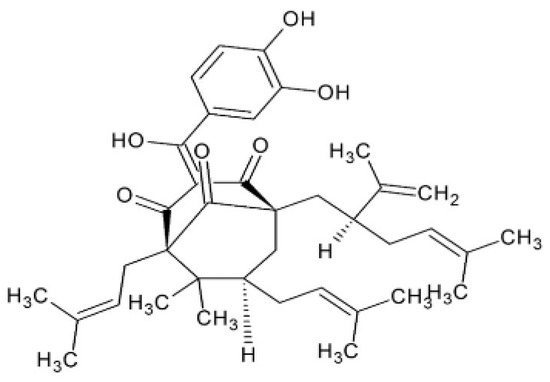
Figure 2.
Chemical structure of garcinol.
2. Mechanisms of Anti-Cancer Activity
The recognition of garcinol as a cell-permeable histone acetyltransferase (HAT) inhibitor [7] provided a molecular explanation for its anti-cancer activity that had been noted in earlier studies [8,9,10]. The major outcomes of garcinol’s ability to cause epigenetic modulation of cancer are depicted in Figure 3. This seminal finding not only clarified garcinol’s mechanism of action but also positioned it as a key epigenetic modulator capable of altering chromatin dynamics and transcriptional control. By inhibiting HAT enzymes such as p300/CBP and PCAF, garcinol affects the acetylation status of multiple transcription factors and histones, thereby regulating a broad range of oncogenic and tumor-suppressive pathways. Subsequent studies have shown that this modulation extends to critical signaling cascades, including NF-κB, STAT3, PI3K/AKT, MAPK, and Wnt/β-catenin, resulting in the suppression of inflammation, angiogenesis, proliferation, and metastasis. Moreover, its HAT-inhibitory activity has been linked to the regulation of microRNAs, epithelial–mesenchymal transition (EMT), and cancer stem cell renewal, establishing garcinol as a multifaceted compound that integrates epigenetic control with transcriptional and signaling network regulation in cancer.
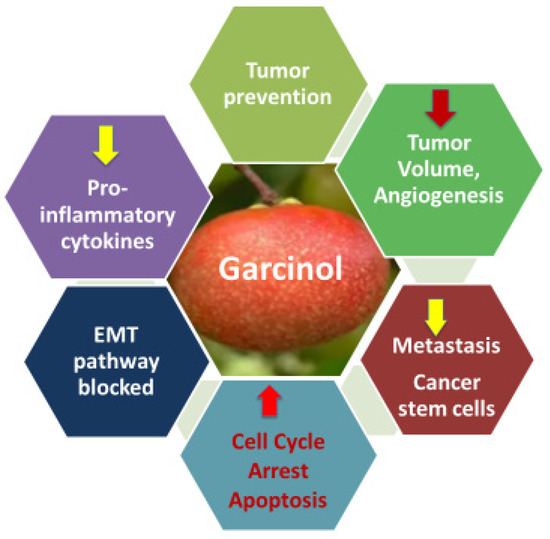
Figure 3.
Outcomes of epigenetic modulation and multifunctional anti-cancer effects of garcinol.
In the pioneering work on HeLa cells [7], garcinol suppressed the expression of more than 70% of evaluated genes, many of which govern cell division, programmed death, and oncogenic processes. Since then, anti-cancer effects have been documented across multiple malignancies—including ovarian, gastric, colon, oral squamous, glioblastoma, hepatocellular, melanoma, breast, pancreatic, and endometrial cancers—underscoring its broad relevance [11,12,13,14,15,16,17,18,19,20,21,22,23,24]. The present review focuses on garcinol’s mechanisms in cancer prevention and therapy, as evidenced in both in vitro and limited in vivo contexts, while excluding studies related solely to obesity, infectious diseases, and dietary supplementation for livestock production.
Subsequent extensive studies revealed that garcinol regulates diverse intracellular pathways through complex interactions with various molecules involved in the cancer development and its progress. Molecular pathways included but are not limited to cell cycle arrest and apoptosis, HAT inhibition, DNA methylation, histone acetylation, downregulation of oncogenic miRNAs, upregulation of tumor-suppressive miRNAs, inhibition of NF-kB, STAT 3, P13/Akt, COX-2, MAPK pathways, inhibition of epithelial-to-mesenchymal transition (EMT), and cancer stem cell targeting. These pathways are illustrated in Figure 4.
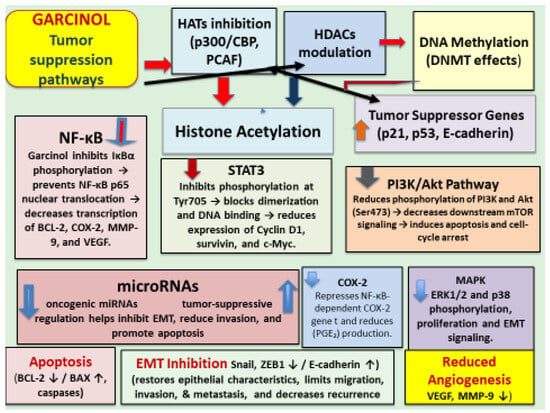
Figure 4.
Schematic illustration of the multi-targeted tumor-suppressive mechanisms of garcinol across epigenetic, transcriptional, and signaling axes.
Cell-cycle arrest and the apoptosis induction ability of garcinol have been widely documented across various tumor models. It exerts a dual regulatory influence on cancer cell proliferation and survival by halting progression at specific checkpoints and initiating apoptosis. These mechanisms have been demonstrated in numerous in vitro systems and confirmed in a few in vivo animal studies. Garcinol commonly blocks the cell cycle at the S-phase, leading to the suppression of cell division and enhanced apoptotic signaling. At the molecular level, this response is linked to decreased expression of cyclin D1 and cyclin D3, inhibition of cyclin-dependent kinases (CDK2, CDK4), and downregulation of the PI3K/AKT pathway, which plays a central role in tumor survival and metastatic progression [25,26,27,28,29].
Additionally, garcinol activates tumor-suppressor proteins such as p53 and inhibits thioredoxin reductase, resulting in elevated intracellular reactive oxygen species (ROS). The accumulation of ROS subsequently stimulates JNK activation and DNA damage signaling, reinforcing p53 function and promoting apoptosis rather than mere growth inhibition [30]. Studies in MCF-7 breast cancer cells further support this mechanism, showing activation of mitochondrial apoptosis through the ROS/JNK/ATF-2/Bcl-2 axis [31,32]. Structurally related analogs, including epigarcinol and isogarcinol, have exhibited similar effects by increasing ROS levels and inducing mitochondrial dysfunction in leukemia and prostate cancer models [33,34].
In rhabdomyosarcoma cells, garcinol was also shown to trigger endoplasmic reticulum (ER) stress, elevating the expression of stress-responsive genes such as DDIT3, DDIT4, TRIB3, and SESN2, which facilitate apoptosis under prolonged stress [35]. Another regulatory mechanism involves upregulation of p21^Waf1/Cip1, a p53-dependent CDK inhibitor that prevents G1–S phase progression. This pathway was highlighted in lung cancer studies, where garcinol caused apoptosis in p53-competent H460 cells and G1-phase arrest in p53-deficient H1299 cells [26,36,37].
Overall, the available evidence demonstrates that garcinol suppresses proliferation and promotes apoptosis through multifaceted signaling interactions, as summarized in Table 1, which lists its key molecular targets and associated outcomes.

Table 1.
Summary of molecular targets and outcomes involved in cell cycle arrest and apoptosis.
Although garcinol is shown to halt cell cycle and promote apoptosis across diverse cancer models, several mechanistic, contextual, and translational gaps persist, including lack of clarity on molecular hierarchy and chromatin context. The hypotheses for the future could be that garcinol induces a bifurcation point where prolonged HAT inhibition stabilizes p21-dependent arrest, and persistent chromatin stress subsequently activates intrinsic apoptotic signaling via mitochondrial depolarization. Whether secondary signaling pathways (PI3K/Akt, MAPK, STAT3) also participate apart from HAT inhibition by garcinol in causing cell cycle arrest and apoptosis needs investigation. Systematic dissection of these unresolved questions will clarify whether garcinol can serve as a prototype epigenetic cell cycle/apoptosis modulator suitable for clinical development.
The NF-κB signaling pathway is known to be constitutively activated in cancer cells, which is a hallmark of numerous malignancies. Suppression of this pathway represents one of the best-established anti-cancer mechanisms of garcinol. The NF-κB network governs the expression of diverse gene families, including pro-inflammatory cytokines (TNF-α, IL-6, IL-1β), anti-apoptotic factors (Bcl-2, Bcl-xL, survivin), and cell cycle regulators such as cyclin D1. Garcinol interferes with upstream events that trigger NF-κB activation, leading to reduced nuclear translocation of p65 and consequently a diminished transcription of pro-inflammatory and survival-related genes.
In breast cancer models, garcinol counteracts estrogen-driven proliferation by lowering acetyl-p65 levels and suppressing cyclin D1, Bcl-2, and Bcl-xL expression, while simultaneously activating caspases and other pro-apoptotic mediators—effects largely attributed to the inhibition of NF-κB signaling [38,39,40,41,42]. Comparable outcomes have been reported in hepatocellular carcinoma cells lacking functional p53, where garcinol initiated death-receptor-mediated apoptosis, elevated the Bax/Bcl-2 ratio, and enhanced caspase activity [43]. In glioblastoma (C6) cells, garcinol treatment reduced NF-κB expression and downstream gene products associated with cell survival and proliferation, including Bcl-2, Bcl-xL, survivin, and cyclin D1 [44] and PDGFR [45].
While garcinol has been shown to block IκBα phosphorylation and p65 nuclear translocation, the precise upstream target(s) responsible for this inhibition remains ambiguous. It remains unclear whether NF-κB inhibition is a downstream result of reduced global histone acetylation or a consequence of the specific disruption of p65 acetylation at Lys310. Since NF-kB also plays a role in immunological activation, a complete blockade may be undesirable. Collectively, the emerging hypothesis is that garcinol does not simply inhibit NF-κB signaling, but rather epigenetically rewires the NF-κB transcriptional network through HAT inhibition, redox modulation, and cross-pathway interference. Elucidating these multi-level mechanisms through integrated systems and translational research will be critical for advancing garcinol, its analogs, or combination drugs toward epigenetic-NF-κB-targeted cancer therapeutics.
The microRNA (miRNA) expression is regulated by garcinol through its dual regulatory role, both as an up regulator of tumor-suppressive miRNAs and as a down regulator of oncogenic miRNAs [Figure 5], depending on the targeted molecular pathways in different types of cancers. It shows this dual role both as an epigenetic and transcriptional modulator. Garcinol causes upregulation of the miR-200 family (miR-200b, miR-200c) in breast cancer cells [13] and in drug-resistant non-small cell lung cancer (NSCLC) cells [46] mainly by reversing the epithelial-to-mesenchymal transition (EMT). Also, garcinol upregulates let-c family miRNAs and miR-218 in breast cancer and NSCLC cells by the suppression of EMT and stemness [46]. miR-200c was upregulated in pancreatic cancer stem-like cells by garcinol targeting Notch1/Oct4 resulting in a loss of stemness [14] and miR-181 was upregulated in glioblastoma cells by inhibiting STAT3 resulting in reduced migration/invasion [15].
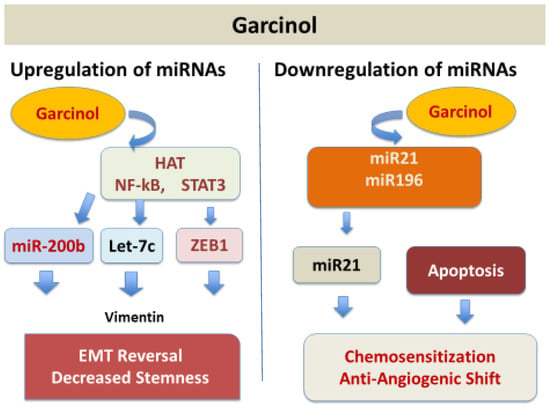
Figure 5.
Garcinol upregulates tumor suppressor miRNAs and downregulates oncogenic miRNAs.
Oncogenic miRNAs including miR-196a, miR-495, miR-605, miR-638, miR-453, and miR-21 were downregulated in pancreatic adenocarcinoma (PANC-1) cells by garcinol reducing anti-apoptotic signaling (PTEN upregulation and Bcl-2 inhibition) and suppressing proliferation and resistance markers with resulting sensitization to chemotherapeutic agents [47].
Results of studies in medulloblastoma and glioblastoma models demonstrated the suppression of platelet-derived growth factor receptor (PDGFR) and NF-κB pathways, respectively, further supporting garcinol as a pluripotent anti-cancer agent [13,45,46,47]. Overall effects on miRNA networks by garcinol could be summarized as reprograming of miRNA networks toward an anti-cancer phenotype, bridging epigenetic modulation with signaling inhibition and reversal of EMT, loss of stemness, and sensitization to chemotherapeutic agents. Beyond apoptosis, garcinol suppressed metastasis by inhibiting the expression of metalloproteinases (MMPs), enzymes that are involved in tumor resistance, by downregulating miRNAs and attenuating STAT3 and AKT phosphorylation [13,14,25,48,49,50].
The molecular basis for the miRNAs’ regulation by garcinol remains poorly defined. It is unclear whether garcinol directly alters miRNA promoter acetylation through HAT inhibition, influences processing by Dicer/Drosha, key enzymes in the miRNA biogenesis pathway, or affects miRNA stability and export. Integrated analyses linking miRNA shifts to downstream signaling and phenotypic outcomes such as EMT reversal and cancer stem cell (CSC) depletion are absent. A mechanistic understanding of how garcinol reprograms miRNA networks is essential for translating these findings into predictive biomarkers and therapeutic strategies.
Vascular endothelial growth factor (VEGF) is a potent signaling protein that stimulates angiogenesis, such as in cancer, by promoting endothelial cell proliferation, migration, and tube formation. It was shown to support cancer progression by enhancing the supply of oxygen and nutrients to the tumor cells. VEGF expression, proliferation, and colony formation were inhibited in oral squamous carcinoma cells by garcinol without harming normal cells [13,47]. Garcinol has been shown to downregulate VEGF by suppressing NF-kB activity [13], STAT3 [51], and by HAT inhibition. As a p300/PCAF inhibitor, garcinol reduces transcriptional programs that favor angiogenic gene expression. p300 is a HIF-1α co-activator at the VEGF promoter [7]. Garcinol’s anti-angiogenesis effect in cancer by inhibiting VFGF has been demonstrated in vitro [13,19,47,48]. The exact regulatory mechanism for suppression of VEGF and angiogenesis by garcinol remains unclear.
Although garcinol has been reported to downregulate VEGF expression through inhibition of NF-κB, STAT3, and HIF-1α pathways, the precise molecular hierarchy and tissue-specific effects remain inadequately characterized. The temporal kinetics of VEGF suppression, its reversibility, and crosstalk with angiogenic regulators such as VEGFR2, PDGF, and FGF signaling are poorly understood. Moreover, no studies have systematically assessed how garcinol’s epigenetic modulation (e.g., HAT inhibition or miRNA restoration) converges on VEGF gene regulation at chromatin or promoter levels. In vivo data are sparse, and dose-dependent anti-angiogenic effects, pharmacokinetics, and tumor vascular normalization outcomes remain to be clarified. Clinical and therapeutic relevance for a combination of garcinol with other anti-VEGF agents such as bevacizumab, sunitinib, and sorafenib in cancer are yet to be studied for their potential to inhibit VEGF.
Epithelial-to-mesenchymal transition (EMT) is a process in which epithelial cells acquire mesenchymal features such as enhanced motility, invasiveness, and apoptosis resistance and its regulation has been shown as an anti-cancer activity of garcinol. As an epigenetic modulator (p300/CBP/PCAF HAT inhibitor), garcinol targets chromatin and reduces transcription of EMT drivers (e.g., Snail, ZEB, Twist) via reduced acetylation of histones and non-histone factors (NF-κB, STAT3). This epigenetic–EMT link of garcinol-mediated miRNA reprogramming that reverts to MET was summarized in a recent review [14]. In both in vitro and in vivo xenograft models for aggressive breast cancer, garcinol reversed EMT to mesenchymal-to-epithelial transition (MET) with increased E-cadherin and loss of vimentin/ZEB1/2, alongside suppression of Wnt/β-catenin signaling [52]. EMT suppression by garcinol has been shown in esophageal, gastric, colorectal, pancreatic, and liver cancers with anti-metastatic phenotypes and apoptosis, positioning EMT control as a cross-tumor mechanism [19]. The mechanistic pathway of garcinol as an epigenetic EMT-modulator offers translational research avenues using garcinol alone or in combination with other drugs that target EMT while tracking the biomarkers.
The anti-cancer effects of garcinol in melanoma correlated with expression of the adhesion molecule T-cadherin, suggesting that biomarker-driven stratification could identify patients more likely to benefit [53,54]. In breast and osteosarcoma cells, garcinol altered histone acetylation patterns, reducing H3K18 acetylation while increasing H4K16 acetylation and activating DNA damage markers such as γH2A.X [55]. Pancreatic cancer research further suggested that garcinol and its analogs can trigger both apoptosis and pyroptosis, mediated through STAT3 inhibition [56].
Pro-inflammatory cytokine production, such as that of TNF-α, IL-1β, IL-6, and IL-8, was inhibited by garcinol. Mechanistically, this effect is primarily mediated through the inhibition of NF-κB and STAT3 signaling pathways, leading to reduced transcription of cytokine genes. In LPS-stimulated macrophages and cancer cell models, garcinol inhibited IκBα phosphorylation and p65 nuclear translocation, thereby attenuating the NF-κB-dependent expression of TNF-α and IL-6 [8,57]. It also downregulated COX-2 and iNOS, further dampening inflammatory cascades. In addition, HAT inhibition by garcinol contributes to the transcriptional repression of cytokine promoters [7]. In animal models of inflammation and carcinogenesis, treatment with garcinol resulted in decreased systemic levels of IL-6 and TNF-α [58], supporting its role as an epigenetically active anti-inflammatory compound with therapeutic potential. By reducing oxidative stress and inflammatory mediator expression including pro-inflammatory cytokines, garcinol may indirectly modulate immune responses which need to be investigated in in vivo animal models.
Synergistic interactions of garcinol with established chemotherapy drugs have been documented in a few in vitro and in vivo studies. Beneficial effects were observed when combined with conventional chemotherapeutics such as paclitaxel, gemcitabine, and cisplatin by enhancing apoptosis, suppressing NF-κB/STAT3 signaling, and overcoming drug resistance. Garcinol’s combination therapy potential lies in augmenting antitumor efficacy, reducing required drug doses, and minimizing toxicity through complementary targeting of epigenetic and signaling pathways. Garcinol enhanced curcumin-induced apoptosis in pancreatic cancer cells [59], also enhanced TRAIL-mediated apoptosis in renal, lung, and liver cancers [60], and potentiated cisplatin efficacy in ovarian cancer [61]. In pancreatic cancer models, garcinol sensitized cells to gemcitabine through microRNA-dependent mechanisms [46,48,50]. Combined with low-dose Taxol, garcinol significantly enhanced apoptosis and suppressed metastasis in 4T1 breast cancer models [62].
Co-administration of garcinol with synergistic compounds inhibits tumor growth more effectively than monotherapy. When combined with HDAC inhibitors such as SAHA (Suberoylanilide Hydroxamic Acid), also known by its clinical name Vorinostat, garcinol provides complementary effects on maintaining histone acetylation balance, leading to a more effective regulation of gene expression [7,63]. In combination with DNA methyltransferase (DNMT) inhibitors like decitabine, it enables dual targeting of both DNA and histone epigenetic marks, thereby promoting the reactivation of silenced tumor suppressor genes [64,65]. Additionally, when used alongside chemotherapeutic agents such as doxorubicin or cisplatin, garcinol sensitizes resistant tumor cells by restoring apoptotic gene expression and overcoming drug resistance mechanisms [52,66,67].
Combinations have yielded encouraging results showing reduced tumor burden and improved drug sensitivity, suggesting a role for garcinol in reducing chemotherapy dosage and associated side effects. Further research is needed to optimize dosing, understand molecular synergy, assess toxicity profiles, and validate the enhanced therapeutic efficacy in clinical settings.
Cancer stem cells (CSCs) have been described as a small subpopulation of cells within the tumor with self-renewal and pluripotency (via Oct4, Nanog, Sox2), resistance to radiation and chemotherapy, EMT profile, and high possibility for cancer potential. Garcinol has been shown to target CSCs by epigenetic, transcriptional, and signaling modulation, leading to loss of stemness, decreased sphere formation, and restored sensitivity to chemotherapeutic agents. However, inhibition of STAT3 signaling appears to be the key molecular pathway by which garcinol targets CSCs. Garcinol inhibited tumor sphere formation, and reduced NOTCH1 and Wnt/β-catenin signaling, resulting in MET in aggressive breast cancer models [52,68,69,70]. In an in vivo experiment, garcinol suppressed tumor growth in STAT3-activated xenografts, reducing CSC markers [69]. Garcinol inhibits pancreatic cancer stem cell characteristics by modulating microRNA expression and the epithelial–mesenchymal transition [71]. These results suggest that garcinol may not only inhibit proliferating tumor cells but also the self-renewing cells that cause relapse and resistance to chemotherapeutic drugs. In summary, the mechanistic link appears to be that STAT3 normally maintains CSC traits by activating stemness genes and garcinol blocks this transcriptional program through both direct inhibition and reduced acetylation by blocking HAT. Despite promising in vitro and xenograft evidence, critical gaps remain. The pharmacokinetic profile of garcinol, its bioavailability in CSC niches, and the potential for synergistic combinations with chemotherapy or immunotherapy remain poorly defined. Future research should focus on in vivo CSC tracing models, patient-derived organoids, and nanoparticle formulations to improve delivery and target engagement. Integration of multi-omics and single-cell sequencing approaches could further clarify the transcriptional and epigenetic networks through which garcinol eradicates CSCs.
Nanoparticle-based research with garcinol-loaded nanoparticles is showing some promise in cancer therapeutic development. Native garcinol suffers from poor aqueous solubility, rapid metabolism, and limited bioavailability, which restrict its clinical translation despite strong in vitro efficacy. To overcome these limitations, researchers have developed nanoparticle-based formulations—including liposomes, polymeric nanoparticles, solid lipid nanoparticles, and PLGA- or chitosan-based nano-carriers—that encapsulate garcinol for controlled release and tumor-targeted delivery. These nanoformulations have demonstrated enhanced cellular uptake, prolonged plasma half-life, and superior cytotoxicity in breast, colon, and lung cancer models compared with free garcinol. Some systems also enable co-delivery with chemotherapeutics or siRNAs, achieving synergistic inhibition of NF-κB, STAT3, and EMT pathways while minimizing systemic toxicity. Vitamin E TPGS emulsified PLGA nanoparticles loaded with garcinol administered to B16F10 melanoma tumor-bearing mice showed in vivo deposition at the tumor site, confirming the efficacy of the formulation [72]. Garcinol-loaded PLGA nanoparticles were found to improve bioavailability and targeted delivery to the tumor site, addressing challenges related to garcinol’s poor solubility and hydrophobicity [73]. Garcinol (GAR)-loaded cationic nanoliposomes showed antitumor efficacy on B16F10 melanoma cells in vitro and in vivo using a B16F10 tumor xenograft mouse model [41]. A recent study with bovine serum albumin-based nanoparticles encapsulating garcinol showed improved solubility and enhanced cellular uptake, demonstrating better delivery to the cancer cells [74]. Another study with pH-sensitive garcinol-loaded PLGA nanoparticles coated with Eudragit S100 (GAR-PLGA-ES100 NPs) targeted for colonic delivery released it at the higher pH of the colon (pH 7.4), demonstrating controlled release and localization potential [75]. However, more such studies are needed to assess the long-term efficacy, pharmacokinetics, and safety of the garcinol-loaded nanoparticle-based agents.
In silico (computational) studies have been reported on the anti-cancer properties of garcinol. In the in silico studies, ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties are predicted computationally (using tools like SwissADME, pkCSM, or ADMETlab) to screen natural compounds. For garcinol, in silico ADMET analysis helps estimate whether it is drug-like, orally bioavailable, non-toxic, and whether it can cross the blood–brain barrier—crucial for evaluating its potential as a therapeutic agent. A recent review summarizes mechanisms of garcinol in gastrointestinal cancer that could help map computational targets and wet-lab endpoints. This and other published work related to in silico research is summarized with references in Section 4.
3. Anti-Cancer Studies in Animal Models
Despite the abundance of in vitro data, relatively few in vivo investigations have been performed. In vivo models remain indispensable for evaluating the therapeutic efficacy, pharmacokinetics, and safety of garcinol. Experiments using different types of laboratory animals, including normal mice and rats, and xenograft or orthotopic tumor models for therapeutic efficacy, provide complementary insights [31,52,59,72,75,76,77,78,79,80,81,82]. In normal rodent models (e.g., AOM/DSS-induced colon carcinogenesis or TPA-induced skin tumorigenesis), garcinol administration has demonstrated suppression of inflammatory mediators (iNOS, COX-2), restoration of antioxidant enzymes (GST, QR, SOD), and reversal of epithelial dysplasia. In xenograft models, such as B16F10 melanoma, MDA-MB-231 breast cancer, or PC-3 prostate carcinoma, garcinol inhibits tumor growth by downregulating NF-κB, STAT3, and PI3K/AKT signaling while inducing apoptosis and reducing the cancer stem cell phenotype.
A key advantage of animal studies is the ability to test different formulations and administration routes—including oral, subcutaneous, intraperitoneal, and nanoparticle-based delivery—to simulate realistic pharmacological exposure. For instance, liposomal or iRGD-targeted garcinol nanoparticles significantly improved tumor accumulation and bioavailability compared with free compounds. Dose ranges between 2.5 and 100 mg/kg body weight have been shown to be effective in reducing tumor burden without systemic toxicity. Such findings validate the pharmacodynamic activity of garcinol and its analogs in a physiological microenvironment, offering critical translational data for clinical development.
However, limitations persist. Garcinol’s poor solubility, rapid metabolism, and variable oral absorption complicate reproducibility across studies. Inter-species differences in metabolism and immune response may also limit direct extrapolation to humans. Moreover, xenograft models often lack an intact immune system, potentially underestimating the compound’s immunomodulatory benefits. Despite these caveats, the convergence of results from multiple in vivo models underscores garcinol’s translational promise as a multi-targeted anti-cancer and chemopreventive agent, warranting further development of optimized analogs and formulations for preclinical and clinical evaluation.
3.1. Lymphoma Models
In a primary effusion lymphoma (PEL) model, NOD/SCID mice engrafted with BCBL1-Luc cells received intraperitoneal administration of garcinol at 2.5 and 25 mg/kg every other day for three weeks. Garcinol suppressed tumor growth in a dose-dependent manner [81]. Weaker tumor signal was observed at week 4 post-treatment. At week 8, all garcinol-treated mice showed significantly reduced tumor signals. The specific mechanism involved was not investigated.
In Dalton’s lymphoma, a T-cell lymphoma of spontaneous origin model, Swiss albino mice were injected with Dalton’s ascites lymphoma (DLA) cells. They were orally administered methanol extracts of garcinol from the fruit (GF), bark (GB), and leaves (GL) of Garcinia morella at dosages of 100–200 mg/kg body weight for 10 days. Significant increase in median survival time (MST), decreased tumor volume, and restoration of hematological and biochemical parameters were reported [76]. No mechanistic pathways have been reported in these studies.
3.2. Liver Cancer—Hepatocellular Carcinoma (HCC)
In xenograft models of liver cancer, athymic nude mice bearing subcutaneous HCC tumors were administered with garcinol at 1–2 mg/kg over four weeks. Tumor volumes were reduced in mice treated with garcinol. Mechanistically, this correlated with inhibition of STAT3 signaling, increased caspase-3 activity, and decreased expression of Bcl-2 [51,82].
3.3. Prostate Cancer
Nude mice injected with prostate cancer PC-3 cells (3 × 106) in 200 μL of a PBS solution were used in the study. Mice received an intraperitoneal injection of garcinol (50 mg/kg/d) in 200 μL of corn oil, or orally either 200 μL of corn oil or garcinol in corn oil (50 mg/kg/d) for 5 days per week. Garcinol reduced tumor mass by >80%, with decreased PanIN3 lesions, increased NK cell activity, and increased Caspase-3 and -9, indicating enhanced apoptosis. Cleavage of PARP decreased anti-apoptotic proteins Bcl-2 and Bcl-xL, and increased pro-apoptotic proteins Bax and Bad [79].
3.4. Pancreatic Cancer
The KPC transgenic pancreatic cancer mouse model (KPC; K-ras and p53 conditional mutant) was used. Mice were divided into the following groups: KC—Control diet; KGr—0.05% Garcinol diet; KGm—Gemcitabine injected; KGG—Garcinol diet + Gemcitabine injected groups. Dietary garcinol delayed the progression of pancreatic intraepithelial neoplasia and enhanced natural killer cell activity. Combination therapy with gemcitabine further improved outcomes, indicating both direct antitumor and immunomodulatory roles [50]. Results showed improved survival, reduced papilloma formation in the fore-stomach, and increased ratios of NK and NKT cells compared to Non-NK lymphocytes in the KGr group. Tumor sizes were decreased; increases in advanced PanIN3 (high-grade pancreatic intraepithelial neoplasia which is a precursor to invasive ductal carcinoma, IDC) were observed in KGr, KGm, and KGG groups. Garcinol with gemcitabine resulted in decreased tumor volume, increased NK cell activity, and increased survival.
3.5. Breast Cancer
Female homozygous ICR SCID mice injected s/c with 5 × 106 MDA-MB-231 cells and used when palpable tumors were observed. Control mice received sesame seed oil only while experimental mice received garcinol by oral gavage (5 mg/d/animal by oral gavage), 6 days per week for 4 weeks. Mice were then sacrificed, and tumors were harvested. Garcinol inhibited NF-κB, vimentin, nuclear β-catenin, and EMT markers, while upregulating miR-200 and let-7 families, reducing tumor vascularization and proliferation [52].
Combined with low-dose Taxol (5 mg/kg, i.p.), garcinol (1 mg/kg, i.g.) was investigated in 4T1 breast cancer models using Balb/c mice injected with metastasis-specific mouse mammary carcinoma 4T1 cells [62]. Garcinol + Taxol enhanced therapeutic efficacy more than garcinol alone. This may be through the induction of Taxol-stimulated G2/M phase arrest and also due to decreased caspase-3/cytosolic Ca2+-independent phospholipase A2 (iPLA2) and nuclear factor-kB (NF- B)/Twist-related protein 1 (Twist1) driven downstream events including EMT.
In another study, Balb/c mice nude mice treated with 17β-estradiol and injected with MCF-7 cells (3 × 106 cells per mouse) at mammary sites were used [32]. Following tumor development, the control group received solvent (0.5% DMSO and 0.5% Tween-80 in normal saline) and the treated mice group received cambogin (10 mg/kg in solvent) via i/p injections every other day. Garcinol significantly enhanced apoptosis and suppressed metastasis. At 35 days post-treatment, tumor weight reduced by 72.0% compared with the control group coinciding with increased TUNEL-positive cells, decreased Bcl-2 protein expression, increased Bax protein expression, induction of JNK/SAPK phosphorylation, and AIF nuclear translocation in the tumors of the cambogin-treated mice. Results suggested that cancer development may be, at least in part, through decreased Bcl-2 and activation of JNK/SAPK, both in an AIF-dependent manner.
Garcinol acts as a degradation device to reduce the suppressive activity of regulatory T cells (44) and to enhance the in vivo antitumor activity of a targeted therapeutic anti-p185(her2/neu) (ERBB2) antibody in MMTV-neu transgenics implanted with neu transformed breast tumor cells [83].
3.6. Colon Cancer
Five-week-old male Albino ICR mice were used in dextran sodium sulfate (DSS)-induced inflammation and colon carcinogenesis models [84]. Controls were fed a regular diet and the treatment group received a garcinol mixed diet (250 or 500 ppm) for 1 week, followed by 2%, w/v DSS in drinking water for 14 days. Garcinol prevented DSS-stimulated shortening of the colon length, formation of aberrant crypt foci, reduced inflammation, nitric oxide synthase, COX-2, and proliferating cell nuclear antigen protein expression.
In another colon carcinogenesis model, the groups were as follows: Group 1—control; Groups 2 through 4 received single i/p injection with Azoxymethane (AOM) (10 mg/kg body weight). One week later, Groups 2 through 4 received 2% DSS in drinking water for 7 days. Groups 3 and 4 were fed garcinol (250 or 500 ppm) for 24 weeks. In this model, garcinol decreased tumor size and tumor incidence in the colon, and decreased COX-2, cyclin D1, and VEGF via inhibition of the extracellular signal-regulated protein kinase 1/2, phosphatidylinositol 3 kinase/Akt/p70 ribosomal S6 kinase, and Wnt/β-catenin signaling pathways.
AOM-induced crypt foci were also suppressed in rats fed garcinol diets [85]. Garcinol reduced the proliferating cell nuclear antigen (PCNA) index in ACF, increased liver Glutathione S-Transferases (GSTs-family of phase II detoxification enzymes) and Quinone Reductase (QR) activities, and reduced O2− and NO generation and the expression of iNOS and COX-2 proteins.
In another experiment [86], ten 5-week-old male C57BL/6J mice in each group of six groups were used. High fat diet (HFD) promoted colitis-associated colon cancer as compared to an AOM/DSS group without the intervention of obesity. Garcinol, 0.05% in diet, reduced obesity-promoted colon carcinogenesis. Microbiota of each group was different and clustered.
More recent nanotechnology approaches encapsulating garcinol into PLGA nanoparticles conjugated with targeting peptides improved colon accumulation and survival in colorectal cancer (CRC)-bearing animals. Sprague-Dawley (SD) rats with dimethyl hydrazine (DMH)-induced CRC were fed with garcinol or encapsulated garcinol in biodegradable polymeric nanoparticle (PLGA)-conjugated with iRGD peptide on the particles’ surface (iRGD-GAR-NP’s) [73]. Biodistribution demonstrated the ability of GAR-NP and iRGD-GAR-NP to accumulate in the colons and the iRGD-GAR-NP reduced CRC tumor progression. Survival increased 166% with iRGD-GAR-NP compared to CRC-bearing animals without treatment.
3.7. Head and Neck Cancers
Five-week-old male athymic nu/nu mice implanted s/c with human head and neck squamous cell carcinoma (HNSCC) (CAL27) cells (2 × 106 cells/100 μL of saline) were used in the study when tumors reached 0.25 cm in diameter. Garcinol decreased growth and survival of HNSCC by modulating various pro-inflammatory signaling pathways with decreased NF-κB, STAT3, MAPKs, p65, Ki-67, and CD31, and apoptosis induction without showing any toxicity [87].
In another similar HNSCC model study, mice were co-administered garcinol with cisplatin [80]. Tumor growth was decreased with garcinol alone and in combination with cisplatin by downregulating some inflammatory biomarkers. The combination was effective in downregulating the expression of various oncogenic gene products involved in HNSCC growth, survival, invasion, and metastasis along with reduced markers of the proliferation index (Ki-67) and microvessel density (CD31). The combination effectively downregulated oncogenic molecules involved in the proliferation (cyclin D1, COX-2), survival (Bcl-xL, survivin), angiogenesis (VEGF), and invasion (MMP-9, ICAM-1) than with either garcinol or cisplatin and further amplified therapeutic efficacy.
3.8. Oral Cancer
Male F344 rats at 7 weeks of age were given 4-Nitroquinoline 1-oxide (4-NQO) at 20 ppm in the drinking water for 8 weeks to induce tongue neoplasms. Dietary garcinol reduced the incidence and multiplicity of 4-NQO-induced tongue neoplasms and/or pre-neoplasms as compared to the control diet, coinciding with reduced BrdU-labeling index, decreased cyclin D1-positive cell ratio, and decreased COX-2 expression, suggesting reduction in cell proliferation activity [88].
Hamster cheek pouch studies similarly showed topical garcinol to reduce DMBA-induced tumors by blocking LTB4 biosynthesis and proliferation [89]. Six to eight-week-old male Syrian golden hamsters were treated with 0.5% 7,12-dimethylbenz[a]anthracene (DMBA) (0.1 mL in mineral oil) topically on the left cheek pouch three times per week for three weeks. Theoretical activity index (Jmax/IC50) was highest with garcinol as compared to other compounds used in the study (Zileuton, ABT-761, Licofelone, Curcumin). Therefore, garcinol was predicted as a potent 5-lipoxygenase (5-Lox) pathway inhibitor, which may potentially be used for oral cancer chemoprevention through topical application. Short-term study showed infiltration of inflammatory cells in the submucosa and reduced leukotriene B4 (LTB4) while the long-term study showed decreased tumor volume coinciding with reduced cancer lesions, cell proliferation, and LTB4 and prostaglandin E2 (PGE2) biosynthesis.
3.9. Glioblastoma (GBM) and Brain Tumors
NOD/SCID mice inoculated with 1 × 106 U87MG cells (human U87MG glioblastoma cells) in the flank subcutaneously were used in this study [90]. The garcinol-treated group (1 mg/kg body weight, suspended in 0.1% DMSO, i/p) showed reduced size of tumors compared to the control group with no effect on body weights. Garcinol resulted in 100% survival compared to 60% in the control group. Garcinol reduced STAT3, pSTAT3, STAT5A, p-STAT5A, Ki-67, and Bcl-xL expression, while Bax was enhanced and miR-181d expression was enhanced 3.52-fold in the U87MG mice treated with garcinol.
GBM tumor development and growth reduction was suggested due to garcinol abrogating STAT3/5A signaling and upregulating hsa-miR-181d, with concomitant suppression of the Ki-67 proliferation index and enhancement of Bax/Bcl-xL apoptotic ratio [90].
3.10. Skin Cancer
CD-1 mice with TPA (12-O-tetradecanoylphorbol-13-acetate)-induced ear inflammation and skin tumors were used [78]. Garcinol was applied topically on the inflamed ear or administered orally. TPA caused the upregulation of pro-inflammatory cytokine IL-6 in the ear skin in a dose-dependent fashion. Oral garcinol reduced UVB-induced ear inflammation and the upregulation of pro-inflammatory cytokine IL-1 beta and IL-6 levels in ears. Garcinol applied to the backs of mice strongly inhibited TPA-induced skin tumor development in mice previously initiated with 7,12-dimethylbenz[a]anthracene (DMBA) with reduced skin tumors/mouse and percent of mice bearing skin tumors.
In a similar study [91], garcinol reduced the TPA-induced expression of inducible nitric oxide synthase (iNOS) and COX-2 and reduced the nuclear translocation of NF-κB and its subsequent DNA binding. This was attributed to blocking the phosphorylation of inhibitor κB α (IκBα) and the p65 subunit. Garcinol also reduced the TPA-induced activation of extracellular signal-regulated kinases (ERK), c-Jun-N-terminal kinases (JNK), p38 mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase (PI3K)/Akt, which are considered to be molecules upstream of NF-κB [91].
3.11. Lung Cancer
Five-week-old NMRI (nu/nu) female mice were injected s/c with A549 cells (2 × 107 in 200 uL of PBS) into the right flank to induce non-small cell lung cancer (NSCLC) [68]. Mice with developed tumors were then injected i/p., with either corn oil (control) or garcinol (15 mg/kg) for 40 days. Tumors in the control group were twice the size of those in the garcinol-treated group.
Garcinol downregulated aldehyde Dehydrogenase 1 Family Member A1 (ALDH1A1) through alterations in the interaction between DNA damage-inducible transcript 3 (DDIT3) and DDIT3-CCAAT-enhancer-binding proteins beta (C/EBPβ), resulting in a decreased binding of C/EBPβ to the endogenous ALDH1A1 promoter. Results suggest that for human patients with NSCLCs showing high ALDH1A1 expression, garcinol could possibly help in reducing this cancer burden [68].
3.12. Nanoparticle Delivery Systems
To overcome solubility issues, garcinol has been encapsulated into biodegradable nanoparticles. These formulations demonstrated improved biodistribution, sustained release, and enhanced tumor targeting in vivo, though large-scale efficacy data remain pending [51,72,75].
B16F10 melanoma tumor-bearing mice were injected via tail vein 99mTc-labeled GAR-NPs (0.03 mL, 8–12 MBq/kg). At 2, 4, 8, and 24 h post-injection, blood samples were collected by cardiac puncture of the sacrificed mice. In another study, B16F10 tumor-bearing mice were injected with FITC-labeled NPs, 40 mg/kg body wt. Tumors were collected from mice sacrificed at 2, 4, and 8 h post-injection to study the intra-tumoral distribution of FITC-labeled nanoformulations. Moderate accumulation of 99mTc-labeled GAR-NPs was detected in the tumor region. GAR-NPs also exhibited substantially high cell binding (B16F10 melanoma cells) and internalization indicating improved bioavailability of GAR as a nanoparticulate suspension.
These results combined with oral administration studies [72] focused on the potential antitumor effect of garcinol nanoparticles and support the use of the nanosystem for an effective delivery and anti-cancer efficacy of garcinol.
4. Summary and Conclusions
Recent advances have strengthened the view of garcinol as a multifunctional epigenetic modulator with direct and indirect effects on tumor progression. Its capacity to inhibit key histone acetyltransferases such as p300/CBP and PCAF positions it among a new class of natural compounds capable of altering chromatin architecture and transcriptional activity. Beyond histone regulation, garcinol also interferes with the acetylation of non-histone proteins, including transcription factors like STAT3, thereby linking its epigenetic effects to signaling pathways that control cell survival, proliferation, and inflammatory responses. Emerging studies further suggest that garcinol influences microRNA and long noncoding RNA expression, integrating chromatin-based and RNA-mediated control of oncogenic networks. Advances in formulation technologies, including nanoparticle-based delivery, are beginning to address its bioavailability challenges, broadening the scope for preclinical and translational exploration.
However, several critical gaps remain. The selectivity of garcinol for individual HAT isoforms and its precise molecular targets in vivo are not yet fully characterized. Quantitative data on pharmacokinetics, pharmacodynamics, and tissue-specific distribution are limited, hindering a clear understanding of dose–response relationships. Mechanistic connections between garcinol-induced epigenetic changes and downstream modulation of noncoding RNAs still need experimental validation. Comparative studies with other established epigenetic drugs and exploration of potential synergies with immunotherapies or chemotherapeutic agents are also lacking. Finally, while cell-based and animal models provide compelling evidence of efficacy, there is an absence of human data confirming target engagement, safety profiles, and biomarker responses. Addressing these gaps will be essential to translating garcinol’s promising epigenetic activity into clinically meaningful outcomes. Beyond its direct cytotoxic properties, emerging evidence suggests that garcinol may influence the tumor microenvironment through the suppression of angiogenic and inflammatory mediators. Several reports describe decreased levels of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β following inhibition of NF-κB signaling, decreased activity of T(reg) cells, and increased NK cell activities. While these findings imply immunomodulatory potential, this area of research is still at an early stage.
Kopytko et al. [14] suggested that histone acetyltransferases (HATs) like p300/CBP support immune evasion by promoting the expression of ligands that dampen antitumor immunity. Since garcinol acts as a natural HAT inhibitor, it may counter this mechanism by enhancing immune recognition. Furthermore, garcinol’s ability to interfere with FOXP3 acetylation could diminish the regulatory T-cell (Treg) suppressive capacity, thereby alleviating immunosuppression within the tumor microenvironment [83]. By downregulating HIF-1α, COX-2, and mPGES-1, key mediators of angiogenesis and immune cell recruitment, garcinol might further shift the tumor microenvironment toward an immune-active state. These actions suggest possible synergy with immune checkpoint blockades, such as anti-PD-1/PD-L1 therapies, which remains an exciting but unexplored avenue. Immune checkpoint proteins, PD-1 (Programmed Cell Death Protein-1), and PD-L1 (Programmed Death Ligand-1), are regulatory molecules that maintain immune homeostasis by either activating or inhibiting immune responses. Tumor cells often exploit these inhibitory checkpoints to suppress antitumor immunity and thus evade immune destruction.
Collectively, the available evidence positions garcinol as a promising candidate for development both as a single-agent therapy and as an adjuvant or sensitizer to existing anti-cancer drugs. Its capacity to influence multiple oncogenic pathways, augment chemotherapeutic responses, and potentially impact chronic inflammatory diseases underscores its value in modern pharmacology. Realizing this potential will require systematic research into its pharmacokinetics, formulation strategies, and safety in clinically relevant models. With sustained interdisciplinary and translational efforts, garcinol could emerge as an important component of next-generation therapeutic regimens, integrating natural product chemistry with precision medicine.
4.1. Anti-Metastatic and Anti-Stem Cell Activity
Garcinol’s ability to impede metastasis is a key therapeutic feature. Garcinol downregulates matrix metalloproteinases (MMPs, MMP-2 and MMP-9) as their overexpression in tumors facilitates local invasion, metastatic dissemination, and angiogenesis. Thus, the significance of garcinol’s effect on MMPs lies at the intersection of its anti-metastatic, anti-invasive, and anti-angiogenic properties in cancers. MMP-9 also releases ECM-bound growth factors (VEGF, TGF-β), enhancing tumor vascularization and immune evasion. Therefore, MMP inhibition is a critical anti-metastatic strategy. Broader therapeutic significance is in its dual targeting as other synthetic MMP inhibitors have failed clinically due to toxicity. Garcinol’s multi-target profile (NF-κB, STAT3, HAT inhibition) achieves MMP suppression in a non-toxic, safer way. This aspect needs further investigation. Garcinol-induced inhibition of EMT provides therapeutic potential by reducing metastasis, drug resistance, and cancer stem cell formation.
Emerging evidence also supports garcinol’s efficacy in targeting cancer stem cells, which are often resistant to conventional therapies. By modulating pathways such as NOTCH1 and inducing mesenchymal-to-epithelial transition (MET), garcinol curtails the tumorigenic and self-renewing capacity of these cells. This property may be particularly valuable in aggressive and treatment-resistant cancers like triple-negative breast cancer and pancreatic cancer; however, this aspect needs extensive clinical research.
4.2. Insights from In Silico Studies
The very few and recent in silico research publications are providing further molecular insights for garcinol’s anti-cancer activity. In an in silico study, anti-breast cancer potential of Garcinia indica phytocompounds including garcinol was evaluated against nine breast cancer-related protein targets. Molecular docking revealed that garcinol exhibited the strongest binding affinity for BRCA2. ADMET and toxicity analyses confirmed a favorable drug likeness and non-toxic profile suggesting that garcinol may serve as a novel anti-cancer agent [92].
In another study [93], the molecular docking and molecular dynamics of garcinol against the p300/CBP associated factor bromodomain (PCAF Brd) was performed as PCAF is one of the promising target proteins for different types of cancers. This study showed that garcinol forms key interactions and has high binding affinity towards PCAF Brd, suggesting its potential as an anti-cancer therapeutic agent. Further, Rizvi et al. [44] showed through molecular docking analysis that garcinol induces apoptotic cell death by inhibiting NF-kB activity in C6 cells by docking NF-kB through important amino acid residues including Pro275, Trp258, Glu225, and Gly225.
More such studies are needed to further delineate and accelerate mechanistic understanding with reduced cost before moving into more in vivo experiments and clinical studies down the line.
4.3. Insights from In Vivo Studies
Despite promising in vitro findings, robust in vivo validation is essential. Studies in animal models involving various cancers have shown significant tumor reduction, enhanced apoptosis, and immune modulation. Topical formulations have also demonstrated anti-cancer and anti-inflammatory effects in skin cancer models. Future comprehensive in vivo and clinical studies will help translate its promising in vitro anti-cancer and epigenetic effects into clinically viable treatments, defining optimal formulations, combination drugs, effective drug delivery systems, dosages, and toxicity while targeting different cancer types.
4.4. Comparison of Garcinol with Other Known Epigenetic Inhibitors
Garcinol represents a distinctive class of natural epigenetic modulators, functioning primarily as a histone acetyltransferase (HAT) inhibitor that alters chromatin structure and gene transcription. Unlike synthetic HAT inhibitors or well-studied HDAC inhibitors such as vorinostat (SAHA) and trichostatin A, which increase histone acetylation, garcinol exerts the opposite effect, reducing the transcriptional activation of oncogenes. Garcinol complements other natural compounds like curcumin and resveratrol, which exhibit broader epigenetic activity through dual HAT/HDAC modulation. A comparison of garcinol with other known epigenetic modulators is shown in Table 2.

Table 2.
Comparison of epigenetic targets, mechanism of action and cancer relevance of garcinol with other known epigenetic modulators.
4.5. Garcinol Toxicity
Multiple studies in laboratory animals have found garcinol to be well-tolerated with no significant toxic effects including no histological or pathological changes to the liver, kidneys, lungs, heart, or esophagus at the doses used in those studies. No cellular toxicity has been reported in many in vitro studies. In an acute safety study using Wistar rats, 40% Garcinol formulation did not produce any adverse effects at a high single dose of 2000 mg/kg and can therefore be classified as GHS Category 5 (unclassified) according to the Globally Harmonized System (GHS) for chemical classification. Furthermore, no treatment-related changes were observed at the highest tested dose of 100 mg/kg/day in the 28-day and 90-day repeated-dose oral toxicity studies, as well as in the reproductive and developmental toxicity assessments [105]. These findings indicate that 40% Garcinol exhibits a low toxicity profile in rodents and showed no observable adverse effects under the experimental conditions tested. Recently, in a clinical trial with human patients with non-alcoholic steatohepatitis, the clinical efficacy and safety of the garcinol, curcuminoids, and piperine (GCP) combination was investigated. Over a 90-day period, no adverse toxicity effects were observed with no significant changes in hematological and clinical laboratory parameters [106].
While the current evidence provides a favorable indication of garcinol’s safety, definitive conclusions require more rigorous and comprehensive investigations. Future studies should encompass long-term human clinical trials and detailed mechanistic toxicity assessments to elucidate its pharmacokinetics, biodistribution, and potential interactions with co-administered therapeutic agents, particularly chemotherapeutic drugs. It is also imperative to evaluate safety parameters across different age groups, sexes, and physiological conditions, including pregnancy and lactation. Furthermore, systematic assessments of toxicity to different body systems and dose–response relationships should be conducted for various formulations and routes of administration. Such multifaceted studies are essential to establish a robust and clinically relevant safety profile for garcinol under diverse therapeutic contexts.
4.6. Advances in Nanotechnology
Nanoparticle-based garcinol formulations are likely to improve bioavailability and solubility as they are hydrophobic with poor aqueous solubility and low bioavailability. Although nanoformulation approaches have substantially improved the solubility and stability of garcinol, several critical gaps remain before clinical translation can be realized. Most current studies are confined to in vitro characterization, with limited in vivo validation of pharmacokinetics, biodistribution, and therapeutic efficacy. Systematic evaluations of toxicity, long-term safety, and optimal dosing strategies are also lacking.
Future research should prioritize in vivo tumor models to confirm therapeutic benefits and elucidate mechanisms of biodistribution and clearance. The scale-up of nanoparticle synthesis under reproducible and regulatory-compliant conditions will be a challenge that must be addressed for translational feasibility. Functionalization of nano-carriers with targeting ligands or antibodies could enhance tumor or immune cell specificity, while co-delivery systems combining garcinol with chemotherapeutic or immunomodulatory agents may further improve efficacy. Ultimately, well-designed preclinical and early-phase clinical trials are needed to establish the safety, pharmacodynamics, and therapeutic potential of garcinol nanoformulations in human cancer management.
4.7. Analogs of Garcinol
Despite garcinol’s potent pleiotropic anti-cancer activity, its clinical translation remains limited by poor aqueous solubility, metabolic instability, and limited target selectivity. These drawbacks underscore the need to design novel analogs and derivatives that retain the benzophenone backbone while improving pharmacokinetics and pharmacodynamics. Structural modifications, such as altering the prenyl side chains, hydroxyl positions, and phenolic substitutions, can enhance HAT selectivity, bioavailability, and tumor targeting, offering a rational path toward next-generation epigenetic therapeutics. Synthetic chemistry efforts have generated few semi-synthetic and fully synthetic garcinol analogs aimed at optimizing potency and reducing off-target effects. Table 3 shown below compares the structure, targets, advantages, and translational limitations of natural analogs (Isogarcinol, Epigarcinol, and Camboginol) with the semi and fully synthetic analogs.

Table 3.
Comparison of natural and synthetic garcinol analogs.
To accelerate translation, future work with garcinol analogs should aim for the following: pursue structure–activity relationship studies linking specific phenolic substitutions to HAT vs. HDAC inhibition profiles; in silico computational docking to predict binding affinities for p300, CBP, and STAT3, guiding analog selection; develop pharmacokinetically optimized analogs (single or combination) validated through in vivo bioavailability and safety studies; and evaluate biomarkers of efficacy, including histone acetylation levels, miRNA signatures, EMT reversal, and CSC elimination, to enable patient stratification and precision-guided therapy.
4.8. Beyond Oncology: A Broader Therapeutic Horizon
Garcinol’s anti-inflammatory and antioxidant properties extend its potential applications to chronic inflammatory conditions, neurodegenerative diseases, and metabolic disorders such as obesity and diabetes. These prospects align with the rising demand for plant-based therapeutics that are both effective and sustainable.
Furthermore, the exploration of garcinol highlights the broader importance of underutilized medicinal plants in drug discovery and development. As interest in phytochemicals grows, garcinol stands as a compelling candidate for integrative and precision medicine approaches.
4.9. Limitations and Future Directions
Although initial in vivo investigations highlight garcinol’s potential as an anti-cancer agent across several tumor models, the available data are not yet sufficient to define standardized dosing protocols or to clarify its complete mechanistic profile. Despite promising preclinical outcomes, clinical translation remains in its infancy. The current evidence base is dominated by in vitro studies, with only limited animal experiments and an absence of human clinical trials. Differences in extraction techniques, formulation strategies, and dosing parameters further complicate the comparison of study results. Consequently, while the mechanistic rationale for garcinol is strong, its therapeutic application in clinical settings remains largely theoretical. Bridging these knowledge gaps through systematic in vivo and clinical research is essential to move garcinol toward practical medical use.
- Administration Routes and Dosage Optimization
The comparative efficacy and safety of oral versus intravenous delivery require rigorous evaluation to determine the optimal administration routes and therapeutic dosages with minimal toxicity.
- 2.
- Pharmacokinetics and Bioavailability
Systematic studies are needed to characterize absorption, metabolism, and tissue distribution. Nanoparticle formulations and conjugation strategies may help overcome solubility and stability challenges. Effects on immunological parameters need to be expanded to delineate changes in circulatory immune cell subsets, pro- and anti-inflammatory cytokines, and changes in immune checkpoint proteins.
- 3.
- Toxicity and Safety Profiles
Comprehensive preclinical safety assessments across different animal models will be necessary to establish tolerability and identify any potential off-target effects.
- 4.
- Standardization of Extraction and Purification
Variations in source material and preparation methods make reproducibility difficult. Development of standardized protocols will be important for consistency in experimental and clinical settings.
- 5.
- Expanded In Vivo Models
Further exploration in genetically engineered and patient-derived xenograft models would provide more clinically relevant insights into efficacy across different tumor types and stages.
- 6.
- Combination Therapies
Given its synergistic effects with agents such as cisplatin, Taxol, gemcitabine, and TRAIL, systematic evaluation of garcinol as an adjuvant therapy could identify contexts where it enhances existing regimens while reducing required drug dosages.
- 7.
- Clinical Translation
Early-phase clinical trials should be prioritized once safety and pharmacokinetics are established, with an initial focus on cancers for which strong preclinical evidence already exists (e.g., pancreatic, breast, and colon cancers).
- 8.
- Effects on immunological parameters
Experiments devoted to studying garcinol’s immunomodulatory effects are almost nonexistent or very limited. Investigations are needed to delineate changes in circulatory immune cell subsets and their functions, pro- and anti-inflammatory cytokines, and the status of immune checkpoint proteins, PD-1 and PD-L1.
Author Contributions
G.P.: Literature search and collection of references, writing of major part of original draft, and final editing. T.S.K.: Literature search, writing—review and editing. S.M.: writing—review and editing. B.K.: Conceptualization, resources, supervision, funding acquisition, project administration, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The work in the Karanam’s laboratory at Tuskegee University was supported by 5U54MD007585-33 (National Institute on Minority Health and Health Disparities), 1UG3HG013553-01 (National Human Genome Research Institute).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sahu, A.; Das, B.; Chatterjee, A. Polyisoprenylated benzophenones from Garcinia pedunculata. Phytochemistry 1989, 28, 1233–1235. [Google Scholar] [CrossRef]
- Kaur, R.; Chattopadhyay, S.K.; Tandon, S.; Sharma, S. Large scale extraction of the fruits of Garcinia indica for the isolation of new and known polyisoprenylated benzophenone derivatives. Ind. Crops Prod. 2012, 37, 420–426. [Google Scholar] [CrossRef]
- Nainegali, B.; Iyyaswami, R.; Belur, P. Simultaneous Extraction of four different Bioactive Compounds from Garcinia indica and their enrichment using Aqueous Two-Phase Systems. Food Bioprod. Process. 2019, 114, 185–195. [Google Scholar] [CrossRef]
- Han, C.M.; Zhou, X.Y.; Cao, J.; Zhang, X.-Y.; Chen, X. 13,14-Dihydroxy groups are critical for the anti-cancer effects of garcinol. Bioorg. Chem. 2015, 60, 123–129. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Cao, J.; Han, C.M.; Li, S.W.; Zhang, C.; Du, Y.D.; Zhou, Q.Q.; Zhang, X.Y.; Chen, X. The C8 side chain is one of the key functional group of Garcinol for its anti-cancer effects. Bioorg. Chem. 2017, 71, 74–80. [Google Scholar] [CrossRef]
- Baky, M.H.; Fahmy, H.; Farag, M.A. Recent Advances in Garcinia cambogia Nutraceuticals in Relation to Its Hydroxy Citric Acid Level. A Comprehensive Review of Its Bioactive Production, Formulation, and Analysis with Future Perspectives. Am. Chem. Soc. Omega 2022, 7, 25948–25957. [Google Scholar] [CrossRef]
- Balasubramanyam, K.; Altaf, M.; Radhika, A.; Swaminathan, V.; Ravindran, A.; Sadhale, P.P.; Kundu, T.K. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J. Biol. Chem. 2004, 279, 33716–33726. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Chang, W.L.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Induction of apoptosis by garcinol and curcumin through cytochrome c release and activation of caspases in human leukemia HL-60 cells. J. Agric. Food Chem. 2001, 49, 1464–1474. [Google Scholar] [CrossRef]
- . Ito, C.; Itoigawa, M.; Miyamoto, Y.; Onoda, S.; Sundar Rao, K.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Furukawa, H. Polyprenylated benzophenones from Garcinia assigu and their potential cancer chemopreventive activities. J. Nat. Prod. 2003, 66, 206–209. [Google Scholar] [CrossRef]
- Matsumoto, K.; Akao, Y.; Kobayashi, E.; Ito, T.; Ohguchi, K.; Tanaka, T.; Iinuma, M. Cytotoxic benzophenone derivatives from Garcinia species display a strong apoptosis-inducing effect against human leukemia cell lines. Biol. Pharm. Bull. 2003, 26, 569–571. [Google Scholar] [CrossRef]
- Saadat, N.; Gupta, S.V. Potential Role of Garcinol as an Anticancer Agent. J. Oncol. 2012, 2012, 647206. [Google Scholar] [CrossRef]
- Liu, C.; Chi-Lui Ho, P.; Wong, F.C.; Sethi, G.; Wang, L.Z.; Goh, B.C. Garcinol: Current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015, 362, 8–14. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Kaur, J.; Aggarwal, D.; Parashar, G.; Chaturvedi Parashar, N.; Kulkarni, S.; Kaur, G.; Sak, K.; Kumar, M.; et al. Garcinol Exhibits Anti-Neoplastic Effects by Targeting Diverse Oncogenic Factors in Tumor Cells. Biomedicines 2020, 8, 103. [Google Scholar] [CrossRef]
- Kopytko, P.; Piotrowska, K.; Janisiak, J.; Tarnowski, M. Garcinol, A Natural Histone Acetyltransferase Inhibitor and New Anti-Cancer Epigenetic Drug. Int. J. Mol. Sci. 2021, 22, 2828. [Google Scholar] [CrossRef] [PubMed]
- Schobert, R.; Biersack, B. Chemical and Biological Aspects of Garcinol and Isogarcinol: Recent Developments. Chem. Biodivers. 2019, 16, e1900366. [Google Scholar] [CrossRef]
- Cao, J.; Han, C.; Zhang, G.; Zhou, X.; Li, S.; Du, Y.; Zhao, S.; Zhang, X.; Chen, X. Synthesis and anticancer activity of 8-allyl garcinol. Chin. J. Org. Chem. 2017, 37, 2086–2093. [Google Scholar] [CrossRef]
- Basha, N.J. Anticancer and anti-Inflammatory Activities of Garcinol and Its Analogs. Polycycl. Aromat. Compd. 2023, 44, 6354–6368. [Google Scholar] [CrossRef]
- Padhye, S.; Ahmad, A.; Oswal, N.; Sarkar, F.H. Emerging role of Garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs. J. Hematol. Oncol. 2009, 2, 38. [Google Scholar] [CrossRef]
- Patwa, N.; Chauhan, R.; Chauhan, A.; Kumar, M.; Ramniwas, S.; Mathkor, D.M.; Saini, A.K.; Tuli, H.S.; Haque, S.; Sláma, P. Garcinol in gastrointestinal cancer prevention: Recent advances and future prospects. J. Cancer Res. Clin. Oncol. 2024, 150, 370. [Google Scholar] [CrossRef]
- Huang, J.; Plass, C.; Gerhauser, C. Cancer chemoprevention by targeting the epigenome. Curr. Drug Targets 2011, 12, 1925–1956. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, D.Q.; Wisnieski, F.; Mota, E.R.D.S.; de Sousa, S.B.M.; da Silva, J.M.C.; Leal, M.F.; Gigek, C.O.; Rasmussen, L.T.; Assumpção, P.P.; Burbano, R.R. Role of histone acetylation in gastric cancer: Implications of dietetic compounds and clinical perspectives. Epigenomics 2019, 11, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Li, Y.; Sarkar, F.H. The bounty of nature for changing the cancer landscape. Mol. Nutr. Food Res. 2016, 60, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tollefsbol, T.O. Impact of Epigenetic Dietary Components on Cancer through Histone Modifications. Curr. Med. Chem. 2015, 22, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Ahmad, A.; Bhat, S.H.; Abuduhier, F.M.; Mustafa, S.K.; Usmani, S. Diet-derived small molecules (nutraceuticals) inhibit cellular proliferation by interfering with key oncogenic pathways: An overview of experimental evidence in cancer chemoprevention. Biol. Futur. 2022, 73, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Guo, C.; Zhang, X.; Wang, X.; Ma, A.H. Garcinol acts as an antineoplastic agent in human gastric cancer by inhibiting the PI3K/AKT signaling pathway. Oncol. Lett. 2020, 20, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Liao, C.H.; Chien, M.H.; Tsai, T.Y.; Lin, J.-K.; Weng, M.S. Induction of p21Waf1/Cip1 by Garcinol via Downregulation of p38-MAPK Signaling in p53-Independent H1299 Lung Cancer. J. Agric. Food Chem. 2014, 62, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Fu, J.; Su, Y.; Zhang, X. Opposite Effects of Garcinol on Tumor Energy Metabolism in Oral Squamous Cell Carcinoma Cells. Nutr. Cancer 2019, 71, 1403–1411. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, Q.; Hou, H.; Gu, J.; Chen, L.; Li, J.; Li, Y.; Wang, T.; Yu, J. Garcinol inhibits the proliferation of endometrial cancer cells by inducing cell cycle arrest. Oncol. Rep. 2020, 45, 630–640. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Sig. Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Shi, Y.; Nikulenkov, F.; Zawacka-Pankau, J.; Li, H.; Gabdoulline, R.; Xu, J.; Eriksson, S.; Hedström, E.; Issaeva, N.; Kel, A.; et al. ROS-dependent activation of JNK converts p53 into an efficient inhibitor of oncogenes leading to robust apoptosis. Cell Death Differ. 2014, 21, 612–623. [Google Scholar] [CrossRef]
- Shen, K.; Xie, J.; Wang, H.; Zhang, H.; Yu, M.; Lu, F.; Zhou, Y.; Chen, Y.; Yang, Y. Cambogin Induces Caspase-Independent Apoptosis through the ROS/JNK Pathway and Epigenetic Regulation in Breast Cancer Cells. Mol. Cancer Ther. 2015, 14, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Lu, F.; Xie, J.; Wu, M.; Cai, B.; Li, Y.; Zhou, Y.; Yang, Y. Cambogin exerts anti-proliferative and pro-apoptotic effects on breast adenocarcinoma through the induction of NADPH oxidase 1 and the alteration of mitochondrial morphology and dynamics. Oncotarget 2016, 7, 50596–50611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pieme, C.A.; Ambassa, P.; Yankep, E.; Biapa, C.P.N.; Penlap, V.N.; Ngogang, J.Y. Epigarcinol and isogarcinol isolated from the root of Garcinia ovalifolia induce apoptosis of human promyelocytic leukemia (HL-60 cells). BMC Res. Notes 2015, 8, 700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Constant Anatole, P.; Guru, S.K.; Sharma, S.C.; Zofou, D.; Tane, P.; Kumar, A.; Bhushan, S. Ethyl acetate fraction of Garcina epunctata induces apoptosis in human promyelocytic cells (HL-60) through the ROS generation and G0/G1 cell cycle arrest: A bioassay-guided approach. Environ. Toxicol. Pharmacol. 2013, 36, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Tomasiak, P.; Janisiak, J.; Rogińska, D.; Stompor-Gorący, M.; Górska-Ponikowska, M.; Knap, N.; Felczykowska, A.; Królicka, A. Garcinol and Anacardic Acid, Natural Inhibitors of Histone Acetyltransferases, Inhibit Rhabdomyosarcoma Growth and Proliferation. Molecules 2023, 28, 5292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.; Zhang, W.; Gao, Y.; Yang, J.; Zhang, Y.; Dong, J.; Zhao, L.; Zhang, J. Senescence-like changes induced by expression of p21Waf1/Cip1 in NIH3T3 cell line. Cell Res. 2002, 12, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Deng, S.; Zhang, M.; Zhang, J.; Peng, J.; Zhang, J.; Nie, Y.; Wu, K.; Shi, Y.; Fan, D. A cyclin-dependent kinase inhibitor (p21WAF1/CIP1) affects thymidine incorporation in human liver cancer cells. Br. J. Cancer 2002, 86, 625–629. [Google Scholar] [CrossRef][Green Version]
- Ye, X.; Yuan, L.; Zhang, L.; Zhao, J.; Zhang, C.; Zhao, D.; Zhu, X.; Liu, W. Garcinol, an acetyltransferase inhibitor, suppresses proliferation of breast cancer cell line MCF-7 promoted by 17β-estradiol. Asian Pac. J. Cancer Prev. 2014, 15, 5001–5007. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Wang, Z.; Wojewoda, C.; Ali, R.; Kong, D.; Maitah, M.Y.; Banerjee, S.; Padhye, S.; Sarkar, F.H. Garcinol-induced apoptosis in prostate and pancreatic cancer cells is mediated by NF-κB signaling. Front. Biosci. 2011, 3, 1483–1492. [Google Scholar]
- Chantree, P.; Martviset, P.; Thongsepee, N.; Tragoolpua, Y.; Sukrong, S.; Roytrakul, S.; Pongcharoen, S.; Punsawad, C. Anti-Inflammatory Effect of Garcinol Extracted from Garcinia dulcis via Modulating NF-κB Signaling Pathway. Nutrients 2023, 15, 575. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Gaonkar, R.H.; Mukhopadhyay, R.; De, A.; Maity, P.; Chattopadhyay, S.; Basu, A.; Ghosh, S.; Kundu, R. Garcinol-loaded novel cationic nanoliposomes: In vitro and in vivo study against B16F10 melanoma tumor model. Nanomedicine 2019, 14, 2045–2065. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Wang, Z.; Ali, R.; Maitah, M.Y.; Kong, D.; Banerjee, S.; Padhye, S.; Sarkar, F.H. Apoptosis-inducing effect of garcinol is mediated by NF-κB signaling in breast cancer cells. J. Cell. Biochem. 2010, 109, 1134–1141. [Google Scholar] [CrossRef]
- Cheng, A.C.; Tsai, M.L.; Liu, C.M.; Lee, M.F.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Garcinol inhibits cell growth in hepatocellular carcinoma Hep3B cells through induction of ROS-dependent apoptosis. Food Funct. 2010, 1, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.M.D.; Almazni, I.A.; Moawadh, M.S.; Alissa, S.A.; Alsharif, K.F.; Aljuhani, N.; Ahmad, I. Targeting NF-kappa B signaling cascades of glioblastoma by a natural benzophenone, garcinol, via in vitro and molecular docking approaches. Front. Chem. 2024, 12, 1352009. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Shen, J.; Wang, F.; Xiao, P.; Jiang, H.; Chen, Y.; Yang, Y. Cambogin is preferentially cytotoxic to cells expressing PDGFR. PLoS ONE 2011, 6, e21370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farhan, M.; Malik, A.; Ullah, M.F.; Afaq, S.; Faisal, M.; Farooqi, A.A.; Biersack, B.; Schobert, R.; Hau, H.; Sarkar, F.H.; et al. Garcinol Sensitizes NSCLC Cells to Standard Therapies by Regulating EMT-Modulating miRNAs. Int. J. Mol. Sci. 2019, 20, 800. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Das, S.N. Garcinol inhibits tumour cell proliferation, angiogenesis, cell cycle progression and induces apoptosis via NF-kappaB inhibition in oral cancer. Tumour Biol. 2016, 37, 7175–7184. [Google Scholar] [CrossRef] [PubMed]
- Ranjbarnejad, T.; Saidijam, M.; Tafakh, M.S.; Ghasemkhani, N.; Najafi, R. Garcinol exhibits anti-proliferative activities by targeting microsomal prostaglandin E synthase-1 in human colon cancer cells. Hum. Exp. Toxicol. 2016, 36, 692–700. [Google Scholar] [CrossRef]
- Duan, Y.T.; Yang, X.A.; Fang, L.Y.; Chen, L.; Wang, Y.C. Anti-proliferative and anti-invasive effects of garcinol from Garcinia indica on gallbladder carcinoma cells. Pharmazie 2018, 73, 413–417. [Google Scholar]
- Saadat, N.; Akhtar, S.; Goja, A.; Razalli, N.H.; Geamanu, A.; David, D.; Shen, Y.; Gupta, S.V. Dietary Garcinol Arrests Pancreatic Cancer in p53 and K-ras Conditional Mutant Mouse Model. Nutr. Cancer 2018, 70, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Sarkar, S.H.; Aboukameel, A.; Ali, S.; Biersack, B.; Seibt, S.; Li, Y.; Bao, B.; Kong, D.; Banerjee, S.; et al. Anticancer action of garcinol in vitro and in vivo is in part mediated through inhibition of STAT-3 signaling. Carcinogenesis 2012, 33, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Sarkar, S.H.; Bitar, B.; Aboukameel, A.; Sethi, S.; Li, Y.; Bao, B.; Kong, D.; Banerjee, S.; Padhye, S.B.; et al. Garcinol Regulates EMT and Wnt Signaling Pathways In Vitro and In Vivo, Leading to Anticancer Activity against Breast Cancer Cells. Mol. Cancer Ther. 2012, 11, 2193–2201. [Google Scholar] [CrossRef]
- Staebler, S.; Hoechst, S.; Thongmao, A.; Fufezan, C.; Knappe, N.; Kanokmedhakul, K.; Kanokmedhakul, S.; Sarin, N.; Schobert, R. The Role of T-Cadherin (CDH13) in Treatment Options with Garcinol in Melanoma. Cancers 2024, 16, 1853. [Google Scholar] [CrossRef] [PubMed]
- Bosserhoff, A.K.; Ellmann, L.; Quast, A.S.; Eberle, J.; Boyle, G.M.; Kuphal, S. Loss of T-cadherin (CDH-13) regulates AKT signaling and desensitizes cells to apoptosis in melanoma. Mol. Carcinog. 2014, 53, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.M.; Abdelghany, M.K.; Messmer, M.; Yue, B.; Deeves, S.E.; Mantelingu, K.; Aslam, A.; Winkler, G.S.; Kundu, T.K.; Chavali, S.; et al. Diffrential effects of garcinol and curcumin on histone and p53 modifications in tumor cells. BMC Cancer 2013, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, J.; Wu, R.; Zhang, Q.; Zhao, Y.; Sun, L.; Liu, H.; Chen, Y.; Li, X. Garcinol and its analogues: Synthesis, cytotoxic activity and mechanistic investigation. Bioorg. Chem. 2023, 133, 106389. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Sarkar, S.H.; Bitar, B.; Ali, S.; Aboukameel, A.; Sethi, S.; Li, Y.; Bao, B.; Kong, D.; Banerjee, S.; et al. Garcinol regulates NF-κB and Nrf2 pathways in pancreatic cancer cells leading to inhibition of cell proliferation and induction of apoptosis. Mol. Cancer Ther. 2010, 9, 1374–1383. [Google Scholar]
- Saadat, N.; Gupta, S.C.; Awasthi, S.; Saxena, N.K.; Sharma, J.; Dasgupta, S.; Tyagi, A.K.; Aggarwal, B.B. Garcinol inhibits inflammatory signaling and tumorigenesis in a mouse skin carcinogenesis model by targeting NF-κB and STAT3 pathways. Cancer Prev. Res. 2015, 8, 327–338. [Google Scholar]
- Parasramka, M.A.; Gupta, S.V. Synergistic Effect of Garcinol and Curcumin on Antiproliferative and Apoptotic Activity in Pancreatic Cancer Cells. J. Oncol. 2012, 2012, 709739, Erratum in J. Oncol. 2019, 2019, 7469284. [Google Scholar] [CrossRef]
- Kim, S.; Seo, S.U.; Min, K.J.; Kwon, T.K. Garcinol EnhancesTRAIL-Induced Apoptotic Cell Death through Up-Regulation of DR5 and Down-Regulation of c-FLIP Expression. Molecules 2018, 23, 1614. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, H.; Zhang, J.; Li, M.; Chen, H.; Xu, W.; Zhao, Y. Garcinol Alone and in Combination With Cisplatin Affect Cellular Behavior and PI3K/AKT Protein Phosphorylation in Human Ovarian Cancer Cells. Dose Response 2020, 18, 1559325820926732. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.H.; Chiou, Y.S.; Kalyanam, N.; Ho, C.T.; Chen, L.C.; Pan, M.H. Garcinol sensitizes breast cancer cells to Taxol through the suppression of caspase-3/iPLA2 and NF-κB/Twist1 signaling pathways in a mouse 4T1 breast tumor model. Food Funct. 2017, 8, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, W.; Chen, W.; Wu, Q.; Liu, H.; Cui, G. Epigenetic modulation of DNA methylation by natural products for cancer prevention and therapy. Pharmacol. Res. 2019, 147, 104343. [Google Scholar]
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Kim, C.; Sikka, S.; Sethi, G. Garcinol, a polyisoprenylated benzophenone modulates multiple pro-inflammatory signaling cascades leading to the suppression of growth and survival of tumor cells. Cancer Lett. 2011, 313, 163–175. [Google Scholar]
- Kumar, R.; Lal, N.; Singh, A.; Meena, R. Garcinol sensitizes cancer cells to chemotherapy by modulating apoptosis and autophagy. Front. Pharmacol. 2016, 7, 185. [Google Scholar]
- Ranjbar, S.; Salehi, F.; Sadeghi, M.M. Natural histone acetyltransferase inhibitors as chemosensitizers: Garcinol potentiates cisplatin and doxorubicin cytotoxicity in resistant cancer cells. Phytother. Res. 2011, 35, 1304–1315. [Google Scholar]
- Wang, J.; Wang, L.; Ho, C.T.; Zhang, K.; Li, D.; Huang, Y.; Zhao, H.; Garban, H.J.; Chen, W.; You, Y. Garcinol from Garcinia indica Downregulates Cancer Stem-like Cell Biomarker ALDH1A1 in Non-small Cell Lung Cancer A549 Cells through DDIT3 Activation. J. Agric. Food Chem. 2017, 65, 3675–3683. [Google Scholar] [CrossRef]
- Huang, C.C.; Lin, C.M.; Huang, Y.J.; Wu, J.Y.; Chen, Y.L.; Chang, J.Y. Garcinol Down regulates Notch 1 signaling via modulating miR-200c and suppresses oncogenic properties of PANC-1 cancer stem-like cells. Biotechnol. Appl. Biochem. 2017, 64, 165–173. [Google Scholar] [CrossRef]
- Huang, W.C.; Kuo, K.T.; Adebayo, B.O.; Wang, C.H.; Chen, L.C.; Lin, Y.S.; Lee, W.H.; Ko, T.P.; Chen, Y.J.; Xue, Y.C.; et al. Garcinol inhibits cancer stem cell-like phenotype via suppression of the Wnt/β-catenin/STAT3 axis signalling pathway in human non-small cell lung carcinomas. J. Nutr. Biochem. 2018, 54, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Parasramka, M.A.; Gupta, S.V. Garcinol inhibits pancreatic cancer stem cell characteristics by modulating microRNA expression and epithelial-mesenchymal transition. Mol. Carcinog. 2013, 52, 450–462. [Google Scholar]
- Gaonkar, R.H.; Ganguly, S.; Dewanjee, S.; Sinha, S.; Tripathy, A.; Jha, N.K.; Kundu, C.N.; Kar, A.; Gaonkar, R. Garcinol loaded vitamin E TPGS emulsified PLGA nanoparticles: Preparation, physicochemical characterization, in vitro and in vivo studies. Sci. Rep. 2017, 7, 530. [Google Scholar] [CrossRef]
- Paul, B.; Gaonkar, R.H.; Dutta, D.; De, A.; Mukhopadhyay, R.; Kundu, R. Inhibitory potential of iRGD peptide-conjugated garcinol-loaded biodegradable nanoparticles in rat colorectal carcinoma. Biomater. Adv. 2022, 134, 112714. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.C.; Mahanti, B.; Ganguly, S.; Majumdar, S. Bovine serum albumin as a nanocarrier for efficient encapsulation of hydrophobic garcinol-A strategy for modifying the in vitro drug release kinetics. Int. J. Biol. Macromol. 2024, 278 Pt 1, 134651. [Google Scholar] [CrossRef]
- Jacob, E.M.; Borah, A.; Pillai, S.C.; Kumar, D.S. Garcinol Encapsulated Ph-Sensitive Biodegradable Nanoparticles: A Novel Therapeutic Strategy for the Treatment of Inflammatory Bowel Disease. Polymers 2021, 13, 862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choudhury, B.; Kandimalla, R.; Bharali, R.; Monisha, J.; Kunnumakkara, A.B.; Kalita, K.; Kotoky, J. Anticancer Activity of Garcinia morella on T-cell Murine Lymphoma Via Apoptotic Induction. Front. Pharmacol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Z.; Shi, K.; Zhang, M.; Li, C.; Wang, L.; Liu, Y.; Zhao, Y.; Lin, J. Polyphenol-Based Self-Assembled Nanomedicine for a Three-Pronged Approach to Reversing Tumor Immunosuppression. Adv. Healthc. Mater. 2024, 14, e2402127. [Google Scholar] [CrossRef]
- Huang, M.T.; Liu, Y.; Badmaev, V.; Ho, C.T. Antiinflammatory and anticancer activities of garcinol. In Dietary Supplements; ASC Symposium Series; American Chemical Society: Washington, DC, USA, 2008; Volume 987, pp. 293–303. [Google Scholar]
- Wang, Y.; Tsai, M.L.; Chiou, L.Y.; Ho, C.T.; Pan, M.H. Antitumor Activity of Garcinol in Human Prostate Cancer Cells and Xenograft Mice. J. Agric. Food Chem. 2015, 63, 9047–9052. [Google Scholar] [CrossRef]
- Li, F.; Shanmugam, M.K.; Siveen, K.S.; Wang, F.; Ong, T.H.; Loo, S.Y.; Swamy, M.M.; Mandal, S.; Kumar, A.P.; Goh, B.C.; et al. Garcinol sensitizes human head and neck carcinoma to cisplatin in a xenograft mouse model despite downregulation of proliferative biomarkers. Oncotarget 2015, 6, 5147–5163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, L.; Zhu, Q.; Zhu, F.; Tan, H.; Xu, W.; Pan, C.; Zhu, C.; Wang, Y.; Zhang, H.; Fu, W.; et al. Identification of viral SIM-SUMO2-interaction inhibitors for treating primary effusion lymphoma. PLoS Pathog. 2019, 15, e1008174. [Google Scholar] [CrossRef]
- Sethi, G.; Chatterjee, S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Wong, K.F.; Prem Kumar, A.; Senapati, P.; Behera, A.K.; Hui, K.M.; et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol. Cancer 2014, 13, 66. [Google Scholar] [CrossRef]
- Du, T.; Nagai, Y.; Xiao, Y.; Greene, M.I.; Zhang, H. Lysosome-dependent p300/FOXP3 degradation and limits Treg cell functions and enhances targeted therapy against cancers. Exp. Mol. Pathol. 2013, 95, 38–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, M.L.; Chiou, Y.S.; Chiou, L.Y.; Wu, J.C.; Ho, C.T.; Pan, M.H. Garcinol suppresses inflammation-associated colon carcinogenesis in mice. Mol. Nutr. Food Res. 2014, 58, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kohno, H.; Shimada, R.; Kagami, S.; Yamaguchi, F.; Kataoka, S.; Ariga, T.; Murakami, A.; Koshimizu, K.; Ohigashi, H. Prevention of colonic aberrant crypt foci by dietary feeding of garcinol in male F344 rats. Carcinogenesis 2000, 21, 1183–1189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, P.S.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Inhibitory Effect of Garcinol on Obesity-Exacerbated, Colitis-Mediated Colon Carcinogenesis. Mol. Nutr. Food Res. 2021, 65, e2100410. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shanmugam, M.K.; Chen, L.; Chatterjee, S.; Basha, J.; Kumar, A.P.; Kundu, T.K.; Sethi, G. Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev. Res. 2013, 6, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Tanaka, T.; Hirose, Y.; Yamaguchi, F.; Kohno, H.; Toida, M.; Hara, A.; Sugie, S.; Shibata, T.; Mori, H. Dietary garcinol inhibits 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in rats. Cancer Lett. 2005, 221, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Lu, Y.; Shim, J.Y.; Sang, S.; Sun, Z.; Chen, X.; Lee, M.J.; Lu, Y.; Ho, C.T.; et al. Chemoprevention of 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster cheek pouch carcinogenesis by a 5-lipoxygenase inhibitor, garcinol. Nutr. Cancer 2012, 64, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Lee, P.M.; Bamodu, O.A.; Su, Y.K.; Fong, I.H.; Yeh, C.T.; Chao, T.Y. Enhanced Hsa-miR-181d/p-STAT3 and Hsa-miR-181d/p-STAT5A Ratios Mediate the Anticancer Effect of Garcinol in STAT3/5A-Addicted Glioblastoma. Cancers 2019, 11, 1888, Erratum in Cancers 2020, 12, 2846. https://doi.org/10.3390/cancers12102846. [Google Scholar] [CrossRef]
- Hung, W.; Liu, C.; Lai, C. Inhibitory effect of garcinol against 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumorigenesis in mice. J. Funct. Foods 2015, 18 Pt A, 432–444. [Google Scholar] [CrossRef]
- Kushwaha, P.; Mehrotra, S.; Ahmad, R. In silico screening, ADMET analysis and computational simulation studies on Garcinia indica Choisy phytoconstituents as prospective anti-breast cancer agents: A critical appraisal of the neglected plant. Biotech 2025, 15, 201. [Google Scholar] [CrossRef]
- Jaganathan, R.; Kumaradhas, P. Binding mechanism of anacardic acid, carnosol and garcinol with PCAF: A comprehensive study using molecular docking and molecular dynamics simulations and binding free energy analysis. J. Cell. Biochem. 2023, 124, 731–742. [Google Scholar] [CrossRef]
- Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Obacunone and garcinol inhibit human colon cancer (SW480) cells by modulating antioxidant enzymes and inducing apoptosis. Biochimie 2011, 93, 234–243. [Google Scholar] [CrossRef]
- Liu, H.L.; Chen, Y.; Cui, G.H.; Zhou, J.F. Curcumin, a potent anti-cancer candidate, modulates epigenetic mechanisms in human cancer. Biofactors 2013, 39, 37–55. [Google Scholar] [CrossRef]
- Duvic, M.; Talpur, R.; Ni, X.; Zhang, C.; Hazarika, P.; Kelly, C.; Chiao, J.H.; Reilly, J.F.; Ricker, J.L.; Richon, V.M.; et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 2007, 109, 31–39. [Google Scholar] [CrossRef]
- Marks, P.A.; Xu, W.S. Histone deacetylase inhibitors: Potential in cancer therapy. J. Cell. Biochem. 2009, 107, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Piekarz, R.L.; Frye, R.; Turner, M.; Wright, J.J.; Allen, S.L.; Kirschbaum, M.H.; Zain, J.; Prince, H.M.; Leonard, J.P.; Geskin, L.J.; et al. Phase II multi-institutional trial of romidepsin in cutaneous T-cell lymphoma. Clin. Cancer Res. 2009, 15, 7908–7915. [Google Scholar] [CrossRef]
- West, A.C.; Johnstone, R.W. New and emerging HDAC inhibitors for cancer treatment. J. Clin. Investig. 2014, 124, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Taylor, S.M. Cellular differentiation, cytidine analogs and DNA methylation. Cell 1980, 20, 85–93. [Google Scholar] [CrossRef]
- Issa, J.P.J. DNA methylation as a therapeutic target in cancer. Clin. Cancer Res. 2007, 13, 1634–1637. [Google Scholar] [CrossRef]
- Yoshida, M.; Kijima, M.; Akita, M.; Beppu, T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 1990, 265, 17174–17179. [Google Scholar] [CrossRef] [PubMed]
- Vigushin, D.M.; Coombes, R.C. Histone deacetylase inhibitors in cancer treatment. Expert Opin. Investig. Drugs 2002, 11, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Majeed, M.; Bani, S.; Bhat, B.; Ansari, M.A.; Azam, A.; Chaudhary, S.S.; Reddy, B.V. Safety profile of 40% Garcinol from Garcinia indica in experimental rodents. Toxicol. Rep. 2018, 5, 750–758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majeed, M.; Nagabhushanam, K.; Noureddin, M.A. Scientifically validated combination of garcinol, curcuminoids, and piperine for mild to moderate nonalcoholic steatohepatitis patients—Results from a randomized, double-blind, placebo-controlled study. Front. Nutr. 2023, 10, 1201186. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S.; Gupta, S.C.; Sung, B. Garcinol: An emerging polyisoprenylated benzophenone with diverse anticancer and anti-inflammatory activities. Mol. Carcinog. 2020, 59, 1287–1302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).