Abstract
The human endometrium, previously considered a sterile environment, is now recognized as a low-biomass but biologically active microbial niche critical to reproductive health. Advances in sequencing technologies, particularly shotgun metagenomics, have provided unprecedented insights into the taxonomic and functional complexity of the endometrial microbiome. While 16S rRNA sequencing has delineated the distinction between Lactobacillus-dominant and non-dominant microbial communities, shotgun metagenomics has revealed additional diversity at the species and strain level, uncovering microbial signatures that remain undetected by amplicon-based approaches. Current evidence supports the association of Lactobacillus dominance with endometrial homeostasis and favorable reproductive outcomes. Dysbiosis, characterized by increased microbial diversity and enrichment of anaerobic taxa such as Gardnerella, Atopobium, Prevotella, and Streptococcus, is linked to chronic endometritis, implantation failure, and adverse IVF results. Beyond compositional differences, the endometrial microbiome interacts with the host through immunological, metabolic, and epigenetic mechanisms. These interactions modulate cytokine signaling, epithelial barrier integrity, and receptivity-associated gene expression, ultimately influencing embryo implantation. However, discrepancies between published studies reflect the lack of standardized protocols for sampling, DNA extraction, and bioinformatic analysis, as well as the inherent challenges of studying low-biomass environments. Factors such as geography, ethnicity, hormonal status, and antibiotic exposure further contribute to interindividual variability. Culturomics approaches complement sequencing by enabling the isolation of viable bacterial strains, offering perspectives for microbiome-based biotherapeutics. Emerging 3D endometrial models provide additional tools to dissect microbiome–host interactions under controlled conditions. Taken together, the growing body of data highlights the potential of endometrial microbiome profiling as a biomarker for reproductive success and as a target for personalized interventions. Future research should focus on integrating multi-omics approaches and functional analyses to establish causal relationships and translate findings into clinical practice. This review gives a new insight into current knowledge on the uterine microbiome and its impact on implantation success, analyzed through the lenses of microbiology, immunology, and oxidative stress.
1. Introduction
The endometrium is the lining of the uterine cavity. The endometrium, a target tissue for sex steroid hormones, consists of two main structural components—the endometrial glands and the specialized endometrial stroma. As part of their physiological function, these two elements proliferate and grow under the influence of ovarian estrogens (first half of the menstrual cycle). Proliferation is stopped by the antagonistic effect of progesterone, which induces endometrial maturation and differentiation (second half of the menstrual cycle). The endometrial microbiome is the presence, quantities, and diversity of different types of microorganisms in the uterus.
A long-time uterine cavity is considered a sterile environment, free from microorganisms, which provides optimal conditions for implantation and development of the embryo. This concept is based basically on methods for microbiological analysis used in the 20th century, when, through the cultivation techniques, no bacterial colonization in the uterus was detected. In the late 1990s and early 2000s, there were isolated culture studies in which bacteria were accidentally isolated from the endometrium or placenta (e.g., during cesarean sections), but these were considered “contamination” [1,2,3,4]. The real turning point came after 2015, when genetic methods obtained data showing that the presence of microbes in the uterus is real and systematic. With the introduction of molecular methods, including 16S rRNA sequencing and later metagenomics, it became possible to discover microorganisms even in low-biomass sites such as the endometrium [5,6,7,8]. Historically, research in this area is tracked in Table 1. The accumulated data changed the paradigm, showing that the endometrium of healthy women can contain diversity from bacteria, often dominated by Lactobacillus spp. This new perspective opens the field for research on the potential role of the microbiome in reproductive health and pathology, including implantation failure, chronic endometritis, and endometriosis [9,10]. According to available data, the rate of colonization of the uterine cavity is relatively low compared to the vagina and cervix [11].

Table 1.
Chronology from “sterile uterus” to a new paradigm.
Today, scientific research related to the endometrial microbiome is the focus of many researchers for several key reasons: (1) Influence on fertility: The endometrial microbiome probably plays a critical role in the successful implantation of the embryo and maintenance of pregnancy. Some studies show that a certain microbial composition in the endometrium can increase or decrease the chances of successful pregnancy, including in vitro fertilization (IVF). (2) Linkage with chronic diseases: Changes in the composition of the endometrial microbiome are related to various chronic gynecological conditions, such as endometriosis and chronic endometritis. Knowledge of the microbiome can help with a better understanding and treatment of these states. (3) Diagnostics and personalized treatment: The analysis of the endometrial microbiome can be used as a diagnostic tool to identify specific abnormalities associated with reproductive health problems. This opens the doors for personalized medicine, where the treatment is adapted to the individual microbiome profile of the patient. (4) Prevention of infections: Imbalances in the microbiome on the endometrium can increase the risk of infections, such as bacterial vaginosis or infectious endometritis. Understanding the microbiome may help in the development of preventive strategies. (5) Role in the immune system answer: The endometrial microbiome can influence local immune response in the uterus, which is important for the protection against pathogens and for toleration of pregnancy.
The research in this area has the potential to revolutionize the approach to diagnostics, treatment, and prevention of different gynecological conditions, such as this one, in a way that significantly improves the quality of life and reproductive outcomes in women. The purpose of this review is a critical analysis of available data and a delineation of the future of juices.
2. Methodological Features and Challenges in Endometrial Research Microbiome
2.1. Taking on Samples: Risk from Contamination (Cervical/Vaginal Transmission)
The sampling method is quite variable across studies, as different endometrial samples (swabs, biopsies, or aspirates) may be obtained during embryo transfer, hysterectomy, or by transcervical uterine sampling using uterine manipulators and cervical dilators, which could further contribute to cross-contamination with the cervical microbiota [25]. During sample collection, the possibility of contamination is a major obstacle to determining a “baseline microbiome”, especially when studying a low-biomass environment such as the uterus. It is highly likely that a normal endometrial microbiome exists, although it may be at very low levels, making it susceptible to contamination during sampling.
2.2. Size of the Study Group and Lack of Healthy Controls
Another key limitation of these studies is the cohort size, strict inclusion and exclusion criteria, and the lack of healthy controls, partly due to the challenges in recruiting patients and the technical challenges of obtaining uterine samples [26].
2.3. Low Biomass on the Endometrial Microbiome
The extent of bacterial presence in the uterus is estimated to be between 100 and 10,000 times less than the vaginal microbiome [5,12,27]. When investigating sites with low bacterial abundance using highly sensitive methods, the risk of amplification of contaminating DNA sequences increases, which can significantly alter the results and lead to misinterpretation.
2.4. Methods for Research
Conventional methods for microbial detection are not suitable for identifying bacteria that are difficult to culture. Sequencing provides an easier, faster, and more informative analysis of bacterial populations. The emergence of new sequencing technologies, such as next-generation sequencing (NGS), has enabled a much more global assessment of the bacterial composition of the uterus than has been achieved with culture-dependent methods alone. NGS enables species-level quantification using the variable regions of the 16S rRNA gene, potentially allowing the determination of the full composition of the uterine microbiome [26,28,29]. But obtaining precise results requires the availability of negative control samples, standardization of 16S amplicons, and bioinformatic analysis [30]. A significant challenge is that different researchers use different regions of the 16S rRNA gene (V1–V2, V3–V4, V4–V5), which leads to variations in taxonomic resolution. Among the main ones, analytical shotgun metagenomics is also an approach that gives more detailed information for taxonomic and functional profiles, but it is more resource-intensive [31]. In some studies, it also applies qPCR panels for directed research on certain bacterial taxa, which, however, do not cover the whole microbial diversity. Table 2 compares the methods for endometrial microbiome analysis.

Table 2.
Research on the endometrial microbiome through different methods—advantages and disadvantages.
2.5. Lack of Standardization → Difficulty in Comparing Results
The absence of unified standardization in protocols for sampling, DNA extraction, and bioinformatics analysis significantly makes it difficult to compare results between different research groups and creates uncertainty regarding the clinical applicability of data. Molina et al., 2021 [31] created methodological considerations and recommendations for good practice when analyzing the endometrial microbiome.
3. Composition and Dynamics of the Endometrial Microbiome: Is There a Connection with Reproductive Health?
In recent years, numerous scientific studies have been reported to determine the composition of the uterine microbiome. The data obtained are not unambiguous, and the definition of a “normal uterine microbiome” is still a subject of discussion. Many researchers discuss the relationship between the bacterial communities in the uterus and the presence of a receptive, fertile endometrium; the course of normal or pathological pregnancy; and the development of uterine tumors.
In recent years, researchers involved in reproduction have focused on the microbiome at different sites of the genital tract to study its role in the pathogenesis, prevention, and treatment of diseases.
Fertility disorders are the driving force behind numerous studies in gynecology and reproductive medicine. The effect of the female genital microflora on the ability to conceive is still unclear due to insufficient and contradictory data. However, it seems that a flora dominated by Lactobacillus spp. plays a key role in determining fertility [32]. Toson et al. [33] recently proposed that the physiological endometrial microbiota should be considered as a group of microorganisms that allows embryo implantation and the birth of a live fetus, regardless of the minimal presence of pathogenic bacteria [33]. Previous studies have defined the normal uterine microbiome in women of childbearing age, where Lactobacillus spp. plays a special role [5]. It is currently difficult to define a consensus “healthy” or “core” uterine microbiota. However, some generalizations can be made from the existing data. The most abundant bacteria consistently belong to the following phyla: Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria [12,26,34]. Within the Firmicutes, the genus Lactobacillus is a very important component in the majority of studies on the uterine microbiome. Other types of bacteria commonly found in endometrial/uterine swabs include Bifidobacterium, Gardnerella, Prevotella, and Streptococcus [6]. Subsequently, abnormal endometrial microbiota is associated with implantation failure, pregnancy loss, and other gynecological and obstetric conditions [35].
Moreno’s team (2016) [6] conducted a comparative study between the endometrial and vaginal microbiota and investigated the functional impact of the composition of the endometrial microbiota on reproductive outcome in patients undergoing in vitro fertilization (IVF). The results suggest that the endometrial and vaginal microbiota may differ in structure and composition in some women. Regarding the endometrial microbiota, all patients were grouped into two groups: the Lactobacillus dominant (LD) group and the non-Lactobacillus dominant (NLD) group. Analysis of the resulting microbiota reflected significant differences in bacterial diversity, with the NLD group showing greater species diversity than the LD group. In contrast to those with LD microbiota, subjects with NLD microbiota had significantly lower implantation rates (60.7% vs. 23.1%), pregnancy rates (70.6% vs. 33.3%), ongoing pregnancy (58.8% vs. 13.3%), and live birth rates (58.8% vs. 6.7%), as well as higher rates of spontaneous abortion. This adverse effect on pregnancy outcomes was more evident in subjects with high percentages of bacteria from the genera Gardnerella and Streptococcus.
Kyono and colleagues (2019) [13] apply 16S sequencing in a Japanese cohort and also find a strong connection between Lactobacillus iners dominance and favorable reproductive results, while the presence of mixed flora is associated with a lower frequency of clinical pregnancy. In other research, however, such as this by Franasiak et al. (2016) [36], it is noted that not all patients with diverse microbiomes show negative reproductive results, which emphasizes heterogeneity in the data. Additional studies reveal geographical and ethnic variations. For example, Chen et al. (2017) [12] in the Chinese population discovered that in some women, Lactobacillus crispatus is dominant, while in others, Streptococcus or Bifidobacterium prevails; the association with fertility is not always unambiguous.
There is a lot of research emerging on the endometrial microbiome and its impact on receptivity. In 2021, Japanese scientists attempted to identify specific vaginal and endometrial microbiota in women with a history of recurrent implantation failure [11]. They concluded that disturbed microbiota communities containing specific bacteria in both the endometrium and vagina are associated with implantation failure. They suggested that the level of vaginal Lactobacillus, but not those in the endometrium, could be a biomarker for recurrent implantation failure (RIF). Two other research groups reported that the flora dominated by Lactobacillus appears to play a major role in determining fertility, and, in particular, Lactobacillus crispatus [32,37]. Despite the unclear causality, increasing evidence suggests that endometrial microbiota profiles may predict recurrent spontaneous abortion [38]. In a recent study, 67 women with two or more previous miscarriages underwent endometrial biopsies taken outside of pregnancy [39]. Analysis of microbiota profiles has shown that subsequent miscarriage is associated with increased relative dominance of Ureaplasma. A similar predictive effect of endometrial microbiota was previously described in a study of 342 women with infertility, which found that endometrial microbiota with predominant lactobacilli is associated with an increased likelihood of live birth [40,41]. Parvanov et al. (2023) investigated this relationship between the endometrial microbiome and the success of the implantation in 30 women after frozen embryo transfer [41]. They compare the endometrial microbiome between women who had become pregnant and non-pregnant women, as embryo transfer with euploid embryos was performed up to 3 months after endometrial biopsy. The authors found that the genus Lactobacillus does not significantly distinguish between the two studied groups, but specific bacterial taxa (Serratia marcescens, Staphylococcus spp., Glutamicibacter spp., and Delftia spp.) have a significantly higher relative abundance in the endometrium of patients with implantation failure. Other authors discuss emerging evidence supporting the theory that dysbiotic endometrial microbiota can indeed modulate key inflammatory pathways needed for successful implantation of the embryo and development of pregnancy [42]. Bellver et al. (2024) [43] studied the microbiota of the digestive and reproductive tracts in infertile women with obesity and found that vaginal and endometrial samples had a similar microbiota with the presence of Lactobacillus, Gardnerella, and Streptococcus, but in obese patients a higher frequency of Streptococcus (>50%) was observed in the endometrial microbiota, which may partly explain their poor reproductive outcomes.
A general trend emerging from multiple 16S-based studies is the distinction between a “Lactobacillus-dominant” and “non-dominant” microbiome profile, which is considered a potential biomarker for reproductive outcome. However, significant discrepancies between studies indicate that NGS-based 16S sequencing alone is not sufficient to fully understand the role of the endometrial microbiome.
During the last decade, shotgun metagenomics has been confirmed as a powerful tool for more deeply researching the endometrial microbiome, building on the restrictions of 16S rRNA sequencing [6,12]. For differences from amplicon approaches, shotgun analysis allows simultaneous taxonomic and functional characterization of microbial communities and reveals greater detail on the level of species and strains [14]. The first metagenomic studies show that although Lactobacillus spp. are often dominant, the endometrial microbiome also contains a number of low-abundant taxa that are not detected with 16S approaches [12].
Several independent studies demonstrate that in women with reproductive problems, specific microbial signatures, including anaerobes such as Gardnerella vaginalis, Atopobium vaginae, Prevotella spp., and Streptococcus, are discovered [40,44]. Shotgun analysis also tracks metabolic potentials of these bacteria, such as, for example, production of biofilm-associated enzymes, lipopolysaccharides, and other factors that could influence endometrial receptivity [45]. At the same time, studies of women with successful implantation and normal reproductive status show a stable presence of Lactobacillus crispatus and L. gasseri, as well as lower microbial diversity [13]. Table 3 shows the microbial profile of the endometrium in health and pathology.

Table 3.
Microbiome profile at health vs. pathology.
Shotgun metagenomics detects both viral and fungal components in the endometrial environment, which usually remain invisible in 16S analyses [14]. Several reports show that herpes viruses and HPV may be found in uterine samples, which raises questions about their role in endometrial homeostasis [14]. Also, metagenomics data reveal potential functional pathways related to inflammation, immunomodulation, and hormonal regulation. Through a metatranscriptomic approach, Sola-Leyva et al. (2021) map the whole alive microbiota, consisting of is of >5300 microorganisms in the endometrium of healthy women [46]. There were significant differences in microbial content in the middle secretory compared to the proliferative phase of the endometrium.
The comparative analysis between different shotgun metagenome studies shows significant heterogeneity. For example, while some authors describe exceptional domination of Lactobacillus in the endometrium [6], others establish a richer microbiome with participation of Bacteroides, Clostridium, and even Escherichia coli [12,14]. These differences are probably owed to geographical and population features, as well as differences in protocols for sampling and bioinformatics analysis [47].
One of the key advantages of shotgun metagenomics is the ability to distinguish viable from nonviable bacteria by functional genes, although this is not yet routinely applied. In addition, metagenomics provides information on antibiotic resistance genes in the endometrial environment, which has direct clinical relevance in the treatment of chronic endometritis [48].
The general consensus among most shotgun studies is that the endometrium represents a low biomass, but biologically active environment in which exists dynamic interaction between bacteria, viruses, and host [14,47]. The evidence indicates that Lactobacillus, the dominant microbiome, is beneficial for implantation, while dysbiotic profiles with participation of anaerobes and conditionally pathogenic bacteria are associated with adverse reproductive outcomes [6,13].
Despite this, there remains a significant need for standardization in the analysis and interpretation of shotgun metagenomics data [31,49]. The lack of unified protocols makes it difficult to make direct comparisons between studies and places into question the reproducibility of results [47]. Future research must include bigger cohorts, control of contamination, and integration of metagenomic with metatranscriptomic and metabolomic data.
In conclusion, shotgun metagenomics offers a unique perspective for understanding the endometrial microbiome, providing not only taxonomic but also functional information. It reveals a more complex and diverse ecosystem than previously thought, and suggests new avenues for clinical intervention, especially in the field of reproductive medicine. In the future, integration with metatranscriptomics and functional analyses will be required to gain a clearer picture of microbial–host interactions (Figure 1).
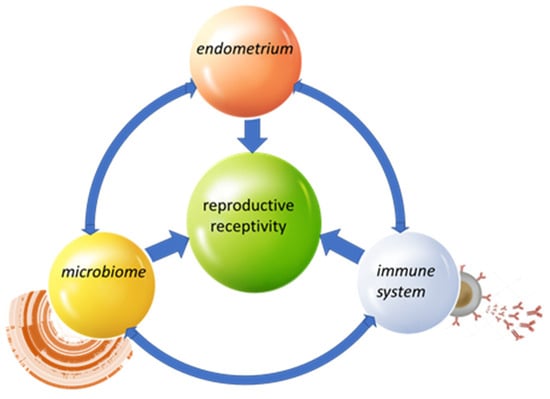
Figure 1.
Key interactions.
Despite the differences in the studies published to date, several main conclusions can be drawn: (1) The endometrium is not a sterile environment. The uterine cavity contains its own microbiome, albeit with a low biomass. (2) Lactobacillus spp. are dominant under physiological conditions. In most cohorts, a trend towards a predominance of Lactobacillus has been observed, which is associated with the maintenance of homeostasis and favorable reproductive potential. (3) Dysbiosis is associated with reproductive disorders. Increased microbial diversity and the presence of anaerobic bacteria such as Gardnerella, Atopobium, and Prevotella are often associated with chronic endometritis, implantation failure, and adverse IVF outcomes. (4) There are significant individual and population differences. Geographic, ethnic, and hormonal factors, as well as antibiotic exposure, may influence the composition of the endometrial microbiome, which explains some of the contradictory results.
4. Study of the Endometrial Microbiome Using Cultivation Methods: Isolation of Pure Cultures of “Beneficial” Microorganisms
Culturomics techniques allow the isolation and identification of viable microorganisms and provide mainly qualitative information on the species identity of the isolated microorganisms, while sequencing methods provide a quantitative profile of the microbiota. The limitations of culture techniques are related to the inability to cultivate some anaerobic or difficult-to-grow species in laboratory conditions [50]. On the other hand, only culture techniques allow the isolation, study, and storage of pure cultures of “good” microorganisms that could be used as probiotic strains and in biotherapy.
To date, culture techniques have been applied in a limited number of studies on endometrial samples, most of which focus on the identification of opportunistic pathogens, such as Escherichia coli, Gardnerella vaginalis, and Streptococcus agalactiae [29,50]. Demonstration of the microbiota in poorly studied anatomical areas remains a challenge.
In a 2025 study involving 50 women, 10 of whom were healthy as a control group and the rest had documented reproductive problems, microbiological analysis was performed by culturing samples from the endometrium, cervical canal, and vagina. In the control group, Lactobacillus representatives were isolated in 7.5% of the endometrial samples, while in women with fertility disorders, the frequency of Lactobacillus isolation was higher, 30% in the endometrium, respectively [51]. The authors compared the culture method with the qPCR method for the identified lactobacilli. The qPCR method showed a much higher positivity, with the frequency of identified lactobacilli in endometrial samples by quantitative real-time PCR in these two groups being 40%.
Cariati’s group [50] (2023) investigated the endometrial microbiota profile in 93 in vitro fertilization (IVF) patients using culturomics-based analysis and MALDI-TOF MS identification. In 74% of the patients, the presence of one or more microorganisms was detected, while in 26% no microbial growth was observed. They found that the phylum Firmicutes was the most frequently isolated taxon, and Lactobacillus spp. significantly correlated with ongoing pregnancy, while the presence of Staphylococcus spp. and Enterobacteriaceae had a negative impact on implantation rates. The following species were identified: L. gasseri, L. crispatus, L. jensenii, L. iners, L. rhamnosus, L. fermentum, L. paracasei, and L. johnsonii [50]. The bacterial population was assessed mainly by growth on solid media, with enrichment broths being used only in the absence of growth on agar media.
In a pilot study [52], the effectiveness of culturomics—a high-throughput cultivation approach—in the analysis of the endometrial microbiome was demonstrated. Ten women with reduced fertility who underwent diagnostic hysteroscopy and endometrial biopsy were included. Despite the low microbial density of the endometrial tissue, 83 bacterial and 2 fungal species were isolated and identified through culturomics, supported by the WASPLab® system. MALDI-TOF mass spectrometry or 16S rRNA sequencing was used to identify the isolates. Two approaches were used to process the samples: one part was directly inoculated onto multiple aerobic and anaerobic media, while the second was subjected to pre-enrichment with two different incubation conditions (aerobic and anaerobic). Most of the isolated species (91%) matched genera already described in previous metagenomic studies of the endometrium. Representatives of the genus Lactobacillus were isolated from all 10 samples, including L. coleohominis, L. crispatus, L. delbrueckii, L. gasseri, L. jensenii, L. iners, and L. vaginalis. L. jensenii was the most frequently isolated species, confirming data from previous metagenomic analyses of the endometrial microbiome [52]. Culturomics provides an opportunity for deeper species differentiation—53 of the total isolated species (62.4%) were described for the first time as part of the endometrial microbiota. In the methodology applied for the isolation and identification of microorganisms by cultivation, the application of different incubation conditions is important: aerobic, anaerobic, or the need for pre-incubation of the samples in broth [33].
The same research team [53] conducted a further study comparing the endometrial and vaginal microbiomes by applying culturomics on paired samples using the WASPLab-assisted culturomics protocol. Ten women with reduced fertility who underwent hysteroscopy and biopsy were included. On average, 28% of the species were detected in both the endometrial biopsy and vaginal swab of the respective patient. Thus, a culturomics-based approach sheds further light on the current understanding of the endometrial microbiome, and the reported data suggest the potential existence of a unique endometrial microbiome that is not a consequence of cross-contamination obtained during sampling. All vaginal and endometrial samples contained one or more members of the genera Lactobacillus, Ligilactobacillus, or Limosilactobacillus. Among the species identified are L. crispatus, L. gasseri, L. iners, L. jensenii, L. vaginalis, Lancefieldella rimae, and Limosilactobacillus fermentum. L. jensenii is predominantly observed in microbiomes with low microbial diversity, while L. iners is more characteristic of microbiomes with high diversity, often associated with dysbiosis. The present data highlight the importance of optimized cultivation conditions and careful selection of the culturomics approach when analyzing the microbiota in sparsely populated microenvironments such as the endometrium [53].
Lactobacillus spp. are the dominant colonizers in healthy women of reproductive age, maintaining an acidic pH (below 4.5) and limiting the spread of pathogens through the production of organic acids, hydrogen peroxide (H2O2), antimicrobial compounds (bacteriocins), biosurfactants, exopolysaccharides, and disruption of biofilms [54,55,56,57,58].
Although the protective function of Lactobacillus in the vaginal ecosystem is well documented, the mechanisms by which this genus affects fertility remain incompletely understood and are the subject of active investigation [33]. Lactobacillus-associated metabolites have demonstrated antagonistic activity against a wide range of bacterial and fungal pathogens, including Candida albicans, Staphylococcus aureus, Streptococcus mutans, Escherichia coli, Pseudomonas aeruginosa, and Salmonella typhimurium [59]. Based on the accumulated data, the selection and application of Lactobacillus strains with optimal characteristics—including the ability to rapidly acidify the environment, effective pathogen suppression, and high adhesive activity to the mucosa—may represent an effective approach to restore microbial balance in the female reproductive tract.
The use of vaginal probiotics composed of well-selected strains isolated from the genital tract may not only help restore a healthy microbiota, but also have a positive impact on the upper parts of the female reproductive system [60]. The reason for this is that standard probiotics are usually not adapted to the specific conditions of the genital tract and fail to sustainably normalize the environment. As a result, the microbiome remains unstable, and dysbiosis-related conditions occur recurrently.
Some authors suggest a link between the intestinal microbiome and the microbiome of the reproductive tract [61]. Sobstyl et al. [62] (2022) commented on data suggesting that gynecological and gastrointestinal dysbiosis may play an active role in the development and metastasis of gynecological neoplasms, such as cervical, endometrial, and ovarian cancer. Baușic et al. [63] (2025) found dysbiosis of the gut microbiota in endometriosis with increased levels of Bacteroidetes and increased inflammatory markers, such as β-glucuronidase and secretory IgA, and suggested a potential link between the gut microbiota and systemic inflammation in endometriosis. Barczyński et al. [64] (2023) investigated the composition of the vaginal and cervical microbiota in patients with endometrial cancer, and their data indicated that Lactobacillus iners was significantly more common in patients with benign disease, while Dialister pneumosintes and Mobiluncus curtisii were more common in patients with cancer. According to the authors, understanding the role of the microbiome in endometriosis could open new avenues for noninvasive diagnostic tools and microbiota-targeted therapies. Advances in understanding the human microbiota have significantly accelerated the development of innovative approaches in live biotherapy, including the use of probiotics and entire communities of microorganisms with therapeutic potential [60,65,66].
5. Therapeutic and Clinical Applications
Disruption of the dominance of Lactobacillus spp. in the vaginal microbiome is associated with increased bacterial diversity and, consequently, a higher risk of gynecological problems, including bacterial vaginosis, infections, and adverse pregnancy outcomes (premature birth, miscarriage) [67].
A probiotic with L. crispatus has been shown to significantly improve vaginal health in women with bacterial vaginosis [68]. This study noted that after treatment, dysbiosis was reduced and the balance of the vaginal microbiota improved, suggesting the possibility of restoring the dominant Lactobacillus flora [68]. Another study evaluated the safety of a vaginal probiotic (L. crispatus CTV-05) in pregnant women at high risk of preterm birth [69]. It was reported that probiotic administration was well tolerated and no serious adverse events were observed, supporting the potential for interventions targeting microbiome therapy to reduce risks during pregnancy [69]. A study by Kadogami, 2020, examined the effect of vaginal probiotics in combination with antibiotic therapy in women with endometrial microbiota disorders (non-Lactobacillus dominated profile) [70]. The authors found that combining vaginal probiotic suppositories (inVag: L. gasseri, L. fermentum, and L. plantarum) with an antibiotic was more effective than single-agent therapy in restoring the endometrial microenvironment. In a 2024 study, Sakamoto et al. [71] reported the effectiveness of combining antibiotics and probiotics (L. acidophilus La-14 and L. rhamnosus HN001 strains) in vaginal formulations for the treatment of abnormal uterine microflora. A recent publication [72] describes the study of a strain of L. crispatus M247 isolated from a probiotic product, which showed strong antimicrobial properties against various pathogens. Its cell-free supernatant (CFS) was found to be more effective than the live strain itself. In in vitro experiments, CFS significantly reduced the growth of pathogens such as Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Streptococcus agalactiae, Enterococcus faecalis, and Candida albicans. This suggests that CFS therapies may be more successful than the oral administration of live bacteria. A study by Vander Donck et al. (2025) showed that different Lactobacillus spp. can stably coexist through metabolic interactions, which provides a basis for future microbiome therapies [73].
Probiotics containing Lactobacillus strains exert their beneficial effects on the host through a complex network of microbiological, immunological, and metabolic mechanisms that collectively support mucosal homeostasis and reproductive health. These bacteria act primarily by competitively excluding pathogenic microorganisms, occupying epithelial adhesion sites, competing for nutrients, and forming biofilms that create a physical and biochemical barrier against harmful species such as Gardnerella, Escherichia coli, and Candida. In addition, Lactobacillus strains produce antimicrobial substances including lactic acid, which lowers the local pH and inhibits the growth of pathogens; hydrogen peroxide (H2O2), which disrupts microbial membranes through oxidative stress; and bacteriocins—small peptides that selectively suppress competing bacteria [54,56,57]. These probiotics also modulate the host immune system by promoting anti-inflammatory cytokine responses (e.g., IL-10), downregulating pro-inflammatory mediators such as IL-6 and TNF-α, stimulating mucosal immunity through enhanced IgA secretion, and interacting with toll-like receptors to support immune tolerance [55,57]. Furthermore, Lactobacillus species strengthen the epithelial barrier by upregulating tight junction proteins, stimulating mucin secretion, and reducing epithelial permeability, thereby preventing pathogen translocation [58,59]. Their metabolic activity contributes to host health through the production of short-chain fatty acids like acetate and butyrate, which possess anti-inflammatory and tissue-repairing properties, as well as by modulating oxidative stress and influencing estrogen metabolism within reproductive tissues. Additionally, Lactobacillus strains engage in cross-talk with other beneficial microorganisms such as Bifidobacterium, fostering a balanced and resilient microbial community that supports vaginal and endometrial homeostasis [56,58]. Altogether, Lactobacillus probiotics are key modulators of the reproductive tract environment, promoting microbial balance, epithelial integrity, and immune regulation.
Although many authors suggest that increasing the level of Lactobacillus occupancy in the uterus leads to improved IVF outcomes, there are no published studies that have directly investigated this relationship. Furthermore, there are no studies comparing the effectiveness of different Lactobacillus spp. contained in probiotics. Considering that the endometrial microbiome can originate from other parts of the body (vaginal, oral, intestinal, etc.), from a general perspective, probiotic treatment in other parts of the body may also be useful for regulating the balance of the endometrial microbiome. There is evidence that the use of probiotics affects various biological processes associated with chronic endometritis [74] and tumorigenesis in endometrial cancer (inflammation, oxidative stress, apoptosis, proliferation, and metastasis) [75].
Based on these studies, several key conclusions can be drawn: first, dysbiosis—defined as a decline in Lactobacillus-dominated microbiota and an enrichment of anaerobes and opportunistic pathogens—correlates with adverse gynecological and reproductive outcomes. Second, Lactobacillus crispatus-based interventions show promising results in terms of symptom reduction and improvement of the microbiome ecosystem. Third, probiotic approaches, according to available data, are safe in specific groups (pregnant women at risk), which is critical for future clinical applications.
However, these studies focused primarily on the vaginal microbiome and vaginal infections, and not explicitly on the endometrial flora and its relationship to reproductive outcomes such as implantation success or live birth. Also, although a reduction in dysbiosis and an enrichment of the vaginal flora have been shown, it is unclear to what extent these effects translate to uterine conditions or directly affect endometrial receptivity.
In conclusion, the body of evidence supports the idea that restoring and maintaining a Lactobacillus-dominant microbiota is a promising strategy for reducing gynecological and reproductive risks. However, gaps remain—especially with regard to direct evidence for the endometrium—requiring future studies with specific endometrial samples and functional assays to form the basis for personalized reproductive medicine based on a microbiome profile.
6. Immunological Mechanisms—Research Methods: Interaction of the Microbiome with the Immune System and the Local Environment
The implantation of the human embryo represents an extremely complex and strictly regulated biological process that needs synchronized interaction between the embryo and the endometrial mucous membrane. The successful implantation is happening in a strictly limited period known as the “implantation window”, during which the endometrium becomes receptive to the blastocyst. The disturbances in this fine balance would lead to repeated implantation failures (RIF), early abortions, or infertility. The endometrial receptivity and the early implantation are also dependent on the communication with the epithelial cells, immune cells, and the microbiome. Because of the ethical and practical limitations, it is impossible to study in vivo in women the basic aspects of the endometrium differentiation and regeneration, and the pathological aspects of the implantation also. The use of different animal models could provide some important insights, but they could not completely reproduce the biology of the human endometrium. To fill the significant hole in understanding these complex communications, the in vitro models of endometrium were established.
The human endometrium contains two different cell types: stromal and epithelial cells. The inner surface of the uterine epithelium is differentiated as luminal epithelium (LE). The first contact of the embryo trophectoderm is with the LE. The invaginations of the epithelium form glands that form glandular epithelia (GE) that produce factors important for the embryo’s survival, the growth of the placenta, and also have an impact on stromal decidualization [76].
6.1. Human Endometrial Epithelial Cell Lines
Since the last decades, the in vitro models of endometrial cell lines like Ishikawa, RL95-2, and HEC-1A have become approved instruments for studying the endometrial receptivity, the blastocyst’s adhesion, and the molecular mechanisms that regulate this process. They are widely used because of their acceptability, stability in culture, and reproducibility of the results, although there are some restrictions according to their tumor origin. During the menstrual cycle, the human endometrium changes its polarity, adhesion, and cytoskeletal organization [77]. Depending on these characteristics, endometrial cell lines with different degrees of polarization were used. The HEC-1 cell line is an example of a non-receptive cell with low adherent properties and high polarization properties [77,78]. A cell line with opposite characteristics, like high receptivity and high adhesive properties, is the RL95-2 epithelial cell line [77,78].
Another well-differentiated human endothelial adenocarcinoma cell line, established more than 20 years ago and widely used as a good model for studying normal endothelial function, is the Ishikawa cell line. This cell line possesses mixed characteristics of LE and GE. With its adhesiveness, moderate polarization, and the expression of functional steroid receptors, the Ishikawa cell line is one of the most used ones [77,78,79]. According to its LE properties, the Ishikawa cell line expresses MUC1 cytokeratins (KRTs) 13 and 18, which makes it a good model for luminal epithelium.
Two other cell lines with LE characteristics are the ECC-1 cell line and the HES line. They also express cytokeratins (KRTs) 13 and 18, estrogen receptors alpha and beta, progesterone receptors A and B, and androgen receptors [78].
KLE is a cell line that was isolated from the endometrium of a 64-day-old female patient with undifferentiated endometrial adenocarcinoma. It is characterized by low receptivity and lower adhesiveness toward trophoblast cells, and that makes it more suitable for studying the molecular pathways of tumorigenesis and cell differentiation than as a model for implantation [80].
The AN3 CA cell line is a human endometrial epithelial cell line developed from poorly differentiated endometrial adenocarcinoma. This line demonstrates low adhesive properties and reduced receptivity, which determines it as a “nonreceptive model”. The AN3 CA line is used for cell signaling research, molecular regulators of cell growth and migration, as well as evaluating the effects of various cytokines and hormones on endometrial epithelial function [81].
These cell lines are usually cultivated under standard conditions—37 °C, 5% CO2, with culture media such as DMEM/F12 or McCoy’s, enriched with 10% FBS and antibiotics. The line RL95-2 requires the addition of insulin, which is important for maintaining its morphology and functional characteristics.
Although there are limitations according to the origin of these cell lines, they are valuable instruments for studying the cellular and molecular mechanisms, including the expression of adhesion molecules, cytokine secretion, and the interaction with the trophoblast cells. They also serve as a basis for the development of more complex models, such as co-culture systems with immune cells and 3D organoids, which more accurately reflect the complex physiology of the endometrium.
6.2. Three-Dimensional Human Endometrial Culture Models
Since the 1980s, the development of the 3D human endometrial culture models has led to significant progress in the research of the human endometrium’s receptivity. Unlike the traditional two-dimensional (2D) cell culture methods, which often lose tissue-specific specificities, 3D models allow the reconstruction of the architecture and functionality of the endothelium. This makes them particularly valuable for studying processes such as blastocyst implantation, the interaction between epithelial, stromal, and immune cells, and the role of the microbiota and inflammatory mediators.
Different types of 3D cultures could be distinguished depending on the organization, the matrix, and cell content: spheroids, organoids and co-culturing systems—assembloids.
Spheroids are 3D aggregates of endometrial cells (epithelial, stromal, or a mixture of them) that are self-organized while cultivated in suspension or on a matrix [82,83]. These organizations imitate the steric organization and cell–cell contacts in the tissue better than the 2D cultures. The spheroids are often used in co-culture with trophoblast cells or embryo spheroids to modulate the early stages of adhesion and invasion of the blastocyst [84]. These systems allow for the evaluation of the expression of the adhesion molecules like integrins, osteopontin, etc., and the role of cytokines and hormones on embryo-endometrium interactions [85].
Organoids are more complex 3D structures that contain primary endometrial epithelial stem cells or progenitor cells, cultivated in the presence of a specific matrix, like Matrigel, and in the presence of medium containing factors supporting cell differentiation [86]. The formed gland-like structures reproduce the cyclical changes in the endometrium under the influence of estrogen and progesterone [87]. The organoids preserved tissue-specific markers, the functional hormonal response, and the secretory activity, and all these features make these structures exceptionally valuable to studying the receptivity, the “implantation window”, and pathological conditions like endometriosis, chronic endometritis, or recurrent implantation failure (RIF) [88,89].
Assembloids are the next most complicated form of 3D models that combine endometrial organoids with stromal cells and/or immune cells in a common 3D structure. This complex culture model reproduces the cell diversity of the endometrium and its dynamics. Assembloids enable the study of interactions between epithelium, stroma, and immune cells, which is critical for successful implantation. They are used to study endometrial pathology, infertility, and to test targeted therapies [88,89].
The main differences between the 3D human endometrial models are summarized in Table 4.

Table 4.
Three-dimensional human endometrial models.
Three-dimensional human endometrial cultures are a powerful tool for studying the mechanisms of implantation and endometrial pathologies. They provide a significantly closer to in vivo environment compared to classical cell lines, but their application still requires standardization and optimization. In the future, these models will likely be integrated with new technologies such as microfluidic systems (organ-on-a-chip) and multiomics analyses to achieve a better understanding and therapeutic application in reproductive medicine.
6.3. Interaction Between the Endometrial Microbiota and Endothelium
The endometrial endothelium functions as both a physical barrier and an active immunological sensor. Epithelial cells express pattern recognition receptors, including Toll-like receptors, which detect microbial components and trigger intracellular signaling cascades leading to the production of cytokines and chemokines such as IL-1b, IL-6, and TNF-a. The immune signaling shapes the local environment to either support or hinder blastocyst adhesion. A key condition for successful embryo implantation is synchronization between the stage of embryo development and the condition of the endometrium. Recently, more and more data have emerged on the importance of the endometrium microbiota for embryo development, its attachment/positioning/invasion, and early pregnancy. Some studies analyze whether the endometrial microbiota can influence the prenatal conditions in the mother and subsequently affect endometrial receptivity [90,91].
The female reproductive tract contains 9–10% of the entire human microbiome population. The diversity of the microbiome is greater from the vagina to the ovaries, with decreasing bacterial diversity from the external to the internal organs [92]. Microbiota diversity in the female genital tract changes throughout a woman’s life, influenced by factors such as age, ethnicity, sexual activity, and overall health status [20]. Hormonal fluctuations of progesterone and estrogen during the menstrual cycle also shape the endometrial microbiota. An increase in the microbial abundance has been observed during the proliferation phase, while the composition appears stable during the days critical for endometrial receptivity [25]. The glycogen deposition in the epithelium of the vagina is caused by estrogen, whereas progesterone-induced cytolysis facilitates its release into the lumen. The liberated glycogen is metabolized by Lactobacillus spp. into lactic acid, thereby maintaining an acidic vaginal environment (pH 3.5–4.5), which is critical for microbial homeostasis and protection against pathogens [93]. It’s already shown that the predominance of Lactobacillus spp. is associated with more favorable reproductive outcomes, while dysbiosis (increase in anaerobes such as Gardnerella, Atopobium, and Prevotella) is associated with chronic endometritis, reduced receptivity, and lower implantation rates [6,26]. Alterations in the microbiome may contribute to the pathogenesis of endometriosis by modifying the local microenvironment. As emphasized in the work of Sirota et al. [94], bacterial-driven uterine inflammation can disrupt the cytokine balance required for proper blastocyst development and successful implantation. In particular, dysbiosis has been associated with increased production of cytokines that promote inflammation (pro-inflammatory), such as IL-6 and TNF-α, which may impair endometrial receptivity, while downregulation of implantation-supporting mediators like leukemia inhibitory factor (LIF) further compromises the adhesion of the blastocyst to the endometrial epithelium [95].
The interaction between the endometrial microbiome and the endothelium occurs through a complex network of immunological, metabolic, and cell-molecular mechanisms that can either support or hinder successful implantation. One major pathway is immunological modulation. Endometrial epithelial cells express TLR2, TLR4, and other pattern recognition receptors that detect bacterial components such as LPS and peptidoglycans [96]. In a state of eubiosis, Lactobacillus spp. help maintain a local environment that suppresses the immune response against specific antigens (a tolerogenic environment) by stimulating the production of anti-inflammatory cytokines such as IL-10 and TGF-β. Dysbiosis, in contrast, promotes elevated levels of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, which may impair endometrial receptivity [97]. The microbiome also influences the activity of uterine natural killer cells (uNK) and M2 macrophages, which are crucial for angiogenesis and immune tolerance during implantation.
Metabolic mechanisms represent another key mode of interaction. Lactobacillus spp. metabolize glycogen into lactic acid, lowering vaginal and endometrial pH and creating a protective antimicrobial environment [90]. Short-chain fatty acids produced by certain bacterial species can regulate the epigenetic programming of endothelial cells and modulate the expression of adhesion molecules. Conversely, dysbiotic bacteria, such as Streptococcus and Staphylococcus, may release metabolites that induce oxidative stress or apoptosis in endometrial cells, disrupting tissue homeostasis. Lactobacillus-dominant microbiota enhances epithelial tight junction integrity, whereas dysbiosis may increase epithelial permeability. Additionally, microbial interactions stimulate the secretion of antimicrobial peptides such as defensins, contributing to local immune homeostasis.
Finally, epigenetic and signaling regulation also play an important role. Microbial metabolites and inflammatory mediators can influence DNA methylation and histone modifications in endothelial cells, altering the expression of genes related to receptivity, angiogenesis, and inflammatory responses. These changes highlight the intricate crosstalk between the microbiome and the endometrium, demonstrating how microbial composition can directly affect implantation outcomes.
6.4. Pro-Inflammatory Molecules, Cytokines, and Implantation Factors
The window of implantation that happens typically between days 20 and 24 of a normal menstrual cycle represents the period during which the endometrium becomes receptive to embryo implantation. During this short period, the luminal epithelium represents the first point of contact with the embryo and plays a decisive role in implantation success [24]. Over the past decade, the application of omics technologies has greatly advanced the understanding of endometrial receptivity. Genomic approaches using high-throughput sequencing have led to the identification of numerous receptivity-associated genes. The Human Gene Expression Endometrial Receptivity database (HGEx-ERdb) currently catalogs over 19,000 endometrial genes, of which 179 have been consistently associated with receptivity across multiple studies [98].
Epigenomics, transcriptomics, and proteomics have provided further insight into the dynamic regulation of endometrial receptivity during the menstrual cycle. Epigenetic modifications, such as DNA methylation and histone modifications, contribute to the cyclic expression of implantation-related genes. Transcriptomic profiling has identified gene expression signatures characteristic of the receptive phase, while proteomics studies reveal post-transcriptional and post-translational regulation of adhesion molecules, cytokines, and growth factors essential for blastocyst attachment. Twelve genes are potentially significant for endometrial receptivity, such as Calpastatin (CAST), Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), Fibroblast Growth Factor Receptor 2 (FGR2), and LIF [99]. Recent studies have validated 19 miRNAs, such as members from the miR-30 and miR-200 families, and 11 of them were upregulated and involved in endometrial receptivity [100]. The human endothelial stromal cells are in extensive communication with the blastocyst and serve as biosensors of the embryo quality. They respond to the embryo-derived hsa-miR-320a by changing their migratory capacity. This function of the endothelial stromal cells serves as an implantation checkpoint. On the other hand, the communication of the endometrium epithelium activates the differentiation of the syncytiotrophoblast and expression of adhesion molecules. The disturbance of the communication between the endometrium and the embryos may also cause problems such as RIF.
Embryo implantation is a highly regulated process that requires a delicate balance between pro-inflammatory signaling, immune tolerance, and tissue remodeling. The endometrium undergoes a transient, inflammatory-like response during the implantation window, with cytokines and growth factors orchestrating successful blastocyst attachment and invasion. Among the key cytokines, IL-1 (α/β) plays an essential role by promoting adhesion molecule expression on endometrial epithelial cells and facilitating extracellular matrix remodeling [101,102]. IL-6 contributes to decidualization, angiogenesis, and trophoblast invasion [101,103], while TNF-α exhibits dual effects: at physiological levels, it supports implantation, but excessive levels can impair trophoblast survival and lead to pregnancy complications [101,104]. Another critical cytokine is LIF (Leukemia Inhibitory Factor), indispensable for endometrial receptivity and blastocyst adhesion [101,102,104]. IFN-γ, largely produced by uterine natural killer (uNK) cells, modulates immune responses and participates in vascular remodeling [105,106]. Similarly, chemokines such as MCP-1 (CCL2) help recruit immune cells to the implantation site, reinforcing the localized inflammatory environment [101].
In addition to cytokines, a broad range of implantation factors contribute to the establishment of receptivity. Integrins (e.g., αvβ3, α4β1) mediate firm blastocyst adhesion to the endometrial epithelium [102,104], while MUC1, a large glycoprotein, is downregulated locally to permit attachment [103,104]. VEGF ensures proper angiogenesis and vascular permeability, supporting early placental development [102,103]. TGF-β regulates immune tolerance and extracellular matrix remodeling [101,104], whereas members of the EGF family stimulate trophoblast proliferation and invasion [101,102]. Transcription factors NOXA10 and NOXA11 further regulate endometrial receptivity by influencing stromal differentiation [103,104]. Enzymes like COX-2 and prostaglandins (PGE2, PGF2a) mediate vascular permeability and decidualization, facilitating embryo implantation [102,106]. Finally, immune mediators such as uNK cells and GM-CSF are central in promoting immune tolerance, vascular remodeling, and trophoblast growth [103,105,106].
Overall, implantation depends on the precise temporal and spatial regulation of these molecules. Insufficient inflammatory signaling can result in poor receptivity and implantation failure, whereas excessive inflammation is linked to recurrent pregnancy loss and pathologies such as endometriosis-related infertility [101,102,106].
6.5. Immunological Markers That Determine the Decidual Immune Profile and the Success of Implantation
The maternal–fetal interface represents a unique immunological environment in which tolerance and defense must be balanced. The decidua contains specialized immune cell populations—primary uterine natural killer (uNK) cells, macrophages, T cells, and dendritic cells—that shape the immune profile required for successful implantation and early placentation [101,106]. The uterine natural killer cells are the most abundant leucocytes in early pregnancy and secrete cytokines such as IFN-γ, VEGF, and angiopoietins, which are critical for spiral artery remodeling and vascular adaptation to support the developing embryo [106,107]. Their activity is modulated by KIR (Killer Cell Immunoglobulin-like Receptors) on maternal uNK cells interacting with HLA-C, HLA-E, and HLA-G molecules expressed by trophoblasts, a process critical for immune tolerance and vascular regulation [107,108]. Dysregulations of this interaction have been linked to recurrent implantation failure and preeclampsia [108].
Macrophages contribute to implantation success by shifting toward an M2 (anti-inflammatory/tolerogenic) phenotype, characterized by the expression of IL-10, TGF-β, and CCL18, which promote tissue remodeling, angiogenesis, and immune tolerance [101,109]. Conversely, excessive M1-like activity, associated with TNF-α and IL-12 production, may impair trophoblast invasion and contribute to pregnancy complications [109].
Regulatory T-cells, defined by CD4+CD25+FoxP3+ expression, expand during the implantation window and secrete IL-10 and TGF-β, both of which are essential for maintaining tolerance to paternal antigens [106,110]. A reduction in frequency of the regulatory T-cells correlates with implantation failure and recurrent pregnancy loss [110].
Another important marker is the expression of HLA-G, a non-classical MHC class I molecule expressed by extravillous trophoblasts. HLA-G interacts with immune cell receptors such as ILT2 and KIR2DL4, suppressing NK and T-cell cytotoxicity, thereby contributing to maternal tolerance [108,110].
Taken together, the decidual immune profile—shaped by the balance between uNK cells, macrophages, Tregs, and trophoblast HLA molecules—acts as a determinant of implantation success. Disruption in any of these immunological markers can shift the environment toward excessive inflammation or inadequate tolerance, leading to impaired implantation outcomes [106,107,108,109,110].
7. Oxidative Stress and the Female Reproductive System
Notwithstanding considerable progress in assisted reproductive technologies (ART), their success rates continue to fall short of expectations. One of the major factors affecting the outcomes of antiretroviral therapy (ART) is oxidative stress (OS) [111].
7.1. The Nature of Oxidative Stress
Oxidative stress is defined as an imbalance between the production of reactive oxygen species (ROS) and antioxidant defenses [112]. The major ROS include superoxide anion (O2•), hydroxyl radical (•OH), and hydrogen peroxide (H2O2). Murphy (2009) [113] found that approximately 2% of the oxygen utilized in mitochondrial energy metabolism is converted to ROS. Increased levels of these molecules have been observed in a number of diseases and under the influence of harmful factors such as smoking, obesity, and unbalanced nutrition [114,115,116]. The present study investigates the physiological and pathological role of ROS in the field of reproduction. Reactive oxygen species (ROS) can induce oxidative damage to cells, leading to disruption of cellular membranes, accelerated aging, immune dysfunction, and alterations in intracellular signaling pathways [117].
Concurrently, controlled levels of ROS are implicated in the normal physiology of the female reproductive system, including folliculogenesis, oocyte maturation, ovulation, steroidogenesis, endometrial changes, and fetoplacental development [118]. However, excessive formation of ROS has been demonstrated to result in oocyte apoptosis, DNA damage, impaired implantation, and even pregnancy loss [119]. Postovulatory oocytes exhibit heightened sensitivity to the effects of ROS. The accrual of oxidative stress has been demonstrated to expedite their senescence, a process typified by alterations in the zona pellucida and the activation of ovoperoxidase [118,120].
With age, oxidative stress progressively increases, correlating with a higher incidence of aneuploidy due to chromosomal abnormalities in aging oocytes [118]. Accumulated damage to DNA, lipids, and proteins impairs cellular function and reproductive capacity [121].
The presence of oxidative stress in follicular fluid and endometrial secretions affects oocyte quality, embryonic development, and embryo transfer success [122,123,124]. Membrane fluidity plays an essential role in the regulation of cellular functions such as transport, enzymatic activity, signal transduction, and cell cycle [125]. At low concentrations, ROS function as secondary mediators in redox-dependent signaling cascades. However, when accumulated, they activate pathways such as mitogen-activated protein kinase (MAPK), which can initiate premature cellular senescence [126]. OS markers are used to predict the outcome of in vitro procedures, with higher antioxidant capacity being associated with an increased chance of clinical pregnancy [127].
Oxidative stress (OS) has a detrimental impact on several key aspects of female reproductive function:
- Oocyte quality: OS induces apoptosis, mitochondrial dysfunction, and chromosomal anomalies, compromising oocyte viability and developmental potential.
- Fertility: It contributes to chromosomal non-disjunction and increases the risk of aneuploidy, particularly in advanced maternal age [128].
- Steroidogenesis: OS suppresses the activity of granulosa cells and impairs the synthesis of essential reproductive hormones such as follicle-stimulating hormone (FSH) and estradiol [118].
Moreover, external factors such as psychological stress and aging exacerbate oxidative imbalance, further impairing fertility outcomes [118].
7.2. Oxidative Stress and Cellular Aging in Endometriosis
The role of ROS in endometriosis, a chronic inflammatory condition affecting 10–15% of women of reproductive age and up to 90% of infertile patients, deserves special attention [129,130,131]. The inflammatory processes that are characteristic of the disease have been shown to stimulate the overproduction of ROS, which in turn leads to damage to cell membranes, immune dysfunction, and impaired implantation [116,132].
Accumulation of ROS and activation of MAPK signaling pathways (ERK1/2, JNK, p38) have been proven in endometriosis, leading to cellular aging and metabolic dysfunction [133,134,135]. Elevated levels of ROS markers have been detected in endometrial, peritoneal, and follicular fluids, as well as in reproductive tissues.
Endometriotic lesions show persistent redox imbalance throughout the menstrual cycle, as well as increased expression of aging markers. Mechanisms include lipid and protein damage, changes in cellular fluidity, and signal transduction associated with aging and inflammation [117].
The relationship between oxidative stress and the female reproductive system is presented in Table 5.

Table 5.
Oxidative stress, biomarkers, and effects on the female reproductive system.
7.3. MAPK Signaling and Aging
The process of ROS-induced growth arrest and the subsequent development of a secretory phenotype associated with senescence (SASP) is facilitated by the activation of the ERK1/2, JNK, and p38 cascades [141]. Increased activity of these pathways, especially ERK1/2, has been reported in endometriosis at different phases of the menstrual cycle [134,135]. This assertion is corroborated by in vitro experiments with stromal cells and GWAS analyses, which identify MAPK and, in particular, ERK1/2, as a pivotal factor in the pathogenesis process [142].
As demonstrated in the studies conducted by Borodkina et al. (2014, 2016), sublethal oxidative stress has been shown to induce premature senescence in endometrial stem cells by activating the p38 pathway [143,144]. The partial restoration of proliferation subsequent to p38 inhibition serves to substantiate the significance of this particular mechanism.
This study investigates the relationship between ROS, MAPK, and endometriotic lesions (Figure 2). The presence of lesions in cases of endometriosis has been shown to be indicative of increased levels of reactive oxygen species (ROS), activated mitogen-activated protein kinase (MAPK) signaling, and elevated levels of markers of cellular senescence. Furthermore, stromal cells from such lesions exhibit an enhanced response to H2O2 compared to controls, thus emphasizing the role of OS in pathogenesis [144,145].
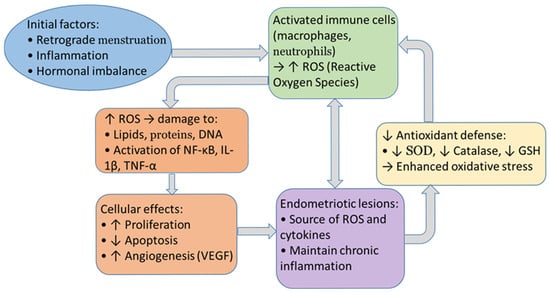
Figure 2.
Cycle between oxidative stress and endometriosis.
According to Malvezzi et al. (2023) [117], H2O2 stimulation activates MAPK pathways and leads to senescence in endometriotic stromal cells. Concurrently, eutopic endometrium from patients diagnosed with endometriosis exhibited diminished levels of ROS and MAPK, accompanied by an augmented prevalence of double-stranded DNA breaks (DSBs) [146].
7.4. Decidualization and Fertility
Deryabin (2021) [147] and Lessey (2017) [148] posit that impaired decidualization in endometriosis, possibly associated with stromal cell senescence, is a factor in reduced fertility. The increased presence of natural killer (NK) cells in the eutopic endometrium of patients with endometriosis indicates chronic stress or tissue damage [149]. Redox homeostasis in the endometrium is imperative. A slight increase in lipid peroxidation has been observed in the premenstrual phase [59,60], with the controlled presence of ROS being necessary to maintain cellular homeostasis.
It is imperative to acknowledge the pivotal role of the cellular antioxidant defense system in preserving cellular homeostasis.
7.5. Antioxidant Defense
The antioxidant system includes both enzymatic (SOD, CAT, GPx) and non-enzymatic components (e.g., glutathione). SOD catalyzes the conversion of superoxide to hydrogen peroxide, while CAT and GPx neutralize it to water and oxygen [136,150].
Higher levels of antioxidant enzymes (e.g., SOD, CAT, TAP) and lower levels of oxidative stress markers (e.g., LPO) have been found to correlate with better IVF outcomes [116]. Furthermore, the role of antioxidant defense in maintaining endometrial receptivity has been demonstrated [151,152].
7.6. Antioxidant Activity of Lactobacilli
Both whole cells and cell lysates of various Lactobacillus spp. have been shown to possess antioxidant properties; however, significant variation in the individual properties of DPPH, TAALA, SOD, and GSH is observed within and among different Lactobacillus strains. In general, all Lactobacillus strains tested, with the exception of L. oris and L. gasseri, exhibited relatively high scores across all antioxidant parameters, suggesting that antioxidant properties are strain-specific. However, it has been demonstrated that only certain Lactobacillus strains possess the capacity to manifest antioxidant activity [153,154,155,156].
A number of studies have reported that certain Lactobacillus strains can be a good natural source of antioxidants, and some strains have also been proven in in vivo studies [157,158]. Probiotic supplementation in rats has been demonstrated to induce the transcription of genes involved in GSH biosynthesis in the intestinal mucosa [159] and to increase GSH synthesis in pancreatic cells [160]. As indicated in the seminal study by Peran et al. (2007) [161], comparable efficacy in restoring GSH levels after oxidative stress has been previously reported in rats supplemented with probiotic strains. Lactobacillus fermentum ME-3 has been demonstrated to elevate the redox ratio of GSH in blood serum [162], while Lactobacillus casei has been shown to curtail lipid peroxidation, thereby enhancing blood lipid metabolism [163].
Certain strains of Lactobacillus have been shown to exhibit elevated antioxidant parameters (Table 6).

Table 6.
Main compounds and enzymes associated with the antioxidant function of LAB.
8. Conclusions
From the scientific research reviewed on the topic, the following main points and guidelines can be summarized:
- Restoration and maintenance of Lactobacillus-dominant microbiota is a promising strategy for reducing gynecological and reproductive risks;
- Probiotic approaches show good results in terms of reducing symptoms and improving the microbiome ecosystem of the reproductive tract, but so far, these studies have focused primarily on the vaginal microbiome and vaginal infections, and not explicitly on the endometrial flora and its relationship with reproductive outcomes;
- The endometrial microbiome may influence the local immune response in the uterus, which is important for protection against pathogens and for tolerating pregnancy;
- Standardization of studies is needed to avoid methodological problems and distortion of results;
- One of the main factors influencing reproductive susceptibility is oxidative stress;
- Some strains of Lactobacillus exhibit relatively high antioxidant parameters, thereby favoring stress management and improving reproductive capacity.
Author Contributions
Conceptualization, G.S. and E.K.; writing—original draft preparation: microbiological and genetic aspects, G.S., cultivation methods, M.G., immunological aspects, N.M., oxidative stress and the female reproductive system, E.K.; writing—review and editing, G.S. and E.K.; supervision, G.S. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Research Fund at the Ministry of Education and Science, Bulgaria, grant number KΠ-06-H83/6 “Investigating the influence of Lactobacillus strains from the endometrial microbiome on factors contributing to successful implantation”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This research was funded by the Scientific Research Fund at the Ministry of Education and Science, Bulgaria, grant number KΠ-06-H83/6, to whom we express our acknowledgments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Paavonen, J.; Critchlow, C.W.; DeRouen, T.; Stevens, C.E.; Kiviat, N.; Brunham, R.C.; Holmes, K.K. Etiology of cervical inflammation. Am. J. Obstet. Gynecol. 1986, 154, 556–564. [Google Scholar] [CrossRef]
- Møller, B.R.; Kristiansen, F.V.; Thorsen, P.; Frost, L.; Mogensen, S.C. Sterility of the uterine cavity. Acta Obstet. Gynecol. Scand. 1995, 74, 216–219. [Google Scholar] [CrossRef]
- Bearfield, C.; Davenport, E.S.; Sivapathasundaram, V.; Allaker, R.P. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG 2002, 109, 527–533. [Google Scholar] [PubMed]
- Stout, M.J.; Conlon, B.; Landeau, M.; Lee, I.; Bower, C.; Zhao, Q.; Mysorekar, I.U. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am. J. Obstet. Gynecol. 2013, 208, 226.e1. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Haick, A.; Nkwopara, E.; Garcia, R.; Rendi, M.; Agnew, K.; Eschenbach, D. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstet. Gynecol. 2015, 212, 611.e1. [Google Scholar] [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Simon, C. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Fang, R.L.; Chen, L.X.; Shu, W.S.; Yao, S.Z.; Wang, S.W.; Chen, Y.Q. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am. J. Transl. Res. 2016, 8, 1581–1592. [Google Scholar]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Van De Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 2016, 4, e1602. [Google Scholar] [CrossRef]
- Altmäe, S.; Rienzi, L. Endometrial microbiome: New hope, or hype? Reprod. Biomed. Online 2021, 42, 1051–1052. [Google Scholar] [CrossRef]
- Cuffaro, F.; Russo, E.; Amedei, A. Endometriosis, pain, and related psychological disorders: Unveiling the interplay among the microbiome, inflammation, and oxidative stress as a common thread. Int. J. Mol. Sci. 2024, 25, 6473. [Google Scholar] [CrossRef]
- Ichiyama, T.; Kuroda, K.; Nagai, Y.; Urushiyama, D.; Ohno, M.; Yamaguchi, T.; Tanaka, A. Analysis of vaginal and endometrial microbiota communities in infertile women with a history of repeated implantation failure. Reprod. Med. Biol. 2021, 20, 334–344. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Jia, H. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Kyono, K.; Hashimoto, T.; Kikuchi, S.; Nagai, Y.; Sakuraba, Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: An analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod. Med. Biol. 2019, 18, 72–82. [Google Scholar] [CrossRef]
- Winters, A.D.; Romero, R.; Gervasi, M.T.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Theis, K.R. Does the endometrial cavity have a molecular microbial signature? Sci. Rep. 2019, 9, 9905. [Google Scholar] [CrossRef]
- Jean, S.; Huang, B.; Parikh, H.I.; Edwards, D.J.; Brooks, J.P.; Kumar, N.G.; Buck, G.A. Multi-omic microbiome profiles in the female reproductive tract in early pregnancy. Infect. Microbes Dis. 2019, 1, 49–60. [Google Scholar] [CrossRef]
- Boroń, D.; Zmarzły, N.; Wierzbik-Strońska, M.; Rosińczuk, J.; Mieszczański, P.; Grabarek, B.O. Recent multiomics approaches in endometrial cancer. Int. J. Mol. Sci. 2022, 23, 1237. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Łaniewski, P.; Adamov, A.; Chase, D.M.; Caporaso, J.G.; Herbst-Kralovetz, M.M. Multi-omics data integration reveals metabolome as the top predictor of the cervicovaginal microenvironment. PLoS Comput. Biol. 2022, 18, e1009876. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Chen, C.; Hao, L.; Li, F.; Song, L.; Zhang, X.; Jia, H. Life history recorded in the vagino-cervical microbiome along with multi-omes. Genom. Proteom. Bioinform. 2022, 20, 304–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Guo, Y.; Jia, L.; Wan, J.; He, T.; Fang, C.; Li, T. Interaction between functionally active endometrial microbiota and host gene regulation in endometrial cancer. Front. Cell Dev. Biol. 2021, 9, 727286. [Google Scholar] [CrossRef] [PubMed]
- Bui, B.N.; van Hoogenhuijze, N.; Viveen, M.; Mol, F.; Teklenburg, G.; de Bruin, J.P.; van de Wijgert, J.H. The endometrial microbiota of women with or without a live birth within 12 months after a first failed IVF/ICSI cycle. Sci. Rep. 2023, 13, 3444. [Google Scholar] [CrossRef] [PubMed]
- Foteinidou, P.; Exindari, M.; Chatzidimitriou, D.; Gioula, G. Endometrial microbiome and its correlation to female infertility: A systematic review and meta-analysis. Acta Microbiol. Hell. 2024, 69, 14–28. [Google Scholar] [CrossRef]
- Su, W.; Gong, C.; Zhong, H.; Yang, H.; Chen, Y.; Wu, X.; Zhao, J. Vaginal and endometrial microbiome dysbiosis associated with adverse embryo transfer outcomes. Reprod. Biol. Endocrinol. 2024, 22, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, X.; Zhao, X.; Du, B. The inconsistent pathogenesis of endometriosis and adenomyosis: Insights from endometrial metabolome and microbiome. mSystems 2025, 10, e00202-25. [Google Scholar] [CrossRef]
- Ye, L.; Dimitriadis, E. Endometrial Receptivity—Lessons from “Omics”. Biomolecules 2025, 15, 106. [Google Scholar] [CrossRef]
- Bwanga, P.K.; Tremblay-Lemoine, P.L.; Timmermans, M.; Ravet, S.; Munaut, C.; Nisolle, M.; Henry, L. The endometrial microbiota: Challenges and prospects. Medicina 2023, 59, 1540. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine microbiota: Residents, tourists, or invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef]
- Moreno, I.; Simon, C. Relevance of assessing the uterine microbiota in infertility. Fertil. Steril. 2018, 110, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Qin, D. Next-generation sequencing and its clinical application. Cancer Biol. Med. 2019, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Korczynska, L.; Zeber-Lubecka, N.; Zgliczynska, M.; Zarychta, E.; Zareba, K.; Wojtyla, C.; Dabrowska, M.; Ciebiera, M. The role of microbiota in the pathophysiology of uterine fibroids—A systematic review. Front. Cell Infect. Microbiol. 2023, 13, 1177366. [Google Scholar] [CrossRef]
- Davidson, I.M.; Nikbakht, E.; Haupt, L.M.; Ashton, K.J.; Dunn, P.J. Methodological approaches in 16S sequencing of female reproductive tract in fertility patients: A review. J. Assist. Reprod. Genet. 2025, 42, 15–37. [Google Scholar] [CrossRef]
- Molina, N.M.; Sola-Leyva, A.; Haahr, T.; Aghajanova, L.; Laudanski, P.; Castilla, J.A.; Altmäe, S. Analysing endometrial microbiome: Methodological considerations and recommendations for good practice. Hum. Reprod. 2021, 36, 859–879. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Ferrari, F.; Ciebiera, M.; Zgliczyńska, M.; Rapisarda, A.M.C.; Vecchio, G.M.; Pino, A.; Angelico, G.; Knafel, A.; Riemma, G.; et al. The role of genital tract microbiome in fertility: A systematic review. Int. J. Mol. Sci. 2022, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Toson, B.; Simon, C.; Moreno, I. The endometrial microbiome and its impact on human conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef]
- Tao, X.; Franasiak, J.M.; Zhan, Y.; Scott, R.T., III; Rajchel, J.; Bedard, J.; Chu, T. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: Next generation sequencing (NGS) analysis of the 16S ribosomal gene. Hum. Microbiome J. 2017, 3, 15–21. [Google Scholar] [CrossRef]
- Moreno, I.; Franasiak, J.M. Endometrial microbiota—New player in town. Fertil. Steril. 2017, 108, 32–39. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Werner, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Scott, R.T. Endometrial microbiome at the time of embryo transfer: Next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef]
- Väinämö, S.; Saqib, S.; Kalliala, I.; Kervinen, K.; Luiro, K.; Niinimäki, M.; Holster, T. Longitudinal analysis of vaginal microbiota during IVF fresh embryo transfer and in early pregnancy. Microbiol. Spectr. 2023, 11, e01650-23. [Google Scholar] [CrossRef]
- Gao, X.; Louwers, Y.; Laven, J.; Schoenmakers, S. Clinical relevance of vaginal and endometrial microbiome investigation in women with repeated implantation failure and recurrent pregnancy loss. Int. J. Mol. Sci. 2024, 25, 622. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yamada, H.; Sasagawa, Y.; Tanimura, K.; Deguchi, M. Uterine endometrium microbiota and pregnancy outcome in women with recurrent pregnancy loss. J. Reprod. Immunol. 2022, 152, 103653. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; Barrionuevo, M.J.; Simon, C. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef]
- Parvanov, D.; Ganeva, R.; Ruseva, M.; Handzhiyska, M.; Tihomirova, T.; Chapanova, S.; Hadjidekova, S. Association between endometrial microbiome and implantation success in women with frozen embryo transfer: Results of a prospective cohort study. Biotechnol. Biotechnol. Equip. 2023, 37, 2250007. [Google Scholar] [CrossRef]
- Odendaal, J.; Black, N.; Bennett, P.R.; Brosens, J.; Quenby, S.; MacIntyre, D.A. The endometrial microbiota and early pregnancy loss. Hum. Reprod. 2024, 39, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Bellver, J.; Gonzalez-Monfort, M.; González, S.; Toson, B.; Labarta, E.; Castillón, G.; Mariani, G.; Vidal, C.; Giles, J.; Cruz, F.; et al. An Analysis of the Digestive and Reproductive Tract Microbiota in Infertile Women with Obesity. Int. J. Mol. Sci. 2024, 25, 12600. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Grau, I.; Perez-Villaroya, D.; Bau, D.; Gonzalez-Monfort, M.; Vilella, F.; Moreno, I.; Simon, C. Taxonomical and functional assessment of the endometrial microbiota in a context of recurrent reproductive failure: A case report. Pathogens 2019, 8, 205. [Google Scholar] [CrossRef]
- Li, F.; Chen, C.; Wei, W.; Wang, Z.; Dai, J.; Hao, L.; Jia, H. The metagenome of the female upper reproductive tract. Gigascience 2018, 7, giy107. [Google Scholar] [CrossRef]
- Sola-Leyva, A.; Andrés-León, E.; Molina, N.M.; Terron-Camero, L.C.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Altmäe, S. Mapping the entire functionally active endometrial microbiota. Hum. Reprod. 2021, 36, 1021–1031. [Google Scholar] [CrossRef]
- Theis, K.R.; Romero, R.; Winters, A.D.; Greenberg, J.M.; Gomez-Lopez, N.; Alhousseini, A.; Hassan, S.S. Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. Am. J. Obstet. Gynecol. 2019, 220, 267.e1. [Google Scholar] [CrossRef] [PubMed]
- Boutriq, S.; González-González, A.; Plaza-Andrades, I.; Laborda-Illanes, A.; Sánchez-Alcoholado, L.; Peralta-Linero, J.; Queipo-Ortuño, M.I. Gut and endometrial microbiome dysbiosis: A new emergent risk factor for endometrial cancer. J. Pers. Med. 2021, 11, 659. [Google Scholar] [CrossRef]
- Balla, B.; Illés, A.; Tobiás, B.; Pikó, H.; Beke, A.; Sipos, M.; Lakatos, P.; Kósa, J. The Role of the Vaginal and Endometrial Microbiomes in Infertility and Their Impact on Pregnancy Outcomes in Light of Recent Literature. Int. J. Mol. Sci. 2024, 25, 13227. [Google Scholar] [CrossRef]
- Cariati, F.; Carotenuto, C.; Bagnulo, F.; Pacella, D.; Marrone, V.; Paolillo, R.; Catania, M.R.; Di Girolamo, R.; Conforti, A.; Strina, I.; et al. Endometrial microbiota profile in in-vitro fertilization (IVF) patients by culturomics-based analysis. Front. Endocrinol. 2023, 14, 1204729. [Google Scholar] [CrossRef]
- Mohammadi, A.; Moini, A.; Falsafi, S.; Feizabadi, M.M. Lactobacilli deficiency in infertile women seeking IVF in Arash Hospital: An imbalance in the genital microbiome. J. Fam. Reprod. Health 2025, 19, 122–127. [Google Scholar] [CrossRef]
- Vanstokstraeten, R.; Mackens, S.; Callewaert, E.; Blotwijk, S.; Emmerechts, K.; Crombé, F.; Soetens, O.; Wybo, I.; Vandoorslaer, K.; Mostert, L.; et al. Culturomics to Investigate the Endometrial Microbiome: Proof-of-Concept. Int. J. Mol. Sci. 2022, 23, 12212. [Google Scholar] [CrossRef]
- Vanstokstraeten, R.; Callewaert, E.; Blotwijk, S.; Rombauts, E.; Crombé, F.; Emmerechts, K.; Soetens, O.; Vandoorslaer, K.; De Geyter, D.; Allonsius, C.; et al. Comparing Vaginal and Endometrial Microbiota Using Culturomics: Proof of Concept. Int. J. Mol. Sci. 2023, 24, 5947. [Google Scholar] [CrossRef]
- Stoyancheva, G.; Marzotto, M.; Dellaglio, F.; Torriani, S. Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch. Microbiol. 2014, 196, 645–653. [Google Scholar] [CrossRef]
- Kovachev, S. Defence factors of vaginal lactobacilli. Crit. Rev. Microbiol. 2018, 44, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Giordani, B.; Abruzzo, A.; Parolin, C.; Foschi, C.; Laghi, L.; Marangoni, A.; Luppi, B.; Vitali, B. Prebiotic activity of vaginal Lactobacilli on Bifidobacteria: From concept to formulation. Microbiol. Spectr. 2023, 11, e02009-22. [Google Scholar] [CrossRef]
- Miko, E.; Barakonyi, A. The role of hydrogen-peroxide (H2O2) produced by vaginal microbiota in female reproductive health. Antioxidants 2023, 12, 1055. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, E.; Menotti, L.; Croatti, V.; Giordani, B.; Parolin, C.; Vitali, B. Protective mechanisms of vaginal Lactobacilli against sexually transmitted viral infections. Int. J. Mol. Sci. 2024, 25, 9168. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Chenoll, E.; Moreno, I.; Sánchez, M.; Garcia-Grau, I.; Silva, Á.; González-Monfort, M.; Genovés, S.; Vilella, F.; Seco-Durban, C.; Simón, C.; et al. Selection of new probiotics for endometrial health. Front. Cell Infect. Microbiol. 2019, 9, 114. [Google Scholar] [CrossRef]
- Pérez-Prieto, I.; Vargas, E.; Salas-Espejo, E.; Lüll, K.; Canha-Gouveia, A.; Pérez, L.A.; Fontes, J.; Salumets, A.; Andreson, R.; Aasmets, O.; et al. Gut microbiome in endometriosis: A cohort study on 1000 individuals. BMC Med. 2024, 22, 294, Correction in BMC Med. 2024, 22, 448. [Google Scholar] [CrossRef]
- Sobstyl, M.; Brecht, P.; Sobstyl, A.; Mertowska, P.; Grywalska, E. The Role of Microbiota in the Immunopathogenesis of Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 5756. [Google Scholar] [CrossRef]
- Baușic, A.; Scurtu, F.; Manu, A.; Matasariu, D.; Brătilă, E. Gut Microbiota Dysbiosis in Endometriosis: A Potential Link to Inflammation and Disease Progression. Int. J. Mol. Sci. 2025, 26, 5144. [Google Scholar] [CrossRef] [PubMed]
- Barczyński, B.; Frąszczak, K.; Grywalska, E.; Kotarski, J.; Korona-Głowniak, I. Vaginal and Cervical Microbiota Composition in Patients with Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 8266. [Google Scholar] [CrossRef]
- Molina, N.M.; Sola-Leyva, A.; Saez-Lara, M.J.; Plaza-Diaz, J.; Tubić-Pavlović, A.; Romero, B.; Altmäe, S. New opportunities for endometrial health by modifying uterine microbial composition: Present or future? Biomolecules 2020, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.; Chan, K.; Kumar, T.; Maissy, E.; Brubaker, L.; Dothard, M.I.; Knight, R. Harnessing the power within: Engineering the microbiome for enhanced gynecologic health. Reprod. Fertil. 2024, 5, e230060. [Google Scholar] [CrossRef] [PubMed]
- Valeriano, V.D.; Lahtinen, E.; Hwang, I.C.; Zhang, Y.; Du, J.; Schuppe-Koistinen, I. Vaginal dysbiosis and the potential of vaginal microbiome-directed therapeutics. Front. Microbiomes 2024, 3, 1363089. [Google Scholar] [CrossRef]
- Mändar, R.; Sõerunurk, G.; Štšepetova, J.; Smidt, I.; Rööp, T.; Kõljalg, S.; Salumets, A. Impact of Lactobacillus crispatus-containing oral and vaginal probiotics on vaginal health: A randomised double-blind placebo controlled clinical trial. Benef. Microbes 2023, 14, 143–152. [Google Scholar] [CrossRef]
- Bayar, E.; MacIntyre, D.A.; Sykes, L.; Mountain, K.; Parks, T.P.; Lee, P.P.; Bennett, P.R. Safety, tolerability, and acceptability of Lactobacillus crispatus CTV-05 (LACTIN-V) in pregnant women at high-risk of preterm birth. Benef. Microbes 2023, 14, 45–56. [Google Scholar] [CrossRef]
- Kadogami, D.; Nakaoka, Y.; Morimoto, Y. Use of a vaginal probiotic suppository and antibiotics to influence the composition of the endometrial microbiota. Reprod. Biol. 2020, 20, 307–314. [Google Scholar] [CrossRef]
- Sakamoto, M.; Tanaka, H.; Enomoto, S.; Takeuchi, H.; Nishioka, M.; Tanaka, K.; Ikeda, T. The efficacy of vaginal treatment for non-Lactobacillus-dominant endometrial microbiota—A case–control study. J. Obstet. Gynaecol. Res. 2024, 50, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, G.; Rosato, R.; Cicchinelli, M.; Iavarone, F.; Urbani, A.; Sanguinetti, M.; Delogu, G.; De Maio, F. The activity of cell-free supernatant of Lactobacillus crispatus M247: A promising treatment against vaginal infections. Front. Cell. Infect. Microbiol. 2025, 15, 1586442. [Google Scholar] [CrossRef] [PubMed]
- Vander Donck, L.; Victor, M.; Van Beeck, W.; Van Rillaer, T.; Dillen, J.; Ahannach, S.; Lebeer, S. Host-independent synergism between Lactobacillus crispatus and other vaginal lactobacilli. Cell Rep. 2025, 44, 9. [Google Scholar] [CrossRef]
- He, X.; Chen, W.; Zhou, X.; Hu, G.; Wei, J.; Liu, Y.; Chen, T. The therapeutic potential of Lactobacillus crispatus for chronic endometritis: A comprehensive clinical trial and experimental investigation. Probiotics Antimicrob. Proteins 2024, 1–19. [Google Scholar] [CrossRef]
- Frąszczak, K.; Barczyński, B.; Kondracka, A. Does Lactobacillus exert a protective effect on the development of cervical and endometrial cancer in women? Cancers 2022, 14, 4909. [Google Scholar] [CrossRef]
- Riaz, M.A.; Kary, F.L.; Jensen, A.; Zeppernick, F.; Meinhold-Heerlein, I.; Konrad, L. Long-Term Maintenance of Viable Human Endometrial Epithelial Cells to Analyze Estrogen and Progestin Effects. Cells 2024, 13, 811. [Google Scholar] [CrossRef]
- Classen-Linke, I.; Buck, V.U.; Sternberg, A.K.; Kohlen, M.; Izmaylova, L.; Leube, R.E. Changes in Epithelial Cell Polarity and Adhesion Guide Human Endometrial Receptivity: How In Vitro Systems Help to Untangle Mechanistic Details. Biomolecules 2025, 15, 1057. [Google Scholar] [CrossRef]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for Study of Human Embryo Implantation: Choice of Cell Lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef]
- Miller, M.M.; Alyea, R.A.; LeSommer, C.; Doheny, D.L.; Rowley, S.M.; Childs, K.M.; Balbuena, P.; Ross, S.M.; Dong, J.; Sun, B.; et al. Editor’s Highlight: Development of an In Vitro Assay Measuring Uterine-Specific Estrogenic Responses for Use in Chemical Safety Assessment. Toxicol. Sci. 2016, 154, 162–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pavlič, R.; Gjorgoska, M.; Hafner, E.; Sinreih, M.; Gajser, K.; Poschner, S.; Jäger, W.; Rižner, T.L. In the Model Cell Lines of Moderately and Poorly Differentiated Endometrial Carcinoma, Estrogens Can Be Formed via the Sulfatase Pathway. Front. Mol. Biosci. 2021, 8, 743403. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Ramanand, S.G.; Cuevas, I.C.; Gao, Y.; Lee, S.; Abbas, A.; Zhang, X.; Kumar, A.; Koduru, P.; Roy, S.; et al. A Distinct Mechanism of Epigenetic Reprogramming Silences PAX2 and Initiates Endometrial Carcinogenesis. J. Clin. Investig. 2025, 135, e190989. [Google Scholar] [CrossRef]
- Wang, H.; Pilla, F.; Martinez-Escribano, S.; Bocca, S.; Oehninger, S.; Horcajadas, J.A. A Novel In Vitro Model of Human Implantation: An Endometrium-like Three-Dimensional (3D) Culture System for Attachment/Invasion of Trophoblast-like Cells. Fertil. Steril. 2010, 94, S64–S65. [Google Scholar] [CrossRef]
- Wang, H.; Pilla, F.; Anderson, S.; Martinez-Escribano, S.; Herrer, I.; Moreno-Moya, J.M.; Musti, S.; Bocca, S.; Oehninger, S.; Horcajadas, J.A. A Novel Model of Human Implantation: 3D Endometrium-like Culture System to Study Attachment of Human Trophoblast (Jar) Cell Spheroids. Mol. Hum. Reprod. 2012, 18, 33–43. [Google Scholar] [CrossRef]
- Stern-Tal, D.; Achache, H.; Jacobs Catane, L.; Reich, R.; Tavor Re’em, T. Novel 3D Embryo Implantation Model within Macroporous Alginate Scaffolds. J. Biol. Eng. 2020, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kodithuwakku, S.P.; Chan, R.W.S.; Yeung, W.S.B.; Yao, Y.; Ng, E.H.Y.; Chiu, P.C.N.; Lee, C.-L. Three-Dimensional Culture Models of Human Endometrium for Studying Trophoblast-Endometrium Interaction during Implantation. Reprod. Biol. Endocrinol. 2022, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Ciprietti, M.; Bueds, C.; Vankelecom, H.; Vriens, J. Organoids as Powerful Models of Endometrium Epithelium in Transcriptomic, Cellular and Functional Mimicry. Cell. Mol. Life Sci. 2025, 82, 272. [Google Scholar] [CrossRef] [PubMed]
- Jabri, A.; Alsharif, M.; Abbad, T.; Taftafa, B.; Mhannayeh, A.; Elsalti, A.; Attia, F.; Mir, T.A.; Saadeldin, I.; Yaqinuddin, A. Endometrial Organoids and Their Role in Modeling Human Infertility. Cells 2025, 14, 829. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Y.; Shen, X.; Zhang, W.; Wang, X.; Wang, K. Evolving from Organoid to Assembloid with Enhanced Cellular Interactions. Cell Organoid. 2025. online ahead of print. Available online: https://www.sciopen.com/article/10.26599/CO.2025.9410010 (accessed on 7 September 2025).
- Kleinová, M.; Varga, I.; Čeháková, M.; Valent, M.; Klein, M. Exploring the Black Box of Human Reproduction: Endometrial Organoids and Assembloids—Generation, Implantation Modeling, and Future Clinical Perspectives. Front. Cell Dev. Biol. 2024, 12, 1482054. [Google Scholar] [CrossRef]
- Benner, M.; Ferwerda, G.; Joosten, I.; Van Der Molen, R.G. How Uterine Microbiota Might Be Responsible for a Receptive, Fertile Endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef]
- Verstraelen, H.; Vieira-Baptista, P.; De Seta, F.; Ventolini, G.; Lonnee-Hoffmann, R.; Lev-Sagie, A. The Vaginal Microbiome: I. Research Development, Lexicon, Defining “Normal” and the Dynamics Throughout Women’s Lives. J. Low. Genit. Tract Dis. 2022, 26, 73–78. [Google Scholar] [CrossRef]
- Karadbhajne, P.; More, A.; Dzoagbe, H.Y. The Role of Endometrial Microbiota in Fertility and Reproductive Health: A Narrative Review. Cureus. 2025. online ahead of print. Available online: https://www.cureus.com/articles/253962-the-role-of-endometrial-microbiota-in-fertility-and-reproductive-health-a-narrative-review (accessed on 8 September 2025).
- Carosso, A.; Revelli, A.; Gennarelli, G.; Canosa, S.; Cosma, S.; Borella, F.; Tancredi, A.; Paschero, C.; Boatti, L.; Zanotto, E.; et al. Controlled Ovarian Stimulation and Progesterone Supplementation Affect Vaginal and Endometrial Microbiota in IVF Cycles: A Pilot Study. J. Assist. Reprod. Genet. 2020, 37, 2315–2326. [Google Scholar] [CrossRef]
- Sirota, I.; Zarek, S.; Segars, J. Potential Influence of the Microbiome on Infertility and Assisted Reproductive Technology. Semin. Reprod. Med. 2014, 32, 35–42. [Google Scholar] [CrossRef]
- Cela, V.; Daniele, S.; Obino, M.E.R.; Ruggiero, M.; Zappelli, E.; Ceccarelli, L.; Papini, F.; Marzi, I.; Scarfò, G.; Tosi, F.; et al. Endometrial Dysbiosis Is Related to Inflammatory Factors in Women with Repeated Implantation Failure: A Pilot Study. J. Clin. Med. 2022, 11, 2481. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Scott, R.T. Endometrial Microbiome. Curr. Opin. Obstet. Gynecol. 2017, 29, 146–152. [Google Scholar] [CrossRef]
- Guo, L.; Guo, A.; Yang, F.; Li, L.; Yan, J.; Deng, X.; Dai, C.; Li, Y. Alterations of Cytokine Profiles in Patients With Recurrent Implantation Failure. Front. Endocrinol. 2022, 13, 949123. [Google Scholar] [CrossRef]
- Bhagwat, S.R.; Chandrashekar, D.S.; Kakar, R.; Davuluri, S.; Bajpai, A.K.; Nayak, S.; Bhutada, S.; Acharya, K.; Sachdeva, G. Endometrial Receptivity: A Revisit to Functional Genomics Studies on Human Endometrium and Creation of HGEx-ERdb. PLoS ONE 2013, 8, e58419. [Google Scholar] [CrossRef] [PubMed]
- Altmäe, S.; Martinez-Conejero, J.A.; Esteban, F.J.; Ruiz-Alonso, M.; Stavreus-Evers, A.; Horcajadas, J.A.; Salumets, A. microRNAs miR-30b, miR-30d, and miR-494 Regulate Human Endometrial Receptivity. Reprod. Sci. 2013, 20, 308–317. [Google Scholar] [CrossRef]
- Altmäe, S.; Koel, M.; Võsa, U.; Adler, P.; Suhorutšenko, M.; Laisk-Podar, T.; Kukushkina, V.; Saare, M.; Velthut-Meikas, A.; Krjutškov, K.; et al. Meta-signature of Human Endometrial Receptivity: A Meta-analysis and Validation Study of Transcriptomic Biomarkers. Sci. Rep. 2017, 7, 10077. [Google Scholar] [CrossRef]
- Dimitriadis, E.; White, C.A.; Jones, R.L.; Salamonsen, L.A. Cytokines, Chemokines and Growth Factors in Endometrium Related to Implantation. Hum. Reprod. Update 2005, 11, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of Implantation: Strategies for Successful Pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Chaudhry, P.; Asselin, E. Bridging Endometrial Receptivity and Implantation: Network of Hormones, Cytokines, and Growth Factors. J. Endocrinol. 2011, 210, 5–14. [Google Scholar] [CrossRef]
- Achache, H.; Revel, A. Endometrial Receptivity Markers, the Journey to Successful Embryo Implantation. Hum. Reprod. Update 2006, 12, 731–746. [Google Scholar] [CrossRef]
- Gnainsky, Y.; Granot, I.; Aldo, P.B.; Barash, A.; Or, Y.; Schechtman, E.; Mor, G.; Dekel, N. Local Injury of the Endometrium Induces an Inflammatory Response That Promotes Successful Implantation. Fertil. Steril. 2010, 94, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Ashary, N.; Tiwari, A.; Modi, D. Embryo Implantation: War in Times of Love. Endocrinology 2018, 159, 1188–1198. [Google Scholar] [CrossRef]
- Moffett, A.; Colucci, F. Uterine NK Cells: Active Regulators at the Maternal-Fetal Interface. J. Clin. Investig. 2014, 124, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Hiby, S.E.; Apps, R.; Sharkey, A.M.; Farrell, L.E.; Gardner, L.; Mulder, A.; Claas, F.H.J.; Walker, J.J.; Redman, C.W.G.; Morgan, L.; et al. Maternal KIR in Combination with Paternal HLA-C Regulate Human Birth Weight. J. Immunol. 2010, 185, 116–1124. [Google Scholar] [CrossRef] [PubMed]
- Houser, B.L. Decidual Macrophages and Their Roles at the Maternal–Fetal Interface. Yale J. Biol. Med. 2012, 85, 105–118. [Google Scholar] [PubMed]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T Cells Mediate Maternal Tolerance to the Fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef]
- Agarwal, S.; Sharma, K.; Swanson, B.G.; Yüksel, G.Ü.; Clark, S. Nonstarter Lactic Acid Bacteria Biofilms and Calcium Lactate Crystals in Cheddar Cheese. J. Dairy Sci. 2006, 89, 1452–1466. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sharma, N.; Chaudhry, R.; Deorari, A.; Paul, V.K.; Gewolb, I.H.; Panigrahi, P. Effects of Oral Lactobacillus GG on Enteric Microflora in Low-Birth-Weight Neonates. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 397–402. [Google Scholar] [CrossRef]
- Murphy, C.; Murphy, S.; O’Brien, F.; O’Donoghue, M.; Boileau, T.; Sunvold, G.; O’Mahony, L. Metabolic Activity of Probiotics—Oxalate Degradation. Vet. Microbiol. 2009, 136, 100–107. [Google Scholar] [CrossRef]
- Montuschi, P.; Barnes, P.; Jackson Roberts, L. Insights into Oxidative Stress: The Isoprostanes. Curr. Med. Chem. 2007, 14, 703–717. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Rahiminejad, M.E.; Moaddab, A.; Ganji, M.; Eskandari, N.; Yepez, M.; Rabiee, S.; Ranjbar, A. Oxidative Stress Biomarkers in Endometrial Secretions: A Comparison between Successful and Unsuccessful In Vitro Fertilization Cycles. J. Reprod. Immunol. 2016, 116, 70–75. [Google Scholar] [CrossRef]
- Malvezzi, H.; Cestari, B.A.; Meola, J.; Podgaec, S. Higher Oxidative Stress in Endometriotic Lesions Upregulates Senescence-Associated p16ink4a and β-Galactosidase in Stromal Cells. Int. J. Mol. Sci. 2023, 24, 914. [Google Scholar] [CrossRef] [PubMed]
- Didziokaite, G.; Biliute, G.; Gudaite, J.; Kvedariene, V. Oxidative Stress as a Potential Underlying Cause of Minimal and Mild Endometriosis-Related Infertility. Int. J. Mol. Sci. 2023, 24, 3809. [Google Scholar] [CrossRef]
- Stein, T.P.; Scholl, T.O.; Schluter, M.D.; Leskiw, M.J.; Chen, X.; Spur, B.W.; Rodriguez, A. Oxidative Stress Early in Pregnancy and Pregnancy Outcome. Free Radic. Res. 2008, 42, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Impact of Oxidative Stress on Male and Female Germ Cells: Implications for Fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [PubMed]
- Ra, K.; Park, S.C.; Lee, B.C. Female Reproductive Aging and Oxidative Stress: Mesenchymal Stem Cell Conditioned Medium as a Promising Antioxidant. Int. J. Mol. Sci. 2023, 24, 5053. [Google Scholar] [CrossRef]
- Oyawoye, O.; Abdel Gadir, A.; Garner, A.; Constantinovici, N.; Perrett, C.; Hardiman, P. Antioxidants and Reactive Oxygen Species in Follicular Fluid of Women Undergoing IVF: Relationship to Outcome. Hum. Reprod. 2003, 18, 2270–2274. [Google Scholar] [CrossRef]
- Combelles, C.M.; Gupta, S.; Agarwal, A. Could Oxidative Stress Influence the In-Vitro Maturation of Oocytes? Reprod. Biomed. Online 2009, 18, 864–880. [Google Scholar] [CrossRef]
- Borowiecka, M.; Wojsiat, J.; Polac, I.; Radwan, M.; Radwan, P.; Zbikowska, H.M. Oxidative Stress Markers in Follicular Fluid of Women Undergoing In Vitro Fertilization and Embryo Transfer. Syst. Biol. Reprod. Med. 2012, 58, 301–305. [Google Scholar] [CrossRef] [PubMed]
- García-Gil, F.A.; Albendea, C.D.; López-Pingarrón, L.; Royo-Dachary, P.; Martínez-Guillén, J.; Piedrafita, E.; Martínez-Díez, M.; Soria, J.; García, J.J. Altered Cellular Membrane Fluidity Levels and Lipid Peroxidation During Experimental Pancreas Transplantation. J. Bioenerg. Biomembr. 2012, 44, 571–577. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Y.; Ozatik, O.; Hassa, H.; Ulusoy, D.; Ogut, S.; Sahin, F. Relationship between oxidative stress and clinical pregnancy in assisted reproductive technology treatment cycles. JARG 2013, 30, 765–772, Correction in JARG 2014, 31, 1569. [Google Scholar] [CrossRef]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Tanbo, T.; Fedorcsak, P. Endometriosis-Associated Infertility: Aspects of Pathophysiological Mechanisms and Treatment Options. Acta Obstet. Gynecol. Scand. 2017, 96, 659–667. [Google Scholar] [CrossRef]
- Amini, L.; Chekini, R.; Nateghi, M.R.; Haghani, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res. Manag. 2021, 2021, 5529741. [Google Scholar] [CrossRef]
- Cacciottola, L.; Donnez, J.; Dolmans, M.M. Oxidative Stress, Mitochondria, and Infertility: Is the Relationship Fully Established? Fertil. Steril. 2021, 116, 306–308. [Google Scholar] [CrossRef]
- Yoshino, O.; Osuga, Y.; Hirota, Y.; Koga, K.; Hirata, T.; Harada, M.; Morimoto, C.; Yano, T.; Nishii, O.; Tsutsumi, O.; et al. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am. J. Reprod. Immunol. 2004, 52, 306–311. [Google Scholar] [CrossRef]
- Yotova, I.Y.; Quan, P.; Leditznig, N.; Beer, U.; Wenzl, R.; Tschugguel, W. Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum. Reprod. 2011, 26, 885–897. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Co-operation between the AKT and ERK signaling pathways may support growth of deep endometriosis in a fibrotic microenvironment in vitro. Hum. Reprod. 2015, 30, 1606–1616. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. RBE 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Ansariniya, H.; Yavari, A.; Javaheri, A.; Zare, F. Oxidative stress-related effects on various aspects of endometriosis. Am. J. Reprod. Immunol. 2022, 88, e13593. [Google Scholar] [CrossRef] [PubMed]
- Bane, K.; Desouza, J.; Shetty, D.; Choudhary, P.; Kadam, S.; Katkam, R.R.; Fernandes, G.; Sawant, R.; Dudhedia, U.; Warty, N.; et al. Endometrial DNA damage response is modulated in endometriosis. Hum. Reprod. 2021, 36, 160–174. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, T.; Xu, L.; Wang, Q.; Li, H.; Wang, X. Extracellular superoxide produced by Enterococcus faecalis reduces endo-metrial receptivity via inflammatory injury. Am. J. Reprod. Immunol. 2021, 86, e13453. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Khan, T.A.; Amjad, S.; Rehman, R. Association of oxidative stress with female infertility—A case control study. J. Pak. Med. Assoc. 2019, 69, 627–631. [Google Scholar]
- Chuang, H.C.; Tsai, K.L.; Tsai, K.J.; Tu, T.Y.; Shyong, Y.J.; Jou, I.M.; Hsu, C.C.; Shih, S.S.; Liu, Y.F.; Lin, C.L. Oxidative Stress Mediates Age-Related Hypertrophy of Ligamentum Flavum by Inducing Inflammation, Fibrosis, and Apoptosis through Activating Akt and MAPK Pathways. Aging 2020, 12, 24168–24183. [Google Scholar] [CrossRef]
- Uimari, O.; Rahmioglu, N.; Nyholt, D.R.; Vincent, K.; Missmer, S.A.; Becker, C.; Morris, A.P.; Montgomery, G.W.; Zondervan, K.T. Genome-Wide Genetic Analyses Highlight Mitogen-Activated Protein Kinase (MAPK) Signaling in the Pathogenesis of Endometriosis. Hum. Reprod. 2017, 32, 780–793. [Google Scholar] [CrossRef]
- Borodkina, A.V.; Shatrova, A.N.; Nikolsky, N.N.; Burova, E.B. The Role of p38 MAP-Kinase in Stress-Induced Senescence of Human Endometrium-Derived Mesenchymal Stem Cells. Cell Tissue Biol. 2016, 10, 365–371. [Google Scholar] [CrossRef]
- Borodkina, A.; Shatrova, A.; Abushik, P.; Nikolsky, N.; Burova, E. Interaction Between ROS Dependent DNA Damage, Mitochondria and p38 MAPK Underlies Senescence of Human Adult Stem Cells. Aging 2014, 6, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Burova, E.; Borodkina, A.; Shatrova, A.; Nikolsky, N. Sublethal Oxidative Stress Induces the Premature Senescence of Human Mesenchymal Stem Cells Derived from Endometrium. Oxidative Med. Cell. Longev. 2013, 2013, 474931. [Google Scholar] [CrossRef]
- Agarwal, A.; Aziz, N.; Rizk, B. Oxidative Stress Endometrium. In Studies on Women’s Health; Springer: New York, NY, USA, 2013; pp. 1–363. [Google Scholar] [CrossRef]
- Deryabin, P.I.; Ivanova, J.S.; Borodkina, A.V. Senescence of Stromal Cells Contributes to Endometrium Dysfunction and Embryo Implantation Failure. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lessey, B.A.; Kim, J.J. Endometrial Receptivity in the Eutopic Endometrium of Women with Endometriosis: It Is Affected, and Let Me Show You Why. Fertil. Steril. 2017, 108, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Poli-Neto, O.B.; Meola, J.; Rosa-e-Silva, J.C.; Tiezzi, D. Transcriptome Meta-Analysis Reveals Differences of Immune Profile Between Eutopic Endometrium from Stage I–II and III–IV Endometriosis Independently of Hormonal Milieu. Sci. Rep. 2020, 10, 313. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants—Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Guerin, P.; El Mouatassim, S.; Menezo, Y. Oxidative Stress and Protection Against Reactive Oxygen Species in the Pre-Implantation Embryo and Its Surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Boomsma, C.M.; Kavelaars, A.; Eijkemans, M.J.C.; Lentjes, E.G.; Fauser, B.C.J.M.; Heijnen, C.J.; Macklon, N.S. Endometrial Secretion Analysis Identifies a Cytokine Profile Predictive of Pregnancy in IVF. Hum. Reprod. 2009, 24, 1427–1435. [Google Scholar] [CrossRef]
- Sun, J.; Hu, X.L.; Le, G.W.; Shi, Y.H. Lactobacilli Prevent Hydroxy Radical Production and Inhibit Escherichia coli and Enterococcus Growth in System Mimicking Colon Fermentation. Lett. Appl. Microbiol. 2009, 50, 264–269. [Google Scholar] [CrossRef]
- Achuthan, A.A.; Duary, R.K.; Madathil, A.; Panwar, H.; Kumar, H.; Batish, V.K. Antioxidative Potential of Lactobacilli Isolated from the Gut of Indian People. Mol. Biol. Rep. 2012, 39, 7887–7897. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Y.; Zhang, L.; Zhang, X.; Huang, L.; Li, D. Antioxidant Activity of Lactobacillus plantarum Strains Isolated from Traditional Chinese Fermented Foods. Food Chem. 2012, 135, 1914–1919. [Google Scholar] [CrossRef]
- Ahmadi, M.F.; Goodarzi, M.T.; Hendi, S.S.; Kasraei, S.; Moghimbeigi, A. Total Antioxidant Capacity of Saliva and Dental Caries. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e553–e556. [Google Scholar] [CrossRef]
- Songisepp, E.; Kals, J.; Kullisaar, T.; Mandar, R.; Hutt, P.; Zilmer, M. Evaluation of the Functional Efficacy of an Antioxidative Probiotic in Healthy Volunteers. Nutr. J. 2005, 4, 22. [Google Scholar] [CrossRef]
- Amaretti, A.; di Nunzio, M.; Pompei, A.; Raimondi, S.; Rossi, M.; Bordoni, A. Antioxidant Properties of Potentially Probiotic Bacteria: In Vitro and In Vivo Activities. Appl. Microbiol. Biotechnol. 2013, 97, 809–817. [Google Scholar] [CrossRef]
- Lutgendorff, F.; Nijmeijer, R.M.; Sandström, P.A.; Trulsson, L.M.; Magnusson, K.E.; Timmerman, H.M. Probiotics Prevent Intestinal Barrier Dysfunction in Acute Pancreatitis in Rats via Induction of Ileal Mucosal Glutathione Biosynthesis. PLoS ONE 2009, 4, e4512. [Google Scholar] [CrossRef]
- Lutgendorff, F.; Trulsson, L.M.; van Minnen, L.P.; Rijkers, G.T.; Timmerman, H.M.; Franzén, L.E. Probiotics Enhance Pancreatic Glutathione Biosynthesis and Reduce Oxidative Stress in Experimental Acute Pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1111–G1121. [Google Scholar] [CrossRef]
- Peran, L.; Camuesco, D.; Comalada, M.; Nieto, A.; Concha, A.; Adrio, J.L. Lactobacillus fermentum, a Probiotic Capable to Release Glutathione, Prevents Colonic Inflammation in the TNBS Model of Rat Colitis. Int. J. Color. Dis. 2006, 21, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Mikelsaar, M.; Zilmer, M. Lactobacillus fermentum ME-3–An Antimicrobial and Antioxidative Probiotic. Microb. Ecol. Health Dis. 2009, 21, 1–27. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Du, R.; Wang, L.; Zhang, H. The Antioxidative Effects of Probiotic Lactobacillus casei Zhang on the Hyperlipidemic Rats. Eur. Food Res. Technol. 2010, 231, 151–155. [Google Scholar] [CrossRef]
- Kullisaar, T.; Songisepp, E.; Aunapuu, M.; Kilk, K.; Arend, A.; Mikelsaar, M.; Zilmer, M. Complete Glutathione System in Probiotic Lactobacillus fermentum ME-3. Appl. Biochem. Microbiol. 2010, 46, 481–486. [Google Scholar] [CrossRef]
- Kim, I.S.; Kim, Y.S.; Yoon, H.S. Glutathione Reductase from Oryza sativa Increases Acquired Tolerance to Abiotic Stresses in a Genetically Modified Saccharomyces cerevisiae Strain. J. Microbiol. Biotechnol. 2012, 22, 1557–1567. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y. Engineering the antioxidative properties of lactic acid bacteria for improving its robustness. Curr. Opin. Biotechnol. 2013, 24, 142–147. [Google Scholar] [CrossRef]
- Al-Madboly, L.A.; Ali, S.M.; Fakharany, E.M.E.; Ragab, A.E.; Khedr, E.G.; Elokely, K.M. Stress-Based Production and Characterization of Glutathione Peroxidase and Glutathione S-Transferase Enzymes from Lactobacillus plantarum. Front. Bioeng. Biotechnol. 2020, 8, 78. [Google Scholar] [CrossRef]
- Lee, M.; Cho, T.; Jantaratnotai, N.; Wang, Y.T.; McGeer, E.; McGeer, P.L. Depletion of GSH in Glial Cells Induces Neurotoxicity: Relevance to Aging and Degenerative Neurological Diseases. FASEB J. 2010, 24, 2533–2545. [Google Scholar] [CrossRef] [PubMed]
- Hamon, E.; Horvatovich, P.; Izquierdo, E.; Bringel, F.; Marchioni, E.; Aoudé-Werner, D.; Ennahar, S. Comparative Proteomic Analysis of Lactobacillus plantarum for the Identification of Key Proteins in Bile Tolerance. BMC Microbiol. 2011, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Jänsch, A.; Korakli, M.; Vogel, R.F.; Gänzle, M.G. Glutathione Reductase from Lactobacillus sanfranciscensis DSM20451T: Contribution to Oxygen Tolerance and Thiol Exchange Reactions in Wheat Sourdoughs. Appl. Environ. Microbiol. 2007, 73, 4469–4476. [Google Scholar] [CrossRef] [PubMed]
- Bryukhanov, A.L.; Klimko, A.I.; Netrusov, A.I. Antioxidant Properties of Lactic Acid Bacteria. Microbiology 2022, 91, 463–478. [Google Scholar] [CrossRef]
- Serrano, J.; Goñi, I.; Saura-Calixto, F. Food antioxidant capacity determined by chemical methods may underestimate the physiological antioxidant capacity. Food Res. Int. 2007, 40, 15–21. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Jastrząb, R.; Graczyk, D.; Siedlecki, P. Molecular and cellular mechanisms influenced by postbiotics. Int. J. Mol. Sci. 2021, 22, 13475. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).