Abstract
In recent years, monoclonal antibodies targeting key cytokines underlying the occurrence of psoriatic skin lesions and joint involvement, i.e., Tumor Necrosis Factor-alpha (TNF-α), Interleukin 17 (IL-17), Interleukin 12 (IL-12), and Interleukin 23 (IL-23), have become more commonly used in the therapy of psoriasis. Due to the high effectiveness, a favorable safety profile, and growing availability of biological treatment methods, the number of patients receiving chronic monoclonal antibody therapy is increasing each year. However, the factors affecting the effectiveness of biological drugs are not fully recognized. The study aimed at analyzing the clinical profile of patients and non-specific inflammatory markers in terms of the response to the psoriasis treatment with IL-17, IL-23, IL-12/23, and TNF-α inhibitors. The analysis involved 185 patients receiving biological therapy in the Department of Dermatology and Venereology at the Medical University of Lodz, which resulted in a total of 222 treatment cycles (TC). The super-response was defined as 100% reduction in the Psoriasis Area and Severity Index (PASI 100), at week 16 (±4 weeks) of therapy. Our study indicates that the chance of achieving a super-response was higher among younger patients with no psoriatic lesions on palms and soles, not suffering from non-alcoholic fatty liver disease, previously treated with methotrexate, and characterized by a higher level of derived Neutrophil-to-Lymphocyte Ratio (dNLR) at the beginning of treatment.
1. Introduction
Psoriasis (PsO) is an autoimmune disease caused by chronic inflammation. The condition is increasingly recognized as a systemic disease associated with a higher risk of cardiovascular disorders, metabolic syndrome, depression, inflammatory bowel disease, non-alcoholic fatty liver disease, and uveitis. Over 30% of psoriatic patients also suffer from joint involvement leading to deformations and destruction of joints [1,2,3,4]. In recent years, monoclonal antibodies targeting key cytokines underlying the occurrence of psoriatic skin lesions and joint involvement, i.e., Tumor Necrosis Factor-alpha (TNF-α), Interleukin 17 (IL-17), Interleukin 12 (IL-12), and Interleukin 23 (IL-23), have become more commonly used in the therapy of psoriasis [5].
Due to the high effectiveness, a favorable safety profile, and growing availability of biological treatment methods, the number of patients receiving chronic monoclonal antibody therapy is increasing every year. However, the factors affecting the effectiveness of biological drugs are not fully recognized [6,7,8,9,10,11,12]. The literature on the subject analyzes the phenomenon of a super-response to treatment with monoclonal antibodies, regarded as near or complete clearance of the skin within a short period after exposure to the therapy. However, no precise definition of this response is provided, and it varies across published studies [6,7,8,9,10,11,12]. Super-response is most often associated with a Psoriasis Area and Severity Index 100 (PASI100) response to the therapy, i.e., complete clearance of the skin, within 12 to 28 weeks [6]. Rompoti et al. define a super-response as achieving Psoriasis Area and Severity Index (PASI) ≤ 1 at weeks 12 and 16 [7], while Feldman et al. as reaching 90% reduction in the Psoriasis Area and Severity Index (PASI90) at week 28 [8]. Some studies define it as both obtaining and maintaining positive clinical results in terms of skin lesions [9,10,11]. Loft et al. describe super-response as achieving and maintaining PASI < 3 skin lesion severity for a period of six to 60 months in the group of bio-naïve patients treated with a single biological drug for at least five years [9]. Similarly, a target defined in two other studies is maintaining PASI100 response for 88–100 [10] or 104 [11] weeks, respectively. The Early Super Response is also described in the literature as achieving PASI100 at week 4 of therapy [12]. Moreover, using the absolute rather than the relative PASI score appears to be of greater value due to the easier translation of research outcomes into everyday clinical practice [13]. Regardless of assumptions and definitions, the objective of currently conducted studies is to identify factors that influence the response to biological drugs, enabling future personalization and reducing the costs of biological treatment methods in the therapy of psoriasis.
The study aimed at analyzing the clinical profile of patients and non-specific inflammatory markers in terms of the response to the psoriasis treatment with IL-17, IL-23, IL-12/23, and TNF-α inhibitors.
2. Results
The analysis involved 185 patients receiving a biological therapy within the B.47 drug program of the Ministry of Health of the Republic of Poland. Some of the patients were treated with more than one biological drug, which resulted in a total of 222 treatment cycles (TC). In the study group, a super-response was observed in 94 patients, accounting for 42.34% of TC. Two patients achieved a super-response twice; in both cases, the therapy was modified due to the secondary treatment failure. Considering treatment cycles, men constituted the majority of the study group (123 TC/55.41%). The average age of patients at the beginning of treatment was 44 ± 14.74 years (super-responders (SR) 40.70 ± 13.38 years vs. non-super-responders (nSR) 46.42 ± 15.16 years), and the average PASI score was 17.57 ± 8.16. A family medical history of psoriasis was reported in 47.06% of all TC and more frequently observed in the super-responder group (SR: 52.69% vs. nSR: 42.97%); however, this observation was not statistically significant (p-value > 0.05). Clinical data of the study group are presented in Table 1.

Table 1.
Clinical data of the study group.
No statistically significant correlations were found between sex, body weight, BMI value, age of disease onset, disease duration before the initiation of biological treatment, initial severity of the skin lesions with PASI and BSA (Body Surface Area) scores at the beginning of the therapy, baseline assessment of quality of life using DLQI (Dermatology Life Quality Index) scale and achieving a super-response at week 16 (±4) of the treatment. However, an older age was associated with a lower chance of a super-response, and with every one-year increase in age, the odds of achieving a super-response decreased by approximately 3.3% (Odds Ratio (OR) = 0.967; 95%Confidence Interval (CI): 0.947–0.988; p = 0.002).
Treatment cycles with IL-23 inhibitors amounted to 51.35% of all cases (risankizumab 71 TC/31.98%, guselkumab 32 TC/14.41%, tildrakizumab 11 TC/4.95%). Monoclonal antibodies targeting IL-17A constituted 26.58% (sekukinumab 37 TC/16.67%, iksekizumab 22 TC/9.9%) and bimekizumab aiming against IL-17A/F 10.36% (23 TC). TNF-α inhibitors comprised 9.01% (adalimumab 18 TC/8.11%), infliximab 2 TC/0.9%) of the analyzed treatment cycles. Ustekinumab, an inhibitor of IL-12/23, with 6 TC accounted for 2.7% of cases. In the study group, 152 treatment cycles were applied to bio-naïve patients representing 68.47% of all TC. No statistically significant association was found between the number of previously received biological drugs and a super-response.
The highest percentage of SR was observed among the patients treated with infliximab (SR: 100% vs. nSR 0%), followed by bimekizumab (SR: 91.30% vs. nSR: 8.70%) and ixekizumab (SR: 59.09% vs. nSR: 40.91%). In the group of patients receiving IL-23 inhibitors, a super-response was more often reported in those undergoing risankizumab therapy (SR: 39.44% vs. nSR: 60.56%) but was comparable to individuals receiving guselkumab therapy (SR: 34.38% vs. nSR: 65.63%). The lowest percentage of SR was observed in the group treated with tildrakizumab (SR: 9.09% vs. nSR: 90.91%), followed by ustekinumab (SR: 16.67% vs. nSR: 83.33%) and adalimumab (SR: 33.33% vs. nSR: 66.67%).
On the basis of multinomial logistic regression analysis, it was determined that ixekizumab and bimekizumab therapy were associated with, respectively, a 14-fold (OR = 14.444; 95% CI: 1.562–133.586; p = 0.0186) and 105-fold (OR = 105; 95% CI: 8.483–1299.596; p = 0.0003) higher odds of achieving a super-response, than the treatment with tildrakizumab. Clinical response to therapy after 24 weeks from the second time point was also assessed. Among the SR, such data were available for 75 of the 94 patients. Super-response was maintained in 82.67% of patients in this group. The highest percentage of SR was observed in the group treated with bimekizumab (100%), followed by sekukinumab (87.5%) and risankizumab (87%). No patient receiving TNF–α inhibitors sustained a PASI100 response at week 40 (±4 weeks). Tildrakizumab was excluded from this analysis due to an insufficiently large study group.
The analysis also included the number, type, and duration of previously received non-biologic therapies (methotrexate (MTX), ciclosporin, acitretin, Psoralen Ultra-Violet A (PUVA) therapy, Narrowband Ultraviolet B (NB-UVB) phototherapy).
A longer duration of methotrexate treatment was associated with a higher chance of a super-response, and with every one-month increase, the odds of achieving a super-response increased by approximately 2.1% (OR = 1.021; 95% CI: 1.002–1.040; p = 0.034). Methotrexate was administered orally or subcutaneously, in doses of 10 to 25 mg/week. The time between the discontinuation of methotrexate therapy and the initiation of biological treatment varied and did not always correspond to the washout period, which could have had an impact on the results obtained. No associations were found between the type or duration of other conventional methods applied before the initiation of biological treatment and achieving a PASI100 response at week 16 (±4 weeks) during monoclonal antibody therapy. The duration of treatment with respective conventional methods in the study group is presented in Table 2.

Table 2.
Duration of treatment with respective conventional methods (in months).
There was no statistically significant relationship between the duration of a biological therapy and SR or nSR group. However, it is worth mentioning that secondary treatment failure was more frequently observed in the nSR group (SR: 7.4% vs. nSR: 19.5%). The incidence rate of adverse reactions resulting in treatment termination amounted to 4.05% and was similar in both groups (SR: 4.3% vs. nSR: 3.9%). More than one adverse reaction was observed in 2.25% of patients.
2.1. Comorbidities
In the analyzed study group, 24.77% of patients suffered from psoriatic arthritis (PsA), which was more often reported in the SR group (SR: 27.66% vs. nSR: 22.66%); however, this disparity was not statistically significant. The study also analyzed co-occurrences of chronic kidney disease and cardiovascular, metabolic, thyroid, and depressive disorders. The incidence of comorbidities diagnosed in the study group is presented in Table 3. It was statistically confirmed that co-occurrence of non-alcoholic fatty liver disease was associated with a more than twofold reduction in the chance of achieving a super-response (OR = 0.443; 95% CI: 0.221–0.931; p = 0.032). No such relationships were determined for other comorbidities. There was also no statistically significant association between nicotinism and response to the biological therapy.

Table 3.
Comorbidities in the study group.
At the beginning of biological treatment, psoriatic skin lesions in special localizations (scalp, palms and soles, anogenital area, nails) were examined. Statistical analysis using a multinomial logistic regression model showed that the occurrence of psoriatic skin lesions on palms and soles was associated with an almost fivefold lower chance of achieving a super-response (OR = 0.222; 95% CI: 0.093–0.528; p = 0.001). No relationships were found for other special localizations.
2.2. Non-Specific Inflammatory Markers
The hematological parameters and calculated non-specific inflammatory markers were analyzed with a multinomial logistic regression. It was confirmed that a thousand per microliter (1000/µL) higher initial levels of neutrophils and lymphocytes were associated with, respectively, a 5.2-fold (OR = 5.201; 95% CI: 1.660–16.295; p = 0.005) and 4.2-fold (OR = 4.150; 95% CI: 1.135–15.182; p = 0.031) greater chance of achieving a super-response after a four-month-long therapy. Simultaneously, a thousand/microliter (1000/µL) higher total level of leukocytes was related to a fourfold (OR = 0.237; 95% CI: 0.081–0.691; p = 0.008) lower chance of reaching a super-responder status. Moreover, aderived Neutrophil-to-Lymphocyte Ratio (dNLR) value higher by one unit prior to treatment initiation was associated with a one and a half times higher chance of achieving a super-response regardless of the monoclonal antibody therapy applied (OR = 1.563; 95% CI: 1.053–2.321; p = 0.027). The Receiver Operating Characteristic (ROC) curve analysis was additionally conducted to evaluate the predictive ability of baseline dNLR. The ROC curve for baseline dNLR is shown in Figure 1. The ROC analysis determined a baseline dNLR cut-off value for a achieving a super response as 1.522 (95% CI: 0.794–1.978) with an Area Under the ROC curve (AUC) of 0.552 (95% CI: 0.474–0.629, p = 0.1891), sensitivity of 0.5106, and specificity of 0.3906. However, the obtained AUC value showed that the role of dNLR as an independent predictor was limited and not statistically significant due to other parameters affecting the predictive model.
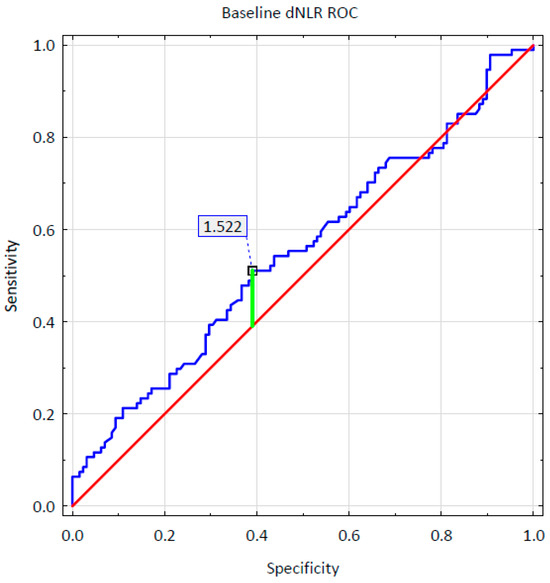
Figure 1.
Baseline dNLR ROC curve.
Using the Mann–Whitney U-test, changes over time in blood-count-derived inflammatory biomarkers were also calculated and compared between the SR and nSR groups. Table 4 presents the definitions and changes in the selected markers for which the differences between SR and nSR groups were statistically significant, between therapy weeks 0 and 16. The box-and-whisker plot for these markers is shown in Figure 2. The decrease in values of Neutrophil-to-Lymphocyte Ratio (NLR), dNLR, Monocyte-to-Lymphocyte Ratio (MLR), Neutrophil-to-Monocyte-to-Lymphocyte Ratio (NMLR), and Systemic Inflammation Response Index (SIRI) biomarkers during biological treatment was statistically significantly higher in the group of super-responders than in the nSR group, which confirms a substantially better response to therapy. A reduction in Aggregate Index of Systemic Inflammation (AISI), Systemic Immune-Inflammation Index (SII), Platelet-to-Lymphocyte Ratio (PLR), and NMR (Neutrophil-to-Monocyte Ratio) parameters over time was also observed; however, no statistically significant disparities were identified between the SR and nSR groups.

Table 4.
Definitions and changes in the selected Blood Count-Derived Inflammatory Markers between week 0 and 16 of the therapy, p-value for Mann–Whitney U-test.
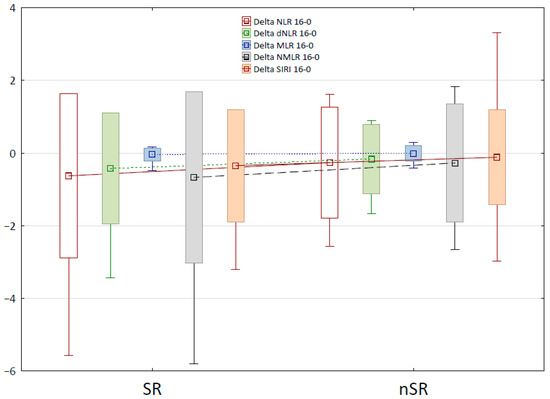
Figure 2.
The box-and-whisker plot for changes in the selected Blood Count-Derived Inflammatory Markers between week 0 and 16 of the therapy.
3. Discussion
Achieving a super-response is currently becoming the most sought-after aim in the treatment of psoriasis. Factors that determine or increase the likelihood of this response are not fully recognized and require further research. Moreover, studies conducted so far vary in terms of the definition of super-response and study groups, which leads to inconsistent results and conclusions. It seems that younger patients more frequently achieve a super-response [6,14,15,16,17] and are characterized by shorter disease duration [8,14,18,19], lower body weight [8,9,15], and Body Mass Index (BMI) value [6,8,9,14,16,17]. They are also presumably distinguished by a lower baseline severity of skin lesions [8,11,14,15,16] and are more often bio-naïve [14,16,18,19,20,21,22]. On the other hand, the aforementioned profile has not been confirmed in a series of research [7,23,24,25,26], and the results of the abovementioned studies are contradictory in terms of different variables. Table 5 presents factors affecting the likelihood of achieving a super-response and findings of various studies.

Table 5.
Factors affecting the likelihood of achieving a super-response and findings of various studies.
Our study found that older patients had a lower chance of achieving a super-response. No statistically significant correlations were identified between sex, body weight, BMI value, age of disease onset, disease duration before biological treatment initiation, the number of different biological drugs received, baseline severity of the skin lesions, and achieving a super-response. However, a relationship between a longer duration of methotrexate therapy and a higher chance of reaching a super-responder status was observed. This result should be interpreted with caution due to differences in the doses applied, routes of drug administration, and the time between discontinuation of MTX-therapy and initiation of biological treatment. No evidence was found on any influence of other previously applied conventional systemic therapies or phototherapy and their duration on the chance of achieving a super-response by patients undergoing biological treatment. Other studies have also reported a lack of impact of previously used systemic non-biological treatments [15,20,24,26]. Nevertheless, two analyses have indicated a higher likelihood of achieving a super-response by patients who received fewer systemic treatment methods prior to biological treatment; however, this observation was not statistically significant [7,15]. To the best of our knowledge, no other studies have assessed the effect of the duration of previously administered systemic non-biologic therapies and phototherapy on achieving a super-responder status.
The type of administered biological drug also has a substantial impact on the clinical response. Our study confirmed that, as compared to tildrakizumab, ixekizumab and bimekizumab therapies were associated with a 14-fold and 105-fold higher odds of achieving a super-response, respectively. Mastorino et al. also noticed that patients treated with IL-17 inhibitors, ixekizumab and brodalumab achieved a super-response more frequently [6]. Similar findings were reported by Morariu et al. [27] and Liu et al. [17], who observed the highest percentage of patients receiving ixekizumab in the SR group. An analysis involving IL-23 inhibitors exclusively showed that patients treated with risankizumab had a statistically significantly higher chance of achieving a super-response compared to those receiving tildrazumab therapy. Similarly, in our study group, among individuals treated with IL-23 inhibitors, the highest percentage of SR was observed for risankizumab (SR: 39.44% vs. nSR: 60.56%), and the lowest for tildrakizumab (SR: 9.09% vs. nSR: 90.91%).
Psoriatic skin lesions in special localizations, i.e., the scalp, nails, anogenital area, folds, palms, and soles, are difficult to treat and significantly affect the patient’s quality of life. Esposito et al. noticed that the scalp and anogenital involvement was more frequently associated with a super-response, while nail and palmoplantar psoriasis were more demanding to treat [20]. According to a study conducted by Gargiulo et al., the non-occurrence of psoriatic changes in special localizations was associated with a higher likelihood of achieving a super-response by patients receiving anti-IL-23 monoclonal antibodies [19]. Some research suggests that nail involvement is a predictive factor indicating a low chance of reaching a super-response [12]. Analysis performed in our study group revealed that psoriatic skin lesions on the palms and soles were associated with a fivefold lower chance of achieving a super-response during biological therapy.
Psoriasis, an immunologically mediated chronic inflammatory disease, promotes co-occurrence of various metabolic diseases, mental disorders, inflammatory bowel disease, or skin diseases [1,2,3,4,28]. For this reason, when selecting a proper treatment strategy all comorbidities, including psoriatic arthritis, need to be considered. It appears that patients with fewer comorbidities have a higher likelihood of achieving a super-response during biological therapy [9,17,20,21,24]. Some studies suggest that the co-occurrence of PsA reduces the chance of reaching the most favorable response to treatment [17,20]; however, this relationship has not been confirmed in other studies [6,9,15,16,19,23]. The results of our study do not prove the influence of PsA on achieving a super-response in terms of skin lesions. Nevertheless, our findings show that the comorbidity of non-alcoholic fatty liver disease decreases the chance of a super-response by over two times. This outcome is consistent with previous studies [17] and the pathogeneses underlying both diseases. In a meta-analysis involving 109,806 participants, it was established that patients with moderate-to-severe psoriasis had a 4.01-times higher chance of metabolic dysfunction-associated steatotic liver disease (MAFLD) compared to non-psoriatic individuals [29]. Furthermore, a greater risk of cirrhosis was reported in individuals with psoriasis and psoriatic arthritis, and the risk of liver disorders grew with the increase in the body surface area affected by psoriasis [30]. The hepatodermal axis is increasingly acknowledged due to the common pathogenesis and mutual exacerbation of both disease entities. Adipose tissue is a source of various proinflammatory cytokines, including adiponectin, leptin, resistin, TNF-α, and IL-6, participating in the pathogenesis of both psoriasis and non-alcoholic fatty liver disease (NFLD). The excessive secretion of these proinflammatory cytokines in the course of psoriasis raises insulin resistance, which is a crucial factor underlying NFLD. Also, the progressing hepatic steatosis causes a further increase in insulin resistance, thus leading to a vicious circle. Moreover, a higher level of IL-17, a key cytokine in the pathogenesis of psoriasis, may be associated with a faster progression of MAFLD to steatohepatitis and even to hepatocellular carcinoma [31].
The importance of genetic background in the pathogenesis of psoriasis is commonly known [5,32]. Liu et al. observed a statistically significantly more frequent family history of psoriasis in a group of SR [17]. Nevertheless, findings obtained in other research do not support this outcome [8,12,23]. In our study, positive family history was also more frequently reported in the SR group; however, this relationship was not statistically confirmed, probably due to the heterogeneity of the study group and the multifactorial pattern of psoriasis inheritance. The source literature provides data on the impact of genetic background on the effectiveness of various biological drugs. It seems that patients carrying HLA-C*06:02/HLA-C*04 alleles have a higher likelihood of achieving a positive clinical response during the ustekinumab therapy [11,33]. Moreover, a relationship was observed between certain polymorphisms and the results of the treatment with anti-TNF drugs [34]. In a study conducted on patients receiving brodalumab, rs495337 (SPATA2), rs6311 (HTR2A), and rs4085613 (LCE3D) polymorphisms were associated with a positive response to the treatment at month 12 of the therapy [35]. The occurrence of eight specific single nucleotide polymorphisms (SNPs) indicates a promising response to sekukinumab therapy. The rs34085293 (DDX58_v1) and rs2304255 (TYK2_v3) SNP variants are in particular related to achieving a super-response in the treatment with the aforementioned IL-17A inhibitor [10]. However, further research is required to determine the genetic profile of patients who develop the most favorable response to the therapy with certain biological drugs.
Regardless of the patient’s genetic profile, it would be beneficial to distinguish between widely available biomarkers that could enable personalization of treatment by selecting the most suitable biological therapy with the highest likelihood of achieving a super-response in the patient. Ziolkowska-Banasik et al. suggested an IL-18/IL-13 serum level ratio as a super-response predictive factor for secukinumab therapy [26]. In another prospective study, it was confirmed that individuals presenting a positive response to the treatment with TNF-α inhibitors were characterized by a lower baseline NLR value compared to non-responders. In patients undergoing adalimumab therapy, lower baseline IL-6 levels were additionally reported. No such correlations were observed in the groups receiving IL-23, IL-12/23, IL-17A, and Interleukin-17 receptor (IL-17R) inhibitors [36].
The literature provides examples of the use of non-specific blood count-derived inflammatory markers as predictive factors in numerous disease entities [37,38,39]. It has been confirmed that biological treatment may cause a decrease in levels of inflammatory markers in the course of psoriasis [40,41,42]. Different markers, i.e., AISI, SIRI, SII, PLR, NLR, dNLR, and MLR, have been positively correlated with the severity of psoriatic skin lesions; however, dNLR appears to be the most reliable one in terms of skin lesion severity among all analyzed blood count-derived inflammatory markers [43]. Our study confirmed that a higher baseline dNLR value was associated with a statistically significantly greater chance of achieving a super-response.However, the AUS value obtained in ROC analysis showed that the role of dNLR as an independent predictor was limited. To the best of our knowledge, there is only one other study that assessed dNLR as a predictive biomarker of response to biological treatment of psoriasis. Morariu et al. reported that achieving a super-responder status was related to higher dNLR and SIRI values at baseline [27].
The main limitations of our study were its retrospective nature and relatively small groups of patients treated with individual biological drugs. Among patients receiving TNF-α inhibitors, the study group included only those undergoing adalimumab and infliximab therapies. In particular, the groups of patients treated with infliximab and ustekinumab were small, which makes it difficult to draw strong conclusions. Another limitation of our study was its single-center nature. A larger, multicenter, prospective study should be conducted to assess the suitability of a dNLR as a predictive biomarker of a super-response in the therapy of psoriasis with individual biological drugs. Moreover, a validation should be performed to establish reference ranges indicating a positive response to biological treatment.
4. Materials and Methods
Our single-center retrospective study involved patients suffering from psoriasis and receiving biological therapy with IL-12/23 (ustekinumab), IL-23 (guselkumab, risankizumab, tyldrakizumab), IL-17A (ixekizumab, sekukinumab), IL-17A/F (bimekizumab), or TNF-α (adalimumab, infliximab) inhibitors in the Department of Dermatology and Venereology, Medical University of Lodz, in the period from 1 March 2015 to 1 March 2025.
The research included patients with diagnosed moderate-to-severe plaque psoriasis (patients with psoriatic lesions severity on the PASI scale > 10 and/or BSA > 10% and/or DLQI > 10 points) who were unsuccessfully treated with at least two conventional methods (methotrexate, ciclosporin, acitretin, PUVA therapy), had contraindications to conventional systemic treatment, or developed adverse reactions during these therapies. The only exception were patients aged under 18 who qualified for biological treatment directly after an unsuccessful topical therapy.
The Psoriasis Area and Severity Index (range from 0 to 72; a higher score is associated with more severe psoriasis), the Body Surface Area scale (range from 0 to 100% of the body surface area affected by psoriatic lesions) and the Dermatology Life Quality Index (range from 0 to 30; a higher value is associated with a lower quality of life) were used to assess the severity of psoriasis.
The following exclusion criteria were defined for this study: active infection at the time of laboratory testing, systemic steroid therapy, pregnancy and lactation period, diagnosed other skin diseases with an inflammatory background, simultaneous treatment with a biological drug and any conventional method, and nail involvement without skin lesions. Patients receiving biological drugs for less than 12 weeks and with lacking data were also excluded from this research. The super-response in our analysis was defined as achieving PASI100 at week 16 (±4 weeks) of therapy. Primary and secondary treatment failures were characterized accordingly to the B.47 drug program financed by the Ministry of Health of the Republic of Poland. A primary failure meant that the patient did not reach at least a 75% reduction in the Psoriasis Area and Severity Index (PASI75) or a 50% reduction in the Psoriasis Area and Severity Index (PASI50) with a simultaneous decrease in the DLQI (Dermatology Life Quality Index) or cDLQI (Children’s Dermatology Life Quality Index) score by at least five points at week 16 (±4 weeks) of treatment. A secondary failure was defined as an increase in the severity of the disease characterized by PASI > 10, BSA > 10% (Body Surface Area), and DLQI > 10 points during two consecutive follow-up appointments.
This retrospective study relied exclusively on medical histories of patients including the severity of psoriatic skin lesions, treatment of psoriasis, anthropometric data, comorbidities, family medical history of psoriasis, and the results of laboratory tests. Peripheral venous blood samples required for laboratory tests were collected from patients in a sitting position before the therapy initiation and at week 16 (±4) of treatment. Hematological inflammatory parameters were analyzed. Based on the results of laboratory tests, the levels of leukocytes, neutrophils, lymphocytes, monocytes and platelets were used to determine and analyze Systemic Immune-Inflammation Index (SII), Systemic Inflammation Response Index (SIRI), Aggregate Index of Systemic Inflammation (AISI), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), Neutrophil-to-Monocyte Ratio (NMR), derived Neutrophil-to-Lymphocyte Ratio (dNLR), Neutrophil-to-Monocyte-to-Lymphocyte Ratio (NMLR), Monocyte-to-Lymphocyte Ratio (MLR) at both time points—at week 0 and 16 (±4 weeks).
This research was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice rules, and all applicable legal regulations. The patients were provided with psoriasis treatment in line with recommendations of medical associations and clinical indications. The study obtained a positive opinion from the Bioethics Committee at the Medical University of Lodz (decision No. RNN/226/25/KE).
Statistical Analysis
The patients were divided into two groups, being assigned either to super-responders or non-super-responders, according to the observed clinical response to therapy. The study aimed to identify and assess clinical and demographic differences between these groups and to determine a potential biomarker for a high likelihood of achieving a super-response by a particular patient. The analysis used widely available blood-count-derived inflammatory markers, i.e., SII, SIRI, AISI, NLR, PLR, NMR, dNLR, NMLR, and MLR. In order to identify the aforementioned possible predictor, biomarkers based on the complete blood count were calculated at week 0 and 16 (±4 weeks) and compared between the groups.
The statistical analysis of the data was conducted using Statistica 13.3 analytics software. Pearson’s chi-squared or Fisher’s exact tests were used, depending on the data, to determine associations between the qualitative variables. To assess factors associated with belonging to the SR group, the multinomial logistic regression analysis was conducted. Changes over time for selected parameters evaluated at both time points were compared between the SR and nSR groups using the Mann–Whitney U-test. ROC analysis was used to identify the cut-off values. p-values < 0.05 were considered statistically significant.
5. Conclusions
The results of our study indicate that individuals who have a higher chance of achieving a super-response are younger patients, with no psoriatic lesions on the palms and soles, not suffering from non-alcoholic fatty liver disease, previously treated with methotrexate, and characterized by a higher level of dNLR at the beginning of treatment. Hematological inflammatory parameters are widely available and inexpensive, and for that reason appear to be perfect biomarkers that enable an early treatment personalization by selecting the most suitable therapy. Further research is required to assess the dNLR biomarker in this respect.
Author Contributions
Conceptualization and methodology, A.H. and A.Ż.; statistical analysis, R.Z.; writing—original draft preparation, A.H.; writing—review and editing, A.Ż. and R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Lodz under grant number 503/1-152-01/503-11-002.
Institutional Review Board Statement
This research was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice rules, and all applicable legal regulations. The study obtained a positive opinion from the Bioethics Committee at the Medical University of Lodz (decision No. RNN/226/25/KE), approval date: 10 September 2025.
Informed Consent Statement
Patient consent was waived due to a positive opinion from the Bioethics Committee of the Medical University of Łódź—No. RNN/226/25/KE Approval Date: 10 September 2025 This research was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice rules, and all applicable legal regulations. The study was retrospective (based on an analysis of medical records), therefore, there was no need to obtain patient consent to participate in the study—The committee declared that this retrospective study did not require special ethical approval and patient consent.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not publicly available due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-17 | Interleukin 17 |
| IL-12 | Interleukin 12 |
| IL-23 | Interleukin 23 |
| Il-6 | Interleukin 6 |
| IL-18 | Interleukin 18 |
| IL-13 | Interleukin 13 |
| IL-17R | Receptor for interleukin 17 |
| PsO | Psoriasis |
| UVB-NB | Narrowband Ultraviolet B |
| PUVA | Psoralen Ultra-Violet A |
| SR | Super-responders |
| nSR | Non-super-responders |
| PsA | Psoriatic arthritis |
| TC | Treatment cycles |
| BMI | Body Mass Index |
| MAFLD | Metabolic dysfunction-associated steatotic liver disease |
| NFLD | Non-alcoholic fatty liver disease |
| IL-17A | Subunit A of interleukin 17 |
| IL-17AF | Subunit A and F of interleukin 17 |
| IL-12/23 | Interleukin 12 and 23 |
| Anti-IL-17A | Antagonist of subunit A of interleukin 17 |
| Anti-TNF | Tumor necrosis factor inhibitor |
| Anti-IL-23 | Interleukin 23 antagonist |
| PASI | Psoriasis Area and Severity Index |
| PASI0 | Baseline Psoriasis Area and Severity Index |
| PASI100 | Complete clearance of psoriatic skin lesion |
| PASI90 | 90% reduction in psoriatic skin lesions |
| PASI75 | 75% reduction in psoriatic skin lesions |
| PASI50 | 50% reduction in psoriatic skin lesions |
| PASI16 | Psoriasis Area and Severity Index score at week 16 (±4) of biological treatment |
| PASI40 | Psoriasis Area and Severity Index score at week 40 (±4) of biological treatment |
| AE | Adverse reactions |
| BSA | Body Surface Area |
| BSA0 | Baseline Body Surface Area |
| BSA16 | Body Surface Area at week 16 (±4) of biological treatment |
| BSA40 | Body Surface Area at week 40 (±4) of biological treatment |
| cDLQI | Children’s Dermatology Life Quality Index |
| DLQI | Dermatology Life Quality Index |
| DLQI0 | Baseline Dermatology Life Quality Index score |
| DLQI16 | Dermatology Life Quality Index score at week 16 (±4) of biological treatment |
| DLQI40 | Dermatology Life Quality Index scoreat week 40 (±4) of biological treatment |
| SII | Systemic Immune-Inflammation Index |
| SIRI | Systemic Inflammation Response Index |
| AISI | Aggregate Index of Systemic Inflammation |
| NLR | Neutrophil-to-Lymphocyte ratio |
| PLR | Platelet-to-Lymphocyte ratio |
| NMR | Neutrophil-to-Monocyte ratio |
| dNLR | derived Neutrophil-to-Lymphocyte Ratio |
| NMLR | Neutrophil-to-Monocyte-to-Lymphocyte Ratio |
| MLR | Monocyte-to-Lymphocyte Ratio |
| SNPs | Single Nucleotide Polymorphisms |
| p | p-value |
| Cl | Confidence Interval |
| OR | Odds Ratio |
References
- Secchiero, P.; Rimondi, E.; Marcuzzi, A.; Longo, G.; Papi, C.; Manfredini, M.; Fields, M.; Caruso, L.; Di Caprio, R.; Balato, A. Metabolic Syndrome and Psoriasis: Pivotal Roles of Chronic Inflammation and Gut Microbiota. Int. J. Mol. Sci. 2024, 25, 8098. [Google Scholar] [CrossRef]
- Martínez-Vidal, M.P.; Jovani, V.; Noguera-Pons, J.R.; Álvarez-Cienfuegos, A. Osteoporosis in psoriatic arthritis: Risk factors, insufficiency fractures and its association with the disease activity. Reumatol. Clin. 2024, 20, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Takami, K.; Higashiyama, M.; Tsuji, S. Osteoporosis and osteopenia in patients with psoriatic arthritis: A single-centre retrospective study. Mod. Rheumatol. 2024, 34, 1252–1257. [Google Scholar] [CrossRef]
- Xu, L.; Cao, Y. The impact of body mass index on the relationship between psoriasis and Osteopenia: A mediating analysis based on NHANES (2003–2006). Arch. Dermatol. Res. 2025, 317, 268. [Google Scholar] [CrossRef] [PubMed]
- Prema, S.S.; Shanmugamprema, D. Systemic Psoriasis: From Molecular Mechanisms to Global Management Strategies. Clin. Rev. Allergy Immunol. 2025, 68, 79. [Google Scholar] [CrossRef] [PubMed]
- Mastorino, L.; Susca, S.; Cariti, C.; Verrone, A.; Stroppiana, E.; Ortoncelli, M.; Dapavo, P.; Ribero, S.; Quaglino, P. “Superresponders” at biologic treatment for psoriasis: A comparative study among IL17 and IL23 inhibitors. Exp. Dermatol. 2023, 32, 2187–2188. [Google Scholar] [CrossRef]
- Rompoti, N.; Politou, M.; Stefanaki, I.; Vavouli, C.; Papoutsaki, M.; Neofotistou, A.; Rigopoulos, D.; Stratigos, A.; Nicolaidou, E. Brodalumab in plaque psoriasis: Real-world data on effectiveness, safety and clinical predictive factors of initial response and drug survival over a period of 104 weeks. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 689–697. [Google Scholar] [CrossRef]
- Feldman, S.R.; Merola, J.F.; Pariser, D.M.; Zhang, J.; Zhao, Y.; Mendelsohn, A.M.; Gottlieb, A.B. Clinical implications and predictive values of early PASI responses to tildrakizumab in patients with moderate-to-severe plaque psoriasis. J. Dermatol. Treat. 2022, 33, 1670–1675. [Google Scholar] [CrossRef]
- Loft, N.; Egeberg, A.; Rasmussen, M.K.; Bryld, L.E.; Nissen, C.V.; Dam, T.N.; Ajgeiy, K.K.; Iversen, L.; Skov, L. Prevalence and characterization of treatment-refractory psoriasis and super-responders to biologic treatment: A nationwide study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1284–1291. [Google Scholar] [CrossRef]
- Morelli, M.; Galluzzo, M.; Madonna, S.; Scarponi, C.; Scaglione, G.L.; Galluccio, T.; Andreani, M.; Pallotta, S.; Girolomoni, G.; Bianchi, L.; et al. HLA-Cw6 and other HLA-C alleles, as well as MICB-DT, DDX58, and TYK2 genetic variants associate with optimal response to anti-IL-17A treatment in patients with psoriasis. Expert Opin. Biol. Ther. 2021, 21, 259–270. [Google Scholar] [CrossRef]
- Talamonti, M.; D’Adamio, S.; Galluccio, T.; Andreani, M.; Pastorino, R.; Egan, C.G.; Bianchi, L.; Galluzzo, M. High-resolution HLA typing identifies a new ‘super responder’ subgroup of HLA-C*06:02-positive psoriatic patients: HLA-C*06:02/HLA-C*04, in response to ustekinumab. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e364–e367. [Google Scholar] [CrossRef]
- Fratton, Z.; Bighetti, S.; Bettolini, L.; Maione, V.; Arisi, M.; Buligan, C.; Stinco, G.; Errichetti, E. Real-World Experience of Bimekizumab in a Cohort of109 Patients Over 48 Weeks and Identification of Predictive Factors for an Early Super Response and Risk of Adverse Events. Psoriasis 2025, 15, 145–158. [Google Scholar] [CrossRef]
- Thomas, S.E.; van den Reek, J.M.P.A.; Seyger, M.M.B.; de Jong, E.M.G.J. How to define a ‘super-responder’ to biologics in psoriasis studies. Br. J. Dermatol. 2023, 189, 621–622. [Google Scholar] [CrossRef]
- Schäkel, K.; Reich, K.; Asadullah, K.; Pinter, A.; Jullien, D.; Weisenseel, P.; Paul, C.; Gomez, M.; Wegner, S.; Personke, Y.; et al. Early disease intervention with guselkumab in psoriasis leads to a higher rate of stable complete skin clearance (‘clinical super response’): Week 28 results from the ongoing phase IIIb randomized, double-blind, parallel-group, GUIDE study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2016–2027. [Google Scholar] [CrossRef]
- Reich, K.; Gordon, K.B.; Strober, B.; Langley, R.G.; Miller, M.; Yang, Y.W.; Shen, Y.K.; You, Y.; Zhu, Y.; Foley, P.; et al. Super-response to guselkumab treatment in patients with moderate-to-severe psoriasis: Age, body weight, baseline Psoriasis Area and Severity Index, and baseline Investigator’s Global Assessment scores predict complete skin clearance. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Mortato, E.; Talamonti, M.; Marcelli, L.; Megna, M.; Raimondo, A.; Caldarola, G.; Bernardini, N.; Balato, A.; Campanati, A.; Esposito, M.; et al. Predictive Factors for Super Responder Status and Long-Term Effectiveness of Guselkumab in Psoriasis: A Multicenter Retrospective Study. Dermatol. Ther. 2025, 15, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, K.; Duan, Y.; Chen, X.; Zhang, M.; Kuang, Y. Characterization and treatment outcomes of biologic therapy in super-responders and biologic-refractory psoriasis patients: A single-center retrospective study in China. J. Am. Acad. Dermatol. 2025, 93, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, S.; Weisenseel, P.; Groß, D.; Ostendorf, R.; Zimmer, S.; Otto, A.; Taut, F.J.H.; Makuc, J.; Jacobsen, S.; Trenkler, N.; et al. Long-Term Impact of Guselkumab on Skin, Sexuality, and Perceived Stigmatization in Patients With Psoriasis in Routine Clinical Practice: Week 76 Effectiveness and Safety Results From the Prospective German Multicenter G-EPOSS Study. J. Dermatol. 2025, 52, 1368–1381. [Google Scholar] [CrossRef]
- Gargiulo, L.; Ibba, L.; Malagoli, P.; Amoruso, F.; Argenziano, G.; Balato, A.; Bardazzi, F.; Burlando, M.; Carrera, C.G.; Damiani, G.; et al. A risankizumab super responder profile identified by long-term real-life observation-IL PSO (ITALIAN LANDSCAPE PSORIASIS). J. Eur. Acad. Dermatol. Venereol. 2024, 38, e113–e116. [Google Scholar] [CrossRef]
- Esposito, M.; Gisondi, P.; Assorgi, C.; Bellinato, F.; Brianti, P.; Burlando, M.; Brunasso, G.; Caccavale, S.; Caldarola, G.; Campione, E.; et al. Super Responder Profile Under Bimekizumab Treatment in Moderate-to-Severe Psoriasis: A Short Term Real-Life Observation-IL PSO (Italian Landscape Psoriasis). Clin. Drug Investig. 2025, 45, 309–315. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, K.; Jian, L.; Duan, Y.; Zhang, M.; Kuang, Y. Comparison between super-responders and non-super-responders in psoriasis under adalimumab treatment: A real-life cohort study on the effectiveness and drug survival over one-year. J. Dermtol. Treat. 2024, 35, 2331782. [Google Scholar] [CrossRef]
- Marcelli, L.; Belcastro, A.; Talamonti, M.; Paganini, C.; Fico, A.; Savastano, L.; Di Raimondo, C.; Vellucci, L.; Bianchi, L.; Galluzzo, M. Characterization of Super-Responder Profile in Chronic Plaque Psoriatic Patients under Guselkumab Treatment: A Long-Term Real-Life Experience. J. Clin. Med. 2024, 13, 5175. [Google Scholar] [CrossRef]
- Ruiz-Villaverde, R.; Vasquez-Chinchay, F.; Rodriguez-Fernandez-Freire, L.C.; Armario-Hita, J.; Pérez-Gil, A.; Galán-Gutiérrez, M. Super-Responders in Moderate-Severe Psoriasis under Guselkumab Treatment: Myths, Realities and Future Perspectives. Life 2022, 12, 1412. [Google Scholar] [CrossRef]
- Mason, K.J.; Alabas, O.A.; Dand, N.; Warren, R.B.; Reynolds, N.J.; Barker, J.N.W.N.; Yiu, Z.Z.N.; Smith, C.H.; Griffiths, C.E.M. BADBIR Study Group. Characteristics of ‘super responders’ and ‘super nonresponders’ to first biologic monotherapy for psoriasis: A nested case-control study. Br. J. Dermatol. 2024, 190, 441–444. [Google Scholar] [CrossRef]
- Menéndez Sánchez, M.; Muñiz de Lucas, A.; Pérez Fernández, E.; Llamas Velasco, M.; Ruiz Genao, D.P.; López Estébaranz, J.L. Super-responders in psoriasis under interleukin 23 inhibitor treatments, experience in two centres. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1321–e1322. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska-Banasik, D.; Pastuszczak, M.; Zawadzinska-Halat, K.; Hadas, E.; Bozek, A. Serum IL-18/IL-13 Ratio Predicts Super Response to Secukinumab in Patients with Psoriasis. Int. J. Mol. Sci. 2025, 26, 6432. [Google Scholar] [CrossRef] [PubMed]
- Morariu, S.H.; Cotoi, O.S.; Tiucă, O.M.; Baican, A.; Gheucă-Solovăstru, L.; Decean, H.; Brihan, I.; Silaghi, K.; Biro, V.; Șerban-Pescar, D.; et al. Blood-Count-Derived Inflammatory Markers as Predictors of Response to Biologics and Small-Molecule Inhibitors in Psoriasis: A Multicenter Study. J. Clin. Med. 2024, 13, 3992. [Google Scholar] [CrossRef] [PubMed]
- Wiala, A.; Elhage, K.G.; Leung, A.; Young, A.T.; Gregory, M.; Adrianto, I.; Zhou, L.; Mi, Q.S.; Kumar, S.; Orcales, F.; et al. Patients with PSOriasis and Suppurative Hidradenitis (PSO-SH) share genetic risk factors and are at risk of increased morbidity. J. Am. Acad. Dermatol. 2025, 92, 1303–1311. [Google Scholar] [CrossRef]
- Untaaveesup, S.; Kantagowit, P.; Ungprasert, P.; Kitlertbanchong, N.; Vajiraviroj, T.; Sutithavinkul, T.; Techataweewan, G.; Eiumtrakul, W.; Threethrong, R.; Chaemsupaphan, T.; et al. The Risk of Metabolic Dysfunction-Associated Steatotic Liver Disease in Moderate-to-Severe Psoriasis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 1374. [Google Scholar] [CrossRef]
- Valencia, O.; López, C.; Vanegas-Duarte, E.; Fillizola, C.; Bejarano Ramírez, D.F.; Cortés Mejía, N.A.; Vera Torres, A. Risk Factors Related to the Development of Nonalcoholic Fatty Liver: A Systematic Review. Can. J. Gastroenterol. Hepatol. 2025, 2025, 9964486. [Google Scholar] [CrossRef]
- Costache, D.O.; Blejan, H.; Cojocaru, D.L.; Ioniță, G.A.; Poenaru, M.; Constantin, M.M.; Costache, A.C.; Căruntu, C.; Balaban, D.V.; Costache, R.S. Intersecting Pathways: Nonalcoholic Fatty Liver Disease and Psoriasis Duet-A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 2660. [Google Scholar] [CrossRef]
- Zhang, M.; Su, W.; Deng, J.; Zhai, B.; Zhu, G.; Gao, R.; Zeng, Q.; Qiu, J.; Bian, Z.; Xiao, H.; et al. Multi-ancestry genome-wide meta-analysis with 472,819 individuals identifies 32 novel risk loci for psoriasis. J. Transl. Med. 2025, 23, 133. [Google Scholar] [CrossRef]
- Talamonti, M.; Galluzzo, M.; van den Reek, J.M.; de Jong, E.M.; Lambert, J.L.W.; Malagoli, P.; Bianchi, L.; Costanzo, A. Role of the HLA-C*06 allele in clinical response to ustekinumab: Evidence from real life in a large cohort of European patients. Br. J. Dermatol. 2017, 177, 489–496. [Google Scholar] [CrossRef]
- Prieto-Pérez, R.; Solano-López, G.; Cabaleiro, T.; Román, M.; Ochoa, D.; Talegón, M.; Baniandrés, O.; López-Estebaranz, J.L.; de la Cueva, P.; Daudén, E.; et al. New polymorphisms associated with response to anti-TNF drugs in patients with moderate-to-severe plaque psoriasis. Pharmacogenomics J. 2018, 18, 70–75. [Google Scholar] [CrossRef]
- Butrón-Bris, B.; Llamas-Velasco, M.; Armesto, S.; Sahuquillo-Torralba, A.; Pujol-Montcusí, J.; Ruiz-Villaverde, R.; Martínez-López, A.; de la Cueva, P.; Romero-Maté, A.; Roustan, G.; et al. Genetic Polymorphisms in Psoriasis: Investigating Genetic Variations for Precise Profiling of Response to Brodalumab in Real-Life Clinical Practice. Actas Dermo-Sifiliogr. 2025, 116, 824–829. [Google Scholar] [CrossRef]
- Andersen, C.S.B.; Kvist-Hansen, A.; Siewertsen, M.; Enevold, C.; Hansen, P.R.; Kaur-Knudsen, D.; Zachariae, C.; Nielsen, C.H.; Loft, N.; Skov, L. Blood Cell Biomarkers of Inflammation and Cytokine Levels as Predictors of Response to Biologics in Patients with Psoriasis. Int. J. Mol. Sci. 2023, 24, 6111. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, C.; Xourafa, A.; Agostino, R.M.; Corigliano, V.; Botindari, A.; Gaudio, A.; Morabito, N.; Allegra, A.; Catalano, A. Exploring the Association Between Platelet Count, the Systemic Immune Inflammation Index, and Fracture Risk in Postmenopausal Women with Osteoporosis: A Cross-Sectional Study. J. Clin. Med. 2025, 14, 5453. [Google Scholar] [CrossRef] [PubMed]
- Firinci, B.; Aydin, C.; Yunluel, D.; Ibrahim, A.; Yigiter, M.; Ahiskalioglu, A. The Role of Systemic Immune-Inflammation Index (SII) in Diagnosing Pediatric Acute Appendicitis. Diagnostics 2025, 15, 1942. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, J.; He, T.; Liu, C. The predictive value of NLR, SII, and complement 3 in treatment response for systemic lupus erythematosus with immune thrombocytopenia. Front. Immunol. 2025, 16, 1606510. [Google Scholar] [CrossRef]
- Tamer, F.; Edek, Y.C.; Aksakal, A.B. Effect of Treatment with Biologic Agents on the Novel Inflammatory Biomarkers Systemic Immune Inflammation Index and Systemic Inflammation Response Index for Psoriasis. Dermatol. Pract. Concept. 2024, 14, e2024065. [Google Scholar] [CrossRef]
- Çelik, M.S.; Aktaş, H. The effect of IL-17 and IL-23 ınhibitors on hematologicalınflammatory parameters in patients with psoriasis vulgaris. Ir. J. Med. Sci. 2025, 194, 1329–1334. [Google Scholar] [CrossRef]
- Kearney, N.; Gorecki, P.; Acciarri, L.; Buyze, J.; Akawung, A.; Merola, J.F.; Kirby, B. Treatment of Plaque Psoriasis with Guselkumab Reduces Systemic Inflammatory Burden as Measured by Neutrophil/Lymphocyte Ratio, Platelet/Lymphocyte Ratio, and Monocyte/Lymphocyte Ratio: A post hoc Analysis of Three Randomised Clinical Trials. Dermatology 2025, 241, 272–286. [Google Scholar] [CrossRef]
- Tiucă, O.M.; Morariu, S.H.; Mariean, C.R.; Tiucă, R.A.; Nicolescu, A.C.; Cotoi, O.S. Impact of Blood-Count-Derived Inflammatory Markers in Psoriatic Disease Progression. Life 2024, 14, 114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).