The Elusive B Cell Antigen in Multiple Sclerosis: Time to Rethink CNS B Cell Functions
Abstract
1. Introduction
1.1. Involvement of B Cells in the Pathogenesis of MS
1.2. Functions of B Cells
1.3. Distinctions from Previously Characterized Antibody-Mediated Demyelinating Disorders
1.4. Examining Antibody-Repertoires: Methodological Challenges
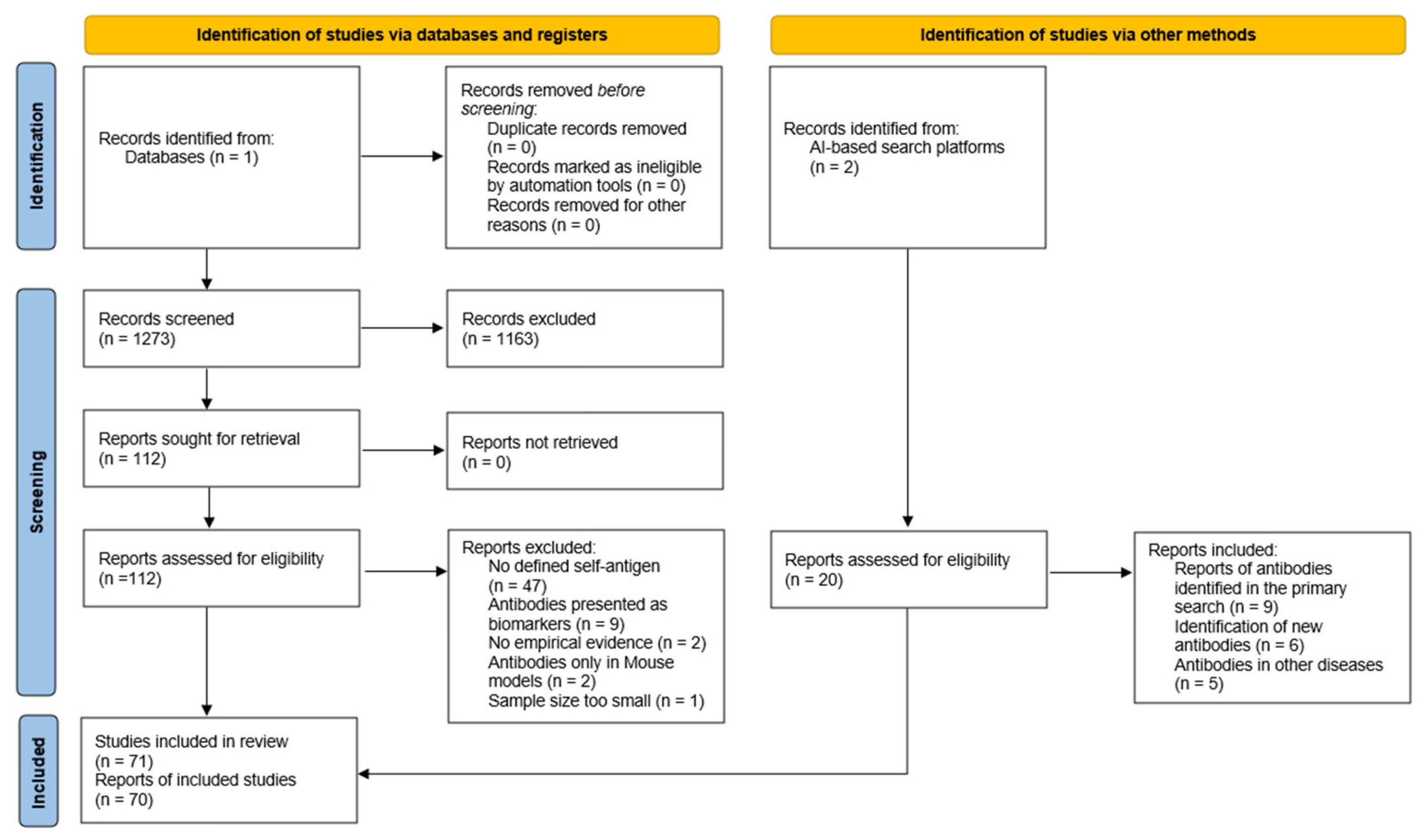
2. Methods
3. Results
3.1. Components of the Myelin Sheath: Proteins and Glycolipids
3.2. Axonal, Cytoskeletal, and Neuronal Antigens
3.3. Heat Shock Proteins
3.4. Ion Channels
3.5. Metabolic Enzymes and Miscellaneous Antigens
3.6. Autoantibodies as Possible Biomarkers
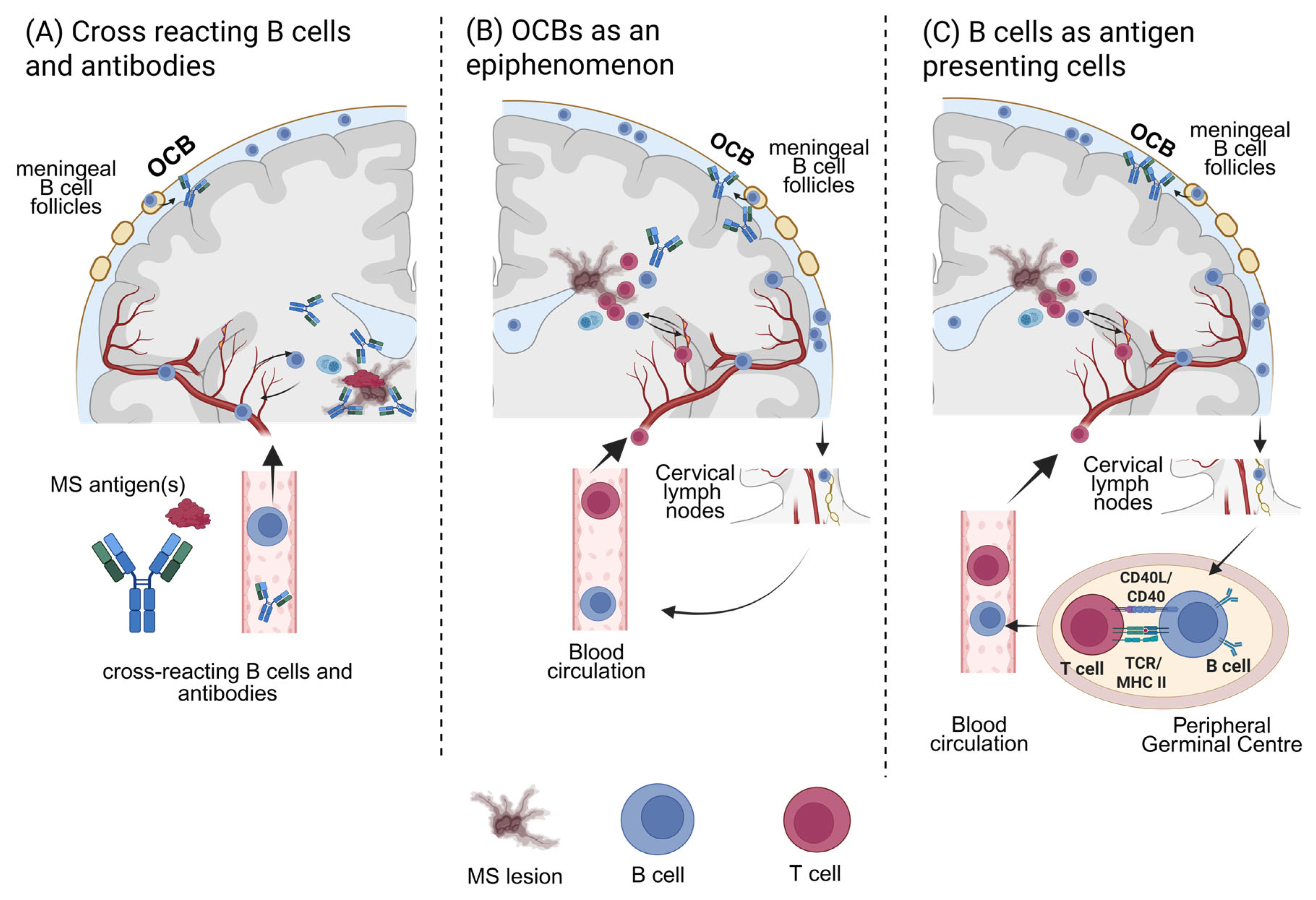
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MS | Multiple Sclerosis |
| CSF | Cerebrospinal fluid |
| CNS | Central nervous system |
| OCB | Oligoclonal Bands |
| BCR | B cell receptor |
| NMOSD | Neuromyelitis spectrum disorder |
| MOGAD | Myelin oligodendrocyte glycoprotein antibody-associated disease |
| AQP4 | Aquaporin-4 |
| MOG | Myelin oligodendrocyte glycoprotein |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| BBB | Blood–brain barrier |
| AI | Artificial Intelligence |
| ELISA | Enzyme-linked Immunosorbent Assay |
| RIA | Radioimmunoassay |
| ELISPOT | Enzyme-linked Immunospot Assay |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
| MaSp | Mass Spectrometry |
| PLP | Proteolipid Protein |
| MBP | Myelin Basic Protein |
| MAG | Myelin-associated Glykoprotein |
| Cx32 | Connexin-32 |
| CNPase | 2′,3′-cyclic nucleotide 3′-phosphodiesterase |
| EAE | Experimental autoimmune Encephalomyelitis |
| EBNA1 | Epstein–Barr virus nuclear antigen 1 |
| OND | Other neurological disorders |
| SLE | Systemic lupus erythematosus |
| MOBP | Myelin Oligodendrocyte Basic Protein |
| ADEM | Acute disseminated encephalomyelitis |
| OSP | Oligodendrocyte-specific Protein |
| RRMS | Relapsing-remitting Multiple Sclerosis |
| PPMS | Primary-progressive Multiple Sclerosis |
| SPMS | Secondary-progressive Multiple Sclerosis |
| CSL | Cerebellar Soluble Lectin |
| HIV | Human Immunodeficiency Virus |
| GlialCAM | Glial cell adhesion molecule |
| ANO2 | Anoctamin 2 |
| CRYAB | Alpha B crystallin |
| GalC | Galactocerebroside |
| GBS | Guillain–Barré syndrome |
| CIDP | Chronic inflammatory demyelinating polyneuropathy |
| Nf | Neurofascins |
| SPAG16 | Sperm-associated antigen 16 |
| Neu5Ac | N-acetylneuraminic acid |
| Neu5Gc | N-glycolylneuraminic acid |
| HSP | Heat shock protein |
| VGKC | Voltage-gated Potassium Channel |
| KIR4.1 | Potassium channel KIR4.1 |
| HTLV-1 | Human T-cell lymphotropic virus |
| RBPJ | Recombination signal binding protein for immunoglobulin kappa J region |
| NIND | Non-inflammatory neurological disorders |
| OIND | Other inflammatory neurological disorders |
| TG6 | Transglutaminase 6 |
| CLIC1 | Chloride intracellular channel protein 1 |
| EBV | Epstein–Barr virus |
References
- Disanto, G.; Morahan, J.M.; Barnett, M.H.; Giovannoni, G.; Ramagopalan, S.V. The evidence for a role of B cells in multiple sclerosis. Neurology 2012, 78, 823–832. [Google Scholar] [CrossRef]
- Kumar, G.; Axtell, R.C. Dual Role of B Cells in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 2336. [Google Scholar] [CrossRef]
- Comi, G.; Bar-Or, A.; Lassmann, H.; Uccelli, A.; Hartung, H.; Montalban, X.; Sørensen, P.S.; Hohlfeld, R.; Hauser, S.L. Expert Panel of the 27th Annual Meeting of the European Charcot Foundation Role of B Cells in Multiple Sclerosis and Related Disorders. Ann. Neurol. 2021, 89, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ruschil, C.; Kemmerer, C.L.; Beller, L.; Gabernet, G.; Kowarik, M.C. Next Generation Sequencing of Cerebrospinal Fluid B Cell Repertoires in Multiple Sclerosis and Other Neuro-Inflammatory Diseases—A Comprehensive Review. Diagnostics 2021, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Patterson, K.R.; Bar-Or, A. Reassessing B cell contributions in multiple sclerosis. Nat. Immunol. 2018, 19, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Blauth, K.; Owens, G.P.; Bennett, J.L. The Ins and Outs of B Cells in Multiple Sclerosis. Front. Immunol. 2015, 6, 565. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.; Vora, A.; Serafini, B.; Nicholas, R.; Puopolo, M.; Reynolds, R.; Aloisi, F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2006, 130, 1089–1104. [Google Scholar] [CrossRef]
- Obermeier, B.; Mentele, R.; Malotka, J.; Kellermann, J.; Kümpfel, T.; Wekerle, H.; Lottspeich, F.; Hohlfeld, R.; Dornmair, K. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat. Med. 2008, 14, 688–693. [Google Scholar] [CrossRef]
- Obermeier, B.; Lovato, L.; Mentele, R.; Brück, W.; Forne, I.; Imhof, A.; Lottspeich, F.; Turk, K.W.; Willis, S.N.; Wekerle, H.; et al. Related B cell clones that populate the CSF and CNS of patients with multiple sclerosis produce CSF immunoglobulin. J. Neuroimmunol. 2011, 233, 245–248. [Google Scholar] [CrossRef]
- Michel, L.; Touil, H.; Pikor, N.B.; Gommerman, J.L.; Prat, A.; Bar-Or, A. B Cells in the Multiple Sclerosis Central Nervous System: Trafficking and Contribution to CNS-Compartmentalized Inflammation. Front. Immunol. 2015, 6, 636. [Google Scholar] [CrossRef]
- Choi, S.R.; Howell, O.W.; Carassiti, D.; Magliozzi, R.; Gveric, D.; Muraro, P.A.; Nicholas, R.; Roncaroli, F.; Reynolds, R. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain 2012, 135, 2925–2937. [Google Scholar] [CrossRef]
- Absinta, M.; Vuolo, L.; Rao, A.; Nair, G.; Sati, P.; Cortese, I.C.M.; Ohayon, J.; Fenton, K.; Reyes-Mantilla, M.I.; Maric, D.; et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology 2015, 85, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Waubant, E.; Arnold, D.L.; Vollmer, T.; Antel, J.; Fox, R.J.; Bar-Or, A.; Panzara, M.; Sarkar, N.; Agarwal, S.; et al. B-Cell Depletion with Rituximab in Relapsing–Remitting Multiple Sclerosis. N. Engl. J. Med. 2008, 358, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; De Seze, J.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef]
- Hauser, S.L.; Bar-Or, A.; Cohen, J.A.; Comi, G.; Correale, J.; Coyle, P.K.; Cross, A.H.; De Seze, J.; Leppert, D.; Montalban, X.; et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N. Engl. J. Med. 2020, 383, 546–557. [Google Scholar] [CrossRef]
- Steinman, L.; Fox, E.; Hartung, H.-P.; Alvarez, E.; Qian, P.; Wray, S.; Robertson, D.; Huang, D.; Selmaj, K.; Wynn, D.; et al. Ublituximab versus Teriflunomide in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2022, 387, 704–714. [Google Scholar] [CrossRef]
- Pieper, K.; Grimbacher, B.; Eibel, H. B-cell biology and development. J. Allergy Clin. Immunol. 2013, 131, 959–971. [Google Scholar] [CrossRef]
- Shen, P.; Fillatreau, S. Antibody-independent functions of B cells: A focus on cytokines. Nat. Rev. Immunol. 2015, 15, 441–451. [Google Scholar] [CrossRef]
- Lund, F.E.; Randall, T.D. Effector and regulatory B cells: Modulators of CD4+ T cell immunity. Nat. Rev. Immunol. 2010, 10, 236–247. [Google Scholar] [CrossRef]
- Avalos, A.M.; Ploegh, H.L. Early BCR Events and Antigen Capture, Processing, and Loading on MHC Class II on B Cells. Front. Immunol. 2014, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Tucker, H.; Drake, L.; Nichol, K.; Drake, J.R. Antigen-B Cell Receptor Complexes Associate with Intracellular major histocompatibility complex (MHC) Class II Molecules. J. Biol. Chem. 2015, 290, 27101–27112. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.N.; Jiang, W.; Bhamidipati, K.; Millican, M.; Macaubas, C.; Hung, S.; Mellins, E.D. The Other Function: Class II-Restricted Antigen Presentation by B Cells. Front. Immunol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.A.; Song, N.; Sadegh-Nasseri, S. How Does B Cell Antigen Presentation Affect Memory CD4 T Cell Differentiation and Longevity? Front. Immunol. 2021, 12, 677036. [Google Scholar] [CrossRef]
- Lennon, V.A.; Wingerchuk, D.M.; Kryzer, T.J.; Pittock, S.J.; Lucchinetti, C.F.; Fujihara, K.; Nakashima, I.; Weinshenker, B.G. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet 2004, 364, 2106–2112. [Google Scholar] [CrossRef]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.S.; Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef]
- Weber, M.S.; Derfuss, T.; Metz, I.; Brück, W. Defining distinct features of anti-MOG antibody associated central nervous system demyelination. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418762083. [Google Scholar] [CrossRef]
- Sechi, E. NMOSD and MOGAD. Continuum 2024, 30, 1052–1087. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.-M.; Zhang, X.-X.; Liu, Y.-O.; Li, S.-Z.; Liu, Z.; Dong, H.-Q. Clinical Features of Patients with Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders. Chin. Med. J. 2016, 129, 2079–2084. [Google Scholar] [CrossRef]
- Vlad, B.; Reichen, I.; Neidhart, S.; Hilty, M.; Lekaditi, D.; Heuer, C.; Eisele, A.; Ziegler, M.; Reindl, M.; Lutterotti, A.; et al. Basic CSF parameters and MRZ reaction help in differentiating MOG antibody-associated autoimmune disease versus multiple sclerosis. Front. Immunol. 2023, 14, 1237149. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; De Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef]
- Ikeguchi, R.; Kanda, N.; Kobayashi, M.; Masui, K.; Nitta, M.; Misu, T.; Muragaki, Y.; Kawamata, T.; Shibata, N.; Kitagawa, K.; et al. CNS B cell infiltration in tumefactive anti-myelin oligodendrocyte glycoprotein antibody-associated disease. Mult. Scler. J.—Exp. Transl. Clin. 2024, 10, 20552173241301011. [Google Scholar] [CrossRef] [PubMed]
- Banwell, B.; Bennett, J.L.; Marignier, R.; Kim, H.J.; Brilot, F.; Flanagan, E.P.; Ramanathan, S.; Waters, P.; Tenembaum, S.; Graves, J.S.; et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023, 22, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Stellmann, J.-P.; Krumbholz, M.; Friede, T.; Gahlen, A.; Borisow, N.; Fischer, K.; Hellwig, K.; Pache, F.; Ruprecht, K.; Havla, J.; et al. Immunotherapies in neuromyelitis optica spectrum disorder: Efficacy and predictors of response. J. Neurol. Neurosurg. Psychiatry 2017, 88, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Traub, J.; Häusser-Kinzel, S.; Weber, M. Differential Effects of MS Therapeutics on B Cells—Implications for Their Use and Failure in AQP4-Positive NMOSD Patients. Int. J. Mol. Sci. 2020, 21, 5021. [Google Scholar] [CrossRef]
- Pitter, J.G.; Nagy, L.; Nagy, B.; Hren, R. Development Perspectives for Curative Technologies in Primary Demyelinating Disorders of the Central Nervous System with Neuromyelitis Optica Spectrum Disorder (NMOSD) and Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD) at the Forefront. J. Pers. Med. 2024, 14, 599. [Google Scholar] [CrossRef]
- Beseler, C.; Vollmer, T.; Graner, M.; Yu, X. The complex relationship between oligoclonal bands, lymphocytes in the cerebrospinal fluid, and immunoglobulin G antibodies in multiple sclerosis: Indication of serum contribution. PLoS ONE 2017, 12, e0186842. [Google Scholar] [CrossRef]
- Yu, X.; Graner, M.; Kennedy, P.G.E.; Liu, Y. The Role of Antibodies in the Pathogenesis of Multiple Sclerosis. Front. Neurol. 2020, 11, 533388. [Google Scholar] [CrossRef]
- Uher, T.; McComb, M.; Galkin, S.; Srpova, B.; Oechtering, J.; Barro, C.; Tyblova, M.; Bergsland, N.; Krasensky, J.; Dwyer, M.; et al. Neurofilament levels are associated with blood–brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult. Scler. J. 2021, 27, 220–231. [Google Scholar] [CrossRef]
- Hansen, C.E.; Kamermans, A.; Mol, K.; Berve, K.; Rodriguez-Mogeda, C.; Fung, W.K.; Van Het Hof, B.; Fontijn, R.D.; Van Der Pol, S.M.A.; Michalick, L.; et al. Inflammation-induced TRPV4 channels exacerbate blood–brain barrier dysfunction in multiple sclerosis. J. Neuroinflamm. 2024, 21, 72. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tsukada, N.; Koh, C.-S.; Yanagisawa, N. Anti-endothelial cell antibodies and circulating immune complexes in the sera of patients with multiple sclerosis. J. Neuroimmunol. 1987, 17, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Gao, C.; Qiu, W.; Hu, X.; Peng, F.; Lu, Z. Antibodies target microvessels in neuromyelitis optica and multiple sclerosis patients. Neurol. Res. 2013, 35, 922–929. [Google Scholar] [CrossRef]
- Nishihara, H.; Shimizu, F.; Kitagawa, T.; Yamanaka, N.; Akada, J.; Kuramitsu, Y.; Sano, Y.; Takeshita, Y.; Maeda, T.; Abe, M.; et al. Identification of galectin-3 as a possible antibody target for secondary progressive multiple sclerosis. Mult. Scler. J. 2017, 23, 382–394. [Google Scholar] [CrossRef]
- Kowarik, M.C.; Astling, D.; Lepennetier, G.; Ritchie, A.; Hemmer, B.; Owens, G.P.; Bennett, J.L. Differential Effects of Fingolimod and Natalizumab on B Cell Repertoires in Multiple Sclerosis Patients. Neurotherapeutics 2021, 18, 364–377. [Google Scholar] [CrossRef]
- Beltrán, E.; Obermeier, B.; Moser, M.; Coret, F.; Simó-Castelló, M.; Boscá, I.; Pérez-Miralles, F.; Villar, L.M.; Senel, M.; Tumani, H.; et al. Intrathecal somatic hypermutation of IgM in multiple sclerosis and neuroinflammation. Brain 2014, 137, 2703–2714. [Google Scholar] [CrossRef]
- Palanichamy, A.; Apeltsin, L.; Kuo, T.C.; Sirota, M.; Wang, S.; Pitts, S.J.; Sundar, P.D.; Telman, D.; Zhao, L.Z.; Derstine, M.; et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci. Transl. Med. 2014, 6, 248ra106. [Google Scholar] [CrossRef]
- Von Büdingen, H.-C.; Kuo, T.C.; Sirota, M.; Van Belle, C.J.; Apeltsin, L.; Glanville, J.; Cree, B.A.; Gourraud, P.-A.; Schwartzburg, A.; Huerta, G.; et al. B cell exchange across the blood-brain barrier in multiple sclerosis. J. Clin. Investig. 2012, 122, 4533–4543. [Google Scholar] [CrossRef]
- Yen, L.; Henao-Díaz, A.; Zimmerman, J.; Giménez-Lirola, L. Considerations on the stability of IgG antibody in clinical specimens. J. Vet. Diagn. Investig. 2025, 37, 13–26. [Google Scholar] [CrossRef]
- Schirmer, L.; Srivastava, R.; Hemmer, B. To look for a needle in a haystack: The search for autoantibodies in multiple sclerosis. Mult. Scler. J. 2014, 20, 271–279. [Google Scholar] [CrossRef]
- Geyer, C.R.; McCafferty, J.; Dübel, S.; Bradbury, A.R.M.; Sidhu, S.S. Recombinant Antibodies and In Vitro Selection Technologies. In Antibody Methods and Protocols; Proetzel, G., Ebersbach, H., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 901, pp. 11–32. ISBN 978-1-61779-930-3. [Google Scholar]
- Zhong, X.; Wang, Y.; Luo, W.; Ma, X.; Sun, X.; Jiang, B.; Qiu, W. Evolution in anti-myelin oligodendrocyte glycoprotein antibody detection and its clinical significance: A narrative review. Ann. Transl. Med. 2023, 11, 287. [Google Scholar] [CrossRef]
- Ayoglu, B.; Mitsios, N.; Kockum, I.; Khademi, M.; Zandian, A.; Sjöberg, R.; Forsström, B.; Bredenberg, J.; Lima Bomfim, I.; Holmgren, E.; et al. Anoctamin 2 identified as an autoimmune target in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Lleixà, C.; Caballero-Ávila, M.; Pascual-Goñi, E.; Martín-Aguilar, L.; Vidal, N.; Tejada, C.; Valdés-Hevia, E.; Zárate, E.; Vesperinas, A.; Collet, R.; et al. Antibodies against the flotillin-1/2 complex in patients with multiple sclerosis. Brain Commun. 2023, 5, fcad109. [Google Scholar] [CrossRef] [PubMed]
- Derfuss, T.; Parikh, K.; Velhin, S.; Braun, M.; Mathey, E.; Krumbholz, M.; Kümpfel, T.; Moldenhauer, A.; Rader, C.; Sonderegger, P.; et al. Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc. Natl. Acad. Sci. USA 2009, 106, 8302–8307. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufunaru, C.; Aromataris, E.; Sears, K.; Sfetc, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Lockwood, C., Porritt, K., Pilla, B., Jordan, Z., Eds.; JBI: Adelaide, Australia, 2024; ISBN 978-0-6488488-2-0. [Google Scholar] [CrossRef]
- Rostami, A.M.; Burns, J.B.; Eccleston, P.A.; Manning, M.C.; Lisak, R.P.; Silberberg, D.H. Search for antibodies to galactocerebroside in the serum and cerebrospinal fluid in human demyelinating disorders. Ann. Neurol. 1987, 22, 381–383. [Google Scholar] [CrossRef]
- Möller, J.R.; Johnson, D.; Brady, R.O.; Tourtellotte, W.W.; Quarles, R.H. Antibodies to Myelin-Associated Glycoprotein (MAG) in the cerebrospinal fluid of multiple sclerosis patients. J. Neuroimmunol. 1989, 22, 55–61. [Google Scholar] [CrossRef]
- Baig, S.; Olsson, T.; Yu-Ping, J.; Höjeberg, B.; Cruz, M.; Link, H. Multiple Sclerosis: Cells Secreting Antibodies Against Myelin-Associated Glycoprotein are Present in Cerebrospinal Fluid. Scand. J. Immunol. 1991, 33, 73–79. [Google Scholar] [CrossRef]
- Link, H.; Baig, S.; Jiang, Y.-P.; Olsson, O.; Höjeberg, B.; Kostulas, V.; Olsson, T. B cells and antibodies in MS. Res. Immunol. 1989, 140, 219–226. [Google Scholar] [CrossRef]
- Latov, N.; Brannagan, T.H.; Sander, H.W.; Gondim, F.D.A.A. Anti-MAG neuropathy: Historical aspects, clinical-pathological correlations, and considerations for future therapeutical trials. Arq. Neuropsiquiatr. 2024, 82, s00431777728. [Google Scholar] [CrossRef]
- Link, H.; Baig, S.; Olsson, O.; Jiang, Y.-P.; Höjeberg, B.; Olsson, T. Persistent anti-myelin basic protein IgG antibody response in multiple sclerosis cerebrospinal fluid. J. Neuroimmunol. 1990, 28, 237–248. [Google Scholar] [CrossRef]
- Mameli, G.; Cossu, D.; Cocco, E.; Masala, S.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Epstein–Barr virus and Mycobacterium avium subsp. paratuberculosis peptides are cross recognized by anti-myelin basic protein antibodies in multiple sclerosis patients. J. Neuroimmunol. 2014, 270, 51–55. [Google Scholar] [CrossRef]
- Olsson, T.; Baig, S.; Jeberg, B.H.; Link, H. Antimyelin basic protein and antimyelin antibody-producing cells in multiple sclerosis. Ann. Neurol. 1990, 27, 132–136. [Google Scholar] [CrossRef]
- Owens, G.P.; Bennett, J.L.; Lassmann, H.; O’Connor, K.C.; Ritchie, A.M.; Shearer, A.; Lam, C.; Yu, X.; Birlea, M.; DuPree, C.; et al. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Ann. Neurol. 2009, 65, 639–649. [Google Scholar] [CrossRef]
- Zanetta, J.-P.; Kuchler, S.; Marschal, P.; Lehmann, S.; Vincendon, G.; Waiter, J.-M.; Rumbach, L.; Tranchant, C. Antibodies to cerebellar soluble lectin CSL in multiple sclerosis. Lancet 1990, 335, 1482–1484. [Google Scholar] [CrossRef] [PubMed]
- Zanetta, J.-P.; Tranchant, C.; Kuchler-Bopp, S.; Lehmann, S.; Warter, J.-M. Presence of anti-CSL antibodies in the cerebrospinal fluid of patients: A sensitive and specific test in the diagnosis of multiple sclerosis. J. Neuroimmunol. 1994, 52, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, L.; Norkrans, G.; Zanetta, J.-P.; Lehmann, S.; Bergström, T. Cerebrospinal fluid anti-cerebellar soluble lectin antibodies in human immunodeficiency virus type 1 infection. J. Neuroimmunol. 1992, 36, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.-G.; Linington, C.; Link, H. Antibodies to myelin-oligodendrocyte glycoprotein in cerebrospinal fluid from patients with multiple sclerosis and controls. J. Neuroimmunol. 1991, 31, 91–96. [Google Scholar] [CrossRef]
- Zhou, D.; Srivastava, R.; Nessler, S.; Grummel, V.; Sommer, N.; Brück, W.; Hartung, H.-P.; Stadelmann, C.; Hemmer, B. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2006, 103, 19057–19062. [Google Scholar] [CrossRef]
- Lalive, P.H.; Menge, T.; Delarasse, C.; Della Gaspera, B.; Pham-Dinh, D.; Villoslada, P.; Von Büdingen, H.-C.; Genain, C.P. Antibodies to native myelin oligodendrocyte glycoprotein are serologic markers of early inflammation in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2006, 103, 2280–2285. [Google Scholar] [CrossRef]
- Mantegazza, R.; Cristaldini, P.; Bernasconi, P.; Baggi, F.; Pedotti, R.; Piccini, I.; Mascoli, N.; La Mantia, L.; Antozzi, C.; Simoncini, O.; et al. Anti-MOG autoantibodies in Italian multiple sclerosis patients: Specificity, sensitivity and clinical association. Int. Immunol. 2004, 16, 559–565. [Google Scholar] [CrossRef][Green Version]
- Sun, J.; Olsson, T.; Wang, W.; Xiao, B.; Kostulas, V.; Fredrikson, S.; Ekre, H.; Link, H. Autoreactive T and B cells responding to myelin proteolipid protein in multiple sclerosis and controls. Eur. J. Immunol. 1991, 21, 1461–1468. [Google Scholar] [CrossRef]
- Owens, G.P.; Fellin, T.J.; Matschulat, A.; Salas, V.; Schaller, K.L.; Given, K.S.; Ritchie, A.M.; Navarro, A.; Blauth, K.; Hughes, E.G.; et al. Pathogenic myelin-specific antibodies in multiple sclerosis target conformational proteolipid protein 1–anchored membrane domains. J. Clin. Investig. 2023, 133, e162731. [Google Scholar] [CrossRef]
- Correale, J.; McMillan, M.; McCarthy, K.; Le, T.; Weiner, L.P. Isolation and characterization of autoreactive moteolioid protein-peptide specific T-cell clones from multiple sclerosis patients. Neurology 1995, 45, 1370–1375. [Google Scholar] [CrossRef]
- Walsh, M.J.; Murray, J.M. Dual implication of 2’,3’-cyclic nucleotide 3’ phosphodiesterase as major autoantigen and C3 complement-binding protein in the pathogenesis of multiple sclerosis. J. Clin. Investig. 1998, 101, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Lovato, L.; Cianti, R.; Gini, B.; Marconi, S.; Bianchi, L.; Armini, A.; Anghileri, E.; Locatelli, F.; Paoletti, F.; Franciotta, D.; et al. Transketolase and 2′,3′-Cyclic-nucleotide 3′-Phosphodiesterase Type I Isoforms Are Specifically Recognized by IgG Autoantibodies in Multiple Sclerosis Patients. Mol. Cell. Proteomics 2008, 7, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, J.M.; Lallone, R.L.; Seitz, R.S.; Ellison, G.W.; Myers, L.W. A humoral response to oligodendrocyte-specific protein in MS: A potential molecular mimic. Neurology 1999, 53, 154. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Kalluri, S.R.; Cepok, S.; Kraus, V.; Buck, D.; Srivastava, R.; Hemmer, B. The antibody response to oligodendrocyte specific protein in multiple sclerosis. J. Neuroimmunol. 2010, 221, 81–86. [Google Scholar] [CrossRef]
- Kaye, J.F.; Kerlero De Rosbo, N.; Mendel, I.; Flechter, S.; Hoffman, M.; Yust, I.; Ben-Nun, A. The central nervous sytem-specific myelin oligodendrocytic basic protein (MOBP) is encephalitogenic and a potential target antigen in multiple sclerosis (MS). J. Neuroimmunol. 2000, 102, 189–198. [Google Scholar] [CrossRef]
- Van Haren, K.; Tomooka, B.H.; Kidd, B.A.; Banwell, B.; Bar-Or, A.; Chitnis, T.; Tenembaum, S.N.; Pohl, D.; Rostasy, K.; Dale, R.C.; et al. Serum autoantibodies to myelin peptides distinguish acute disseminated encephalomyelitis from relapsing–remitting multiple sclerosis. Mult. Scler. J. 2013, 19, 1726–1733. [Google Scholar] [CrossRef]
- Ilyas, A.; Chen, Z.-W.; Cook, S.D. Antibodies to sulfatide in cerebrospinal fluid of patients with multiple sclerosis. J. Neuroimmunol. 2003, 139, 76–80. [Google Scholar] [CrossRef]
- Brennan, K.M.; Galban-Horcajo, F.; Rinaldi, S.; O’Leary, C.P.; Goodyear, C.S.; Kalna, G.; Arthur, A.; Elliot, C.; Barnett, S.; Linington, C.; et al. Lipid arrays identify myelin-derived lipids and lipid complexes as prominent targets for oligoclonal band antibodies in multiple sclerosis. J. Neuroimmunol. 2011, 238, 87–95. [Google Scholar] [CrossRef]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.-S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.-S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Sattarnezhad, N.; Kockum, I.; Thomas, O.G.; Liu, Y.; Ho, P.P.; Barrett, A.K.; Comanescu, A.I.; Wijeratne, T.U.; Utz, P.J.; Alfredsson, L.; et al. Antibody reactivity against EBNA1 and GlialCAM differentiates multiple sclerosis patients from healthy controls. Proc. Natl. Acad. Sci. USA 2025, 122, e2424986122. [Google Scholar] [CrossRef] [PubMed]
- Vietzen, H.; Kühner, L.M.; Berger, S.M.; Furlano, P.L.; Bsteh, G.; Berger, T.; Rommer, P.; Puchhammer-Stöckl, E. Accumulation of Epstein-Barr virus–induced cross-reactive immune responses is associated with multiple sclerosis. J. Clin. Investig. 2024, 134, e184481. [Google Scholar] [CrossRef] [PubMed]
- Vasilenko, N.; Tieck, M.P.; Michel, T.; Schembecker, S.; Schwarz, P.; Guenther, A.; Ruschil, C.; Poli, S.; Ziemann, U.; Giede-Jeppe, A.; et al. In-depth analysis of serum antibodies against Epstein-Barr virus lifecycle proteins, and EBNA1, ANO2, GlialCAM and CRYAB peptides in patients with multiple sclerosis. Front. Immunol. 2024, 15, 1487523. [Google Scholar] [CrossRef]
- Zamecnik, C.R.; Sowa, G.M.; Abdelhak, A.; Dandekar, R.; Bair, R.D.; Wade, K.J.; Bartley, C.M.; Kizer, K.; Augusto, D.G.; Tubati, A.; et al. An autoantibody signature predictive for multiple sclerosis. Nat. Med. 2024, 30, 1300–1308. [Google Scholar] [CrossRef]
- Mathey, E.K.; Derfuss, T.; Storch, M.K.; Williams, K.R.; Hales, K.; Woolley, D.R.; Al-Hayani, A.; Davies, S.N.; Rasband, M.N.; Olsson, T.; et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J. Exp. Med. 2007, 204, 2363–2372. [Google Scholar] [CrossRef]
- Mathew, T.; Garg, S.; Majumdar, P.; Bhardwaj, S.; Kannoth, S.; Mathai, A.; Selvam, S.; George, M.; Murgod, U.; Kamath, V.; et al. Neurofascin 155, 186 and 140 Antibodies in Patients with Multiple Sclerosis: A Pilot Prospective Exploratory Study. Neurol. India 2025, 73, 533–537. [Google Scholar] [CrossRef]
- Devaux, J.J.; Miura, Y.; Fukami, Y.; Inoue, T.; Manso, C.; Belghazi, M.; Sekiguchi, K.; Kokubun, N.; Ichikawa, H.; Wong, A.H.Y.; et al. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology 2016, 86, 800–807. [Google Scholar] [CrossRef]
- Somers, V.; Govarts, C.; Somers, K.; Hupperts, R.; Medaer, R.; Stinissen, P. Autoantibody Profiling in Multiple Sclerosis Reveals Novel Antigenic Candidates. J. Immunol. 2008, 180, 3957–3963. [Google Scholar] [CrossRef]
- De Bock, L.; Somers, K.; Fraussen, J.; Hendriks, J.J.A.; Van Horssen, J.; Rouwette, M.; Hellings, N.; Villar, L.M.; Álvarez-Cermeño, J.C.; Espiño, M.; et al. Sperm-Associated Antigen 16 Is a Novel Target of the Humoral Autoimmune Response in Multiple Sclerosis. J. Immunol. 2014, 193, 2147–2156. [Google Scholar] [CrossRef]
- Boronat, A.; Sepúlveda, M.; Llufriu, S.; Sabater, L.; Blanco, Y.; Gabilondo, I.; Solà, N.; Villoslada, P.; Dalmau, J.; Graus, F.; et al. Analysis of antibodies to surface epitopes of contactin-2 in multiple sclerosis. J. Neuroimmunol. 2012, 244, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Trendelenburg, G.; Scharf, M.; Denno, Y.; Brakopp, S.; Teegen, B.; Probst, C.; Wandinger, K.P.; Buttmann, M.; Haarmann, A.; et al. Identification of the flotillin-1/2 heterocomplex as a target of autoantibodies in bona fide multiple sclerosis. J. Neuroinflamm. 2017, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Boligan, K.F.; Oechtering, J.; Keller, C.W.; Peschke, B.; Rieben, R.; Bovin, N.; Kappos, L.; Cummings, R.D.; Kuhle, J.; Von Gunten, S.; et al. Xenogeneic Neu5Gc and self-glycan Neu5Ac epitopes are potential immune targets in MS. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e676. [Google Scholar] [CrossRef] [PubMed]
- Padler-Karavani, V.; Tremoulet, A.H.; Yu, H.; Chen, X.; Burns, J.C.; Varki, A. A Simple Method for Assessment of Human Anti-Neu5Gc Antibodies Applied to Kawasaki Disease. PLoS ONE 2013, 8, e58443. [Google Scholar] [CrossRef]
- Gao, Y.L.; Raine, C.S.; Brosnan, C.F. Humoral response to hsp 65 in multiple sclerosis and other neurologic conditions. Neurology 1994, 44, 941. [Google Scholar] [CrossRef]
- Quintana, F.J.; Farez, M.F.; Viglietta, V.; Iglesias, A.H.; Merbl, Y.; Izquierdo, G.; Lucas, M.; Basso, A.S.; Khoury, S.J.; Lucchinetti, C.F.; et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2008, 105, 18889–18894. [Google Scholar] [CrossRef]
- Quintana, F.J.; Farez, M.F.; Izquierdo, G.; Lucas, M.; Cohen, I.R.; Weiner, H.L. Antigen microarrays identify CNS-produced autoantibodies in RRMS. Neurology 2012, 78, 532–539. [Google Scholar] [CrossRef]
- van Noort, J.M.; van Sechel, A.C.; Bajramovic, J.J.; Ouagmiri, M.E.; Polmant, C.H.; Lassmannt, H.; Ravid, R. The small heat-shock protein αB-crystallin as candidate autoantigen in multiple sclerosis. Nature 1995, 375, 798–801. [Google Scholar] [CrossRef]
- Thomas, O.G.; Bronge, M.; Tengvall, K.; Akpinar, B.; Nilsson, O.B.; Holmgren, E.; Hessa, T.; Gafvelin, G.; Khademi, M.; Alfredsson, L.; et al. Cross-reactive EBNA1 immunity targets alpha-crystallin B and is associated with multiple sclerosis. Sci. Adv. 2023, 9, eadg3032. [Google Scholar] [CrossRef]
- Van Noort, J.M.; Verbeek, R.; Meilof, J.F.; Polman, C.H.; Amor, S. Autoantibodies against alpha B-crystallin, a candidate autoantigen in multiple sclerosis, are part of a normal human immune repertoire. Mult. Scler. J. 2006, 12, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, M.; Ristori, G.; Buttinelli, C.; Fiori, P.; Falcone, M.; Britton, W.; Adams, E.; Paone, G.; Grasso, M.G.; Pozzilli, C. The immune response to Mycobacterial 70-kDa heat shock proteins frequently involves autoreactive T cells and is quantitatively disregulated in multiple sclerosis. J. Neuroimmunol. 1996, 65, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Minota, S.; Cameron, B.; Welch, W.J.; Winfield, J.B. Autoantibodies to the constitutive 73-kD member of the hsp70 family of heat shock proteins in systemic lupus erythematosus. J. Exp. Med. 1988, 168, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Aslam, M.; Kalluri, S.R.; Schirmer, L.; Buck, D.; Tackenberg, B.; Rothhammer, V.; Chan, A.; Gold, R.; Berthele, A.; et al. Potassium Channel KIR4.1 as an Immune Target in Multiple Sclerosis. N. Engl. J. Med. 2012, 367, 115–123. [Google Scholar] [CrossRef]
- Brickshawana, A.; Hinson, S.R.; Romero, M.F.; Lucchinetti, C.F.; Guo, Y.; Buttmann, M.; McKeon, A.; Pittock, S.J.; Chang, M.-H.; Chen, A.-P.; et al. Investigation of the KIR4.1 potassium channel as a putative antigen in patients with multiple sclerosis: A comparative study. Lancet Neurol. 2014, 13, 795–806. [Google Scholar] [CrossRef]
- Chastre, A.; Hafler, D.A.; O’Connor, K.C. Evaluation of KIR4.1 as an Immune Target in Multiple Sclerosis. N. Engl. J. Med. 2016, 374, 1495–1496. [Google Scholar] [CrossRef]
- Banki, K.; Colombo, E.; Sia, F.; Halladay, D.; Mattson, D.H.; Tatum, A.H.; Massa, P.T.; Phillips, P.E.; Perl, A. Oligodendrocyte-specific expression and autoantigenicity of transaldolase in multiple sclerosis. J. Exp. Med. 1994, 180, 1649–1663. [Google Scholar] [CrossRef]
- Colombo, E.; Banki, K.; Tatum, A.H.; Daucher, J.; Ferrante, P.; Murray, R.S.; Phillips, P.E.; Perl, A. Comparative analysis of antibody and cell-mediated autoimmunity to transaldolase and myelin basic protein in patients with multiple sclerosis. J. Clin. Investig. 1997, 99, 1238–1250. [Google Scholar] [CrossRef][Green Version]
- Niland, B.; Miklossy, G.; Banki, K.; Biddison, W.E.; Casciola-Rosen, L.; Rosen, A.; Martinvalet, D.; Lieberman, J.; Perl, A. Cleavage of Transaldolase by Granzyme B Causes the Loss of Enzymatic Activity with Retention of Antigenicity for Multiple Sclerosis Patients. J. Immunol. 2010, 184, 4025–4032. [Google Scholar] [CrossRef]
- Archelos, J.J.; Trotter, J.; Previtali, S.; Weißbrich, B.; Toyka, K.V.; Hartung, H. Isolation and characterization of an oligodendrocyte precursor–derived B-cell epitope in multiple sclerosis. Ann. Neurol. 1998, 43, 15–24. [Google Scholar] [CrossRef]
- Querol, L.; Clark, P.L.; Bailey, M.A.; Cotsapas, C.; Cross, A.H.; Hafler, D.A.; Kleinstein, S.H.; Lee, J.-Y.; Yaari, G.; Willis, S.N.; et al. Protein array–based profiling of CSF identifies RBPJ as an autoantigen in multiple sclerosis. Neurology 2013, 81, 956–963. [Google Scholar] [CrossRef]
- Mangé, A.; Lacombe, J.; Bascoul-Mollevi, C.; Jarlier, M.; Lamy, P.-J.; Rouanet, P.; Maudelonde, T.; Solassol, J. Serum Autoantibody Signature of Ductal Carcinoma In Situ Progression to Invasive Breast Cancer. Clin. Cancer Res. 2012, 18, 1992–2000. [Google Scholar] [CrossRef]
- Rouwette, M.; Noben, J.; Van Horssen, J.; Van Wijmeersch, B.; Hupperts, R.; Jongen, P.J.; Verbeek, M.M.; De Deyn, P.P.; Stinissen, P.; Somers, V. Identification of coronin-1a as a novel antibody target for clinically isolated syndrome and multiple sclerosis. J. Neurochem. 2013, 126, 483–492. [Google Scholar] [CrossRef]
- Kister, A.; Kister, I. Overview of myelin, major myelin lipids, and myelin-associated proteins. Front. Chem. 2023, 10, 1041961. [Google Scholar] [CrossRef] [PubMed]
- Krugmann, B.; Radulescu, A.; Appavou, M.-S.; Koutsioubas, A.; Stingaciu, L.R.; Dulle, M.; Förster, S.; Stadler, A.M. Membrane stiffness and myelin basic protein binding strength as molecular origin of multiple sclerosis. Sci. Rep. 2020, 10, 16691. [Google Scholar] [CrossRef] [PubMed]
- Boggs, J.M. Myelin basic protein: A multifunctional protein. Cell. Mol. Life Sci. 2006, 63, 1945–1961. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Rosenzweig, A.; Zweiman, B.; Moskovitz, A.; Lisak, R. Recovery of myelin basic protein reactive T cells from spinal cords of Lewis rats with autoimmune encephalomyelitis. J. Immunol. 1984, 132, 2690–2692. [Google Scholar] [CrossRef]
- Pette, M.; Fujita, K.; Kitze, B.; Whitaker, J.N.; Albert, E.; Kappos, L.; Wekerle, H. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology 1990, 40, 1770. [Google Scholar] [CrossRef]
- Möller, J.R.; Yanagisawa, K.; Brady, R.O.; Tourtellotte, W.W.; Quarles, R.H. Myelin-associated glycoprotein in multiple sclerosis lesions: A quantitative and qualitative analysis. Ann. Neurol. 1987, 22, 469–474. [Google Scholar] [CrossRef]
- Schluesener, H.J.; Sobel, R.A.; Linington, C.; Weiner, H.L. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J. Immunol. 1987, 139, 4016–4021. [Google Scholar] [CrossRef]
- Genain, C.P.; Nguyen, M.H.; Letvin, N.L.; Pearl, R.; Davis, R.L.; Adelman, M.; Lees, M.B.; Linington, C.; Hauser, S.L. Antibody facilitation of multiple sclerosis-like lesions in a nonhuman primate. J. Clin. Investig. 1995, 96, 2966–2974. [Google Scholar] [CrossRef]
- Bischof, F.; Bins, A.; Dürr, M.; Zevering, Y.; Melms, A.; Kruisbeek, A.M. A Structurally Available Encephalitogenic Epitope of Myelin Oligodendrocyte Glycoprotein Specifically Induces a Diversified Pathogenic Autoimmune Response. J. Immunol. 2004, 173, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Holz, A.; Bielekova, B.; Martin, R.; Oldstone, M.B.A. Myelin-Associated Oligodendrocytic Basic Protein: Identification of an Encephalitogenic Epitope and Association with Multiple Sclerosis. J. Immunol. 2000, 164, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- De Rosbo, N.K.; Kaye, J.F.; Eisenstein, M.; Mendel, I.; Hoeftberger, R.; Lassmann, H.; Milo, R.; Ben-Nun, A. The Myelin-Associated Oligodendrocytic Basic Protein Region MOBP15–36 Encompasses the Immunodominant Major Encephalitogenic Epitope(s) for SJL/J Mice and Predicted Epitope(s) for Multiple Sclerosis-Associated HLA-DRB1*1501. J. Immunol. 2004, 173, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Morgan, L.; Brammer, M.; Mirsky, R. Galactocerebroside is expressed by non-myelin-forming Schwann cells in situ. J. Cell Biol. 1985, 101, 1135–1143. [Google Scholar] [CrossRef]
- Saida, T.; Saida, K.; Dorfman, S.H.; Silberberg, D.H.; Sumner, A.J.; Manning, M.C.; Lisak, R.P.; Brown, M.J. Experimental Allergic Neuritis Induced by Sensitization with Galactocerebroside. Science 1979, 204, 1103–1106. [Google Scholar] [CrossRef]
- Eckhardt, M. The Role and Metabolism of Sulfatide in the Nervous System. Mol. Neurobiol. 2008, 37, 93–103. [Google Scholar] [CrossRef]
- Stadelmann, C.; Albert, M.; Wegner, C.; Brück, W. Cortical pathology in multiple sclerosis. Curr. Opin. Neurol. 2008, 21, 229–234. [Google Scholar] [CrossRef]
- Singh, S.; Dallenga, T.; Winkler, A.; Roemer, S.; Maruschak, B.; Siebert, H.; Brück, W.; Stadelmann, C. Relationship of acute axonal damage, Wallerian degeneration, and clinical disability in multiple sclerosis. J. Neuroinflamm. 2017, 14, 57. [Google Scholar] [CrossRef]
- Klein, C.J.; Lennon, V.A.; Aston, P.A.; McKeon, A.; O’Toole, O.; Quek, A.; Pittock, S.J. Insights From LGI1 and CASPR2 Potassium Channel Complex Autoantibody Subtyping. JAMA Neurol. 2013, 70, 229. [Google Scholar] [CrossRef]
- Paterson, R.W.; Zandi, M.S.; Armstrong, R.; Vincent, A.; Schott, J.M. Clinical relevance of positive voltage-gated potassium channel (VGKC)-complex antibodies: Experience from a tertiary referral centre. J. Neurol. Neurosurg. Psychiatry 2014, 85, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Hedström, A.K.; Huang, J.; Michel, A.; Butt, J.; Brenner, N.; Hillert, J.; Waterboer, T.; Kockum, I.; Olsson, T.; Alfredsson, L. High Levels of Epstein–Barr Virus Nuclear Antigen-1-Specific Antibodies and Infectious Mononucleosis Act Both Independently and Synergistically to Increase Multiple Sclerosis Risk. Front. Neurol. 2020, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Ligers, A.; Dyment, D.A.; Willer, C.J.; Sadovnick, A.D.; Ebers, G.; Risch, N.; Hillert, J. Evidence of Linkage with HLA-DR in DRB1*15-Negative Families with Multiple Sclerosis. Am. J. Hum. Genet. 2001, 69, 900–903. [Google Scholar] [CrossRef] [PubMed]
- UniProt_Coronin1a. UniProt n.d. Available online: https://www.uniprot.org/uniprotkb/P31146/entry (accessed on 22 July 2025).
- Cristofanilli, M.; Gratch, D.; Pagano, B.; McDermott, K.; Huang, J.; Jian, J.; Bates, D.; Sadiq, S.A. Transglutaminase-6 is an autoantigen in progressive multiple sclerosis and is upregulated in reactive astrocytes. Mult. Scler. J. 2017, 23, 1707–1715. [Google Scholar] [CrossRef]
- Sadatipour, B.T.; Greer, J.M.; Pender, M.P. Increased circulating antiganglioside antibodies in primary and secondary progressive multiple sclerosis. Ann. Neurol. 1998, 44, 980–983. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Li, R.; Lin, Y.; Fu, Y.; Yan, Y.; Zhu, W.; Wang, N.; Zhang, Z.; Xu, G. Anti-ganglioside Antibodies in Guillain-Barre Syndrome: A Novel Immunoblotting-Panel Assay. Front. Neurol. 2021, 12, 760889. [Google Scholar] [CrossRef]
- Pardo, E.; Cárcamo, C.; Uribe-San Martín, R.; Ciampi, E.; Segovia-Miranda, F.; Curkovic-Peña, C.; Montecino, F.; Holmes, C.; Tichauer, J.E.; Acuña, E.; et al. Galectin-8 as an immunosuppressor in experimental autoimmune encephalomyelitis and a target of human early prognostic antibodies in multiple sclerosis. PLoS ONE 2017, 12, e0177472. [Google Scholar] [CrossRef]
- Podbielska, M.; Macala, J.; Jakubiak-Augustyn, A.; Szulc, Z.M.; Fortuna, W.; Budrewicz, S.; Jaskiewicz, E.; Bilinska, M.; Hogan, E.L.; Pokryszko-Dragan, A. Ceramide is implicated in humoral peripheral and intrathecal autoimmune response in MS patients. Mult. Scler. Relat. Disord. 2023, 71, 104565. [Google Scholar] [CrossRef]
- Karaaslan, Z.; Şengül-Yediel, B.; Yüceer-Korkmaz, H.; Şanlı, E.; Gezen-Ak, D.; Dursun, E.; Timirci-Kahraman, Ö.; Baykal, A.T.; Yılmaz, V.; Türkoğlu, R.; et al. Chloride intracellular channel protein-1 (CLIC1) antibody in multiple sclerosis patients with predominant optic nerve and spinal cord involvement. Mult. Scler. Relat. Disord. 2023, 78, 104940. [Google Scholar] [CrossRef]
- Lehmann-Horn, K.; Kinzel, S.; Weber, M. Deciphering the Role of B Cells in Multiple Sclerosis—Towards Specific Targeting of Pathogenic Function. Int. J. Mol. Sci. 2017, 18, 2048. [Google Scholar] [CrossRef]
- Winger, R.C.; Zamvil, S.S. Antibodies in multiple sclerosis oligoclonal bands target debris. Proc. Natl. Acad. Sci. USA 2016, 113, 7696–7698. [Google Scholar] [CrossRef]
- Brändle, S.M.; Obermeier, B.; Senel, M.; Bruder, J.; Mentele, R.; Khademi, M.; Olsson, T.; Tumani, H.; Kristoferitsch, W.; Lottspeich, F.; et al. Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 7864–7869. [Google Scholar] [CrossRef]
- Chen, F.; Wang, L.; Hong, J.; Jiang, J.; Zhou, L. Unmasking bias in artificial intelligence: A systematic review of bias detection and mitigation strategies in electronic health record-based models. J. Am. Med. Inform. Assoc. 2024, 31, 1172–1183. [Google Scholar] [CrossRef]
- Kumar, L. Fairness in Artificial Intelligence: A Comprehensive Review of Bias Detection: A Systematic Literature. Int. Sci. J. Eng. Manag. 2025, 4, 1–9. [Google Scholar] [CrossRef]
| Location/Function | Self- Antigen | Initial Identification | Method | Molecular Mimicry Reported | Supporting Evidence * | Contradictory Evidence * | Status |
|---|---|---|---|---|---|---|---|
| Myelin Sheath | GalC | 1987 [57] | RIA | No | D/B [57] | Non-specific | |
| MAG | ≤1989 [58] | RIA | No | L/C [59], L/B [60] | L/C [58], R/OD [61] | Non-specific | |
| MBP | ≤1990 [62] | ELISPOT | Yes: EBNA1 [63] | L/P [63], L/B/NS [64] | L/B [62], L/B [60], N/O/CS [65] | Non-specific | |
| CSL | 1990 [66] | Western blot | No | L/C [66], L/C [67] | L/C/OD [68] | Non-specific | |
| MOG | ≤1991 [69] | ELISA | No | L/B [69], N/P [70], N/P [71] | N/O/CS [65], U/B [72], R/OD [27] | Non-specific | |
| PLP | ≤1991 [73] | ELISPOT | No | N/C [74] | L/B [73], L/P [75], N/O/CS [65] | Provisionally | |
| CNPase | ≤1998 [76] | Western blot | No | L/B [76], L/B [77] | Controversial | ||
| OSP | 1999 [78] | Western blot | Yes: Common pathogen peptides [78] | L/P [78] | N/B [79] | Controversial | |
| MOBP | ≤2000 [80] | Proliferation assay (T-cells) | No | L/P [81] | Non-specific | ||
| Sulfatide | 2003 [82] | ELISA | No | D/C [82], D/C [83] | Non-specific | ||
| GlialCAM | 2022 [84] | Microarray | Yes: EBNA1 [85] | L/P [84], L/P [85] L/P [86] | L/P [87], L/P [88] | Controversial | |
| Neuronal | Neurofascin | 2007 [89] | Western blot | No | LN/P/SP [89], L/P/CS [90] | LN/P/OD [91], N/B [53] | Non-specific |
| SPAG16 | 2008 [92] | ELISA | No | L/C [92], L/P [93] | Non-specific | ||
| Contactin-2a | 2009 [54] | Western blot | No | LN/B/SR+LF [94] | L/B [54] | Controversial | |
| Flotilin-1/2-Heterocomplex | 2017 [95] | Immunoprecipitation | No | N/P/LF [95], N/B/LF [53] | Controversial | ||
| Neu5Ac | 2020 [96] | Microarray | Yes: Neu5Gc [96] | D/P [96] | D/C [96], D/P/OD [97] | Non-specific | |
| Heat Shock Proteins | HSP60 | ≤1994 [98] | Western blot | No | L/P/SR [99], L/C/SR [100] | L/B [98] | Non-specific |
| CRYAB | 1995 [101] | Western blot | Yes: EBNA1 [102] | L/P [102] | L/P [103], L/B [77] | Controversial | |
| HSP70 | ≤1996 [104] | Proliferation assay (T-cells) | No | L/P/SR [99], L/C/SR [100] | L/P/OD [105] | Non-specific | |
| Ion Channels | Kir4.1 | 2012 [106] | ELISA | No | N/P [106] | N/B [107], N/P [108] | Controversial |
| ANO2 | 2016 [52] | Bead-Based Antigen Array | Yes: EBNA1 [85] | L/P [52], L/P [86] | N/B [53] | Controversial | |
| Miscellaneous | Transaldolase | 1994 [109] | Western blot | Yes: HTLV-1 Peptides [109] | L/P [109], L/B [110], L/P [111] | L/B [77] | Controversial |
| Alu-peptides | 1998 [112] | Phage display | No | L/B [112] | Non-specific | ||
| Transketolase | 2008 [77] | 2D-Immunoblotting | No | L/B [77] | Provisionally | ||
| RBPJ | 2013 [113] | Microarray | No | LN/C/LF [113] | L/P/OD [114] | Non-specific | |
| Coronin-1a | 2013 [115] | SDS-PAGE/ Mass Spectrometry | No | L/C [115] | Non-specific |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mailaender, F.; Vasilenko, N.; Tieck, M.P.; Schembecker, S.; Kowarik, M.C. The Elusive B Cell Antigen in Multiple Sclerosis: Time to Rethink CNS B Cell Functions. Int. J. Mol. Sci. 2025, 26, 10771. https://doi.org/10.3390/ijms262110771
Mailaender F, Vasilenko N, Tieck MP, Schembecker S, Kowarik MC. The Elusive B Cell Antigen in Multiple Sclerosis: Time to Rethink CNS B Cell Functions. International Journal of Molecular Sciences. 2025; 26(21):10771. https://doi.org/10.3390/ijms262110771
Chicago/Turabian StyleMailaender, Florian, Nicole Vasilenko, Maria P. Tieck, Sonja Schembecker, and Markus C. Kowarik. 2025. "The Elusive B Cell Antigen in Multiple Sclerosis: Time to Rethink CNS B Cell Functions" International Journal of Molecular Sciences 26, no. 21: 10771. https://doi.org/10.3390/ijms262110771
APA StyleMailaender, F., Vasilenko, N., Tieck, M. P., Schembecker, S., & Kowarik, M. C. (2025). The Elusive B Cell Antigen in Multiple Sclerosis: Time to Rethink CNS B Cell Functions. International Journal of Molecular Sciences, 26(21), 10771. https://doi.org/10.3390/ijms262110771




