Abstract
Hybridization and polyploidization are key evolutionary forces shaping fish biodiversity. But their interaction with environmental factors, such as temperature, remains poorly understood. This study examined how maternal genome composition and incubation water temperature influence the hatching success, ploidy structure, morphology and early growth of polyploid Cobitis larvae. Crosses were performed using triploid Cobitis females with three genomic compositions (EEN, EET and ETN), representing combinations of genomes from C. elongatoides (E), C. taenia (T) and C. tanaitica (N), and diploid C. taenia males as sperm donors. Fertilized eggs were incubated at 18 °C, 22 °C and 28 °C. Triploid and tetraploid offspring occurred in comparable proportions on average across all groups, but developmental abnormalities were significantly more observed in tetraploid larvae. Females with EET and ETN genomes achieved higher hatching success than those with the EEN genome. Temperature had a pronounced effect on developmental timing and success: hatching occurred earliest at 28 °C, but survival decreased and abnormalities were most frequent. These results highlight genome- and temperature-dependent trade-offs in early development of Cobitis hybrids, providing new insight into reproductive dynamics and the potential resilience of polyploid systems under climate warming.
1. Introduction
In poikilothermic animals such as teleost fish, ambient temperature modulates growth, development, and reproduction by acting on multiple levels of the brain–pituitary–gonad axis [1,2]. Warmer-than-optimal temperatures can affect every stage of reproductive development, from gametogenesis to spawning, altering both timing and success rates [1]. Experimental studies across diverse taxa, including Atlantic salmon Salmo salar [3], rainbow trout Oncorhynchus mykiss [4], European catfish Silurus glanis [5], Nile tilapia Oreochromis niloticus [6] and aquarium species such as spotted scat Scatophagus argus [7] and Korean rockfish Sebastes schlegelii [8] consistently demonstrate that temperature critically influences gonad maturation, fertilization, and embryonic survival.
Polyploidy, the presence of more than two chromosome sets, is a major evolutionary event that occurred repeatedly in both plants and animals. Polyploidization has played a pivotal role in vertebrate evolution. Ancient whole-genome duplications (WGDs), likely of autopolyploid origin, were fundamental to early vertebrate diversification [9,10,11]. In contrast, recent polyploidization events, particularly those of allopolyploid origin, are rare in vertebrates and have been described mainly in fish and amphibians [12,13]. The genus Cobitis, which includes naturally occurring hybrids with different ploidy levels, therefore provides an important model for studying the mechanisms and consequences of hybridization and polyploidization processes [14,15,16,17]. These small benthic fishes, usually about 10 cm in length, comprise nearly 110 species distributed across Eurasia and North Africa [18], including 33 species in Europe. At least three of these species occur in Central Europe—the spined loach (C. taenia), the Danubian loach (C. elongatoides, EE) and C. tanaitica, NN—which form complexes of natural hybrids that include numerous polyploid lineages [19]. Triploid (3n) Cobitis females carry different genomic combinations depending on their parental species. Within the European range, the most common genomic compositions are EEN, EET, ETN, ETT and ENN [19]. In Poland, mixed diploid—polyploid populations consist mainly of diploid (2n) hybrids (ET) and 3n hybrid females with the EEN, EET, ETN and ETT genomes, whereas allotetraploids (4n) of both sexes rarely exceed [20,21,22]. Although C. tanaitica has not been found in Polish waters, its genome persists in 3n and 4n hybrids [19,20]. These hybrid forms are morphologically similar and can be distinguished only through cytogenetic or molecular analyses [23]. The origin of these hybrids is associated with postglacial dispersal and secondary contact between previously isolated populations derived from Ponto-Caspian refugia. Following the retreat of the glaciers, these populations recolonized Central and Eastern Europe, where contact zones among C. taenia, C. elongatoides, and C. tanaitica were established, promoting the formation of hybrid and clonal lineages. Mito-nuclear discordance, resulting from historical hybridization and mitochondrial capture, explains the presence of C. tanaitica mitochondrial haplotypes within Cobitis populations dominated by other nuclear genomes [24].
Reproductive biology in Cobitis is equally complex. These fishes spawn in batches, from May to July [25]. Diploid and triploid hybrid males are typically sterile, whereas females reproduce clonally through gynogenesis or, less frequently, sexually to produce 4n offspring of both sexes [22,26,27]. Gynogenetic reproduction is triggered by sperm activation without genetic contribution, and it is supported by premeiotic genome endoreplication, which restores diploidy in clonal oocytes before meiosis [28]. Unlike triploids, tetraploid females are rarely fertile and produce fewer eggs than diploids and triploids [25]. In our previous study, 4n progeny obtained from induced spawning experiments showed markedly lower survival than clonal 3n ones during early development [29]. In that study, Juchno et al. [29] demonstrated that the initial proportions of 3n and 4n individuals hatching were comparable; however, the number of tetraploids decreased significantly over time, reaching levels similar to those found in the environment. Furthermore, some polyploid offspring exhibited various developmental deformities during embryogenesis and larval stages [29].
Fish of the genus Cobitis thus provide a unique model for studying also how environmental factors, particularly water temperature, influence reproductive outcomes. Polyploid organisms are known for their ability to tolerate environmental fluctuations, and in some taxa polyploidy has been linked to enhanced resilience to thermal stress. For example, certain polyploid plants show increased cold tolerance [30], or benefit from post–fire warming [31]. Moreover, 3n hybrid Pelophylax frogs exhibit faster growth and greater body mass than diploids at lower temperatures [32]. Triploid Cobitis hybrids possess larger nuclei and cells, as well as a significantly lower metabolic rate than diploids [33], which may influence their developmental response to temperature. In the context of ongoing climate warming [34], understanding how water incubation temperature influences reproduction in polyploid Cobitis is essential for predicting the persistence of their lineages, which spawn across a broad thermal range 18–28 °C [35]. Despite extensive research on fish reproduction under varying thermal regimes, the effects of temperature and genome composition in natural hybrid polyploid systems remain poorly understood. To our knowledge, no previous study has compared hatching success, ploidy structure, and early larval development among allotriploid Cobitis females with known genomic composition under controlled temperature regimes (18 °C, 22 °C, and 28 °C).
The present study aimed to determine how maternal genomic composition and incubation temperature affect reproductive success, developmental stability, and early larval growth in polyploid Cobitis hybrids. We hypothesized that (1) developmental success, including hatching rate, frequency of abnormalities, and larval body length, differs among 3n females with distinct genomic constitutions (EEN, EET, ETN) (2) incubation temperature affects both hatching success and offspring phenotype, and (3) the proportion of gynogenetic (3n) and sexually derived (4n) progeny remains similar during the first few days after hatching across temperatures but may vary among genomic lineages depending on their reproductive mode preference.
2. Results
2.1. Female Genome Structure
Based on molecular and karyotype analyses, the triploid parental females were classified according to their inferred parental subgenomes: C. taenia (T), C. elongatoides (E) and C. tanaitica (N). These were assigned to three genomic groups: EEN, EET and ETN. These groups possess the following numbers of chromosomes: 3n = 75, 3n = 74* and 3n = 74, respectively. Of the 20 triploid parental females analyzed, 5 exhibited an EEN composition, 5 an EET composition and 10 an ETN composition.
2.2. Hatching Success
Overall, none of the tested temperatures precluded offspring production. Furthermore, a significant effect of temperature and genomic composition on developmental success has been demonstrated. The duration of embryonic development varied; larvae incubated at 28 °C hatched after three days, those incubated at 22 °C hatched after four days, and those incubated at 18 °C hatched after five days post-fertilization. Table 1 shows the number of eggs, the number of larvae hatched, and the resulting hatching success rates for each family across the three 3n female genomes: EEN, EET and ETN—at three experimental temperatures: 18 °C, 22 °C and 28 °C. Regardless of genome composition, the mean hatching success rates were 32.9%, 31.7%, and 14.8% at 18 °C, 22 °C, and 28 °C, respectively. However, the mean hatching success of offspring was 10.3% for the EEN genome, 31.7% for the EET genome and 31.9% for the ETN genome, regardless of water temperature.

Table 1.
Number of eggs, hatched larvae, and hatching success (%) of offspring produced by allotriploid Cobitis females of different genome compositions (EEN, EET, and ETN) fertilized by diploid C. taenia males at three incubation temperatures (18 °C, 22 °C, and 28 °C). The table summarizes variation in reproductive performance according to female genome composition and incubation temperature.
Offspring of EEN females generally had low hatching success. Among offspring derived from ETN females, hatching success was 16.8% at 18 °C, followed by 7.7% at 22 °C and 6.4% at 28 °C. No significant differences were found within the EEN genome at different temperatures (p > 0.05). However, we emphasize that this lack of difference may be caused by interfamilial differences where several families (F35M35, F37M37, F39M39 and F47M47) indeed showed no hatching in one or two temperature groups, whereas one family, F35M35, showed consistently high success across temperatures, particularly at 18 °C (39.5%).
In the EET group, the mean hatching success was 31.2% at 18 °C, 49.8% at 22 °C, and 14.2% at 28 °C. Statistically significant differences were found between hatching success at 22 °C and 28 °C (p < 0.05). The F72M72 family stands out in this group, showing high efficiency at all temperatures (up to 84.5% at 18 °C).
Among offspring derived from ETN females, hatching success was 41.8% at 18 °C, 34.6% at 22 °C, and 19.3% at 28 °C. However, there were no statistically significant differences between the three temperatures (p > 0.05). Some ETN families (e.g., F78M78) exhibited very high hatching success at all temperatures. Others (e.g., F5M5 and F10M10) showed complete hatching failure at the highest temperature only, while some (e.g., F12M12) exhibited no hatching at 18 °C and 28 °C.
At temperatures of 18 °C and 28 °C, there were no statistically significant differences in hatching success between the studied genomes (p > 0.05). However, at 22 °C, EEN females exhibited significantly lower hatching success compared to EET and ETN females (p < 0.05).
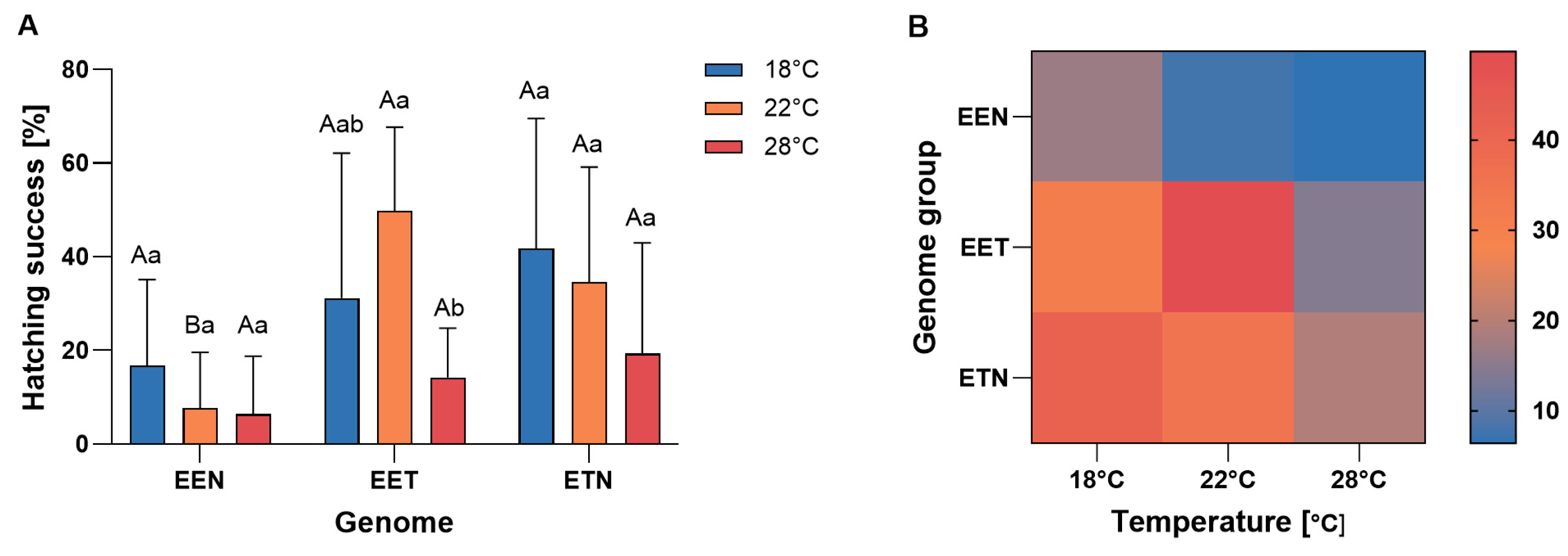
Interaction between maternal genome composition and incubation temperature was observed in terms of hatching success (Figure 1). Offspring of ETN females exhibited higher hatching success at 18 °C, whereas offspring of EET females demonstrated higher hatching success at 22 °C. In contrast, EEN offspring exhibited significantly reduced hatching success at 22 °C and 28 °C, achieving minimal success at higher temperatures. Overall, ETN and EET females produced offspring with a similar mean hatching success rate, while EEN females exhibited the lowest rate, particularly at the highest temperature (Figure 1).

Figure 1.
(A) Mean frequency of hatching success (%) of Cobitis progeny according to female genome composition (EEN, EET, and ETN) and incubation water temperature (18 °C, 22 °C or 28 °C). Bars represent mean ± SD. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test (p < 0.05). Different uppercase letters indicate significant differences (p < 0.05) among genotypes within a given temperature, and different lowercase letters indicate significant differences (p < 0.05) among temperatures within each genotype (B) Heatmap illustrates hatching success (%) across genotypes (EEN, EET, ETN) and incubation water temperature (18 °C, 22 °C and 28 °C). Color intensity represents mean hatching success for each genome × temperature combination.
2.3. Ploidy and Phenotype of Progeny
The ploidy status of randomly selected progeny during the three days post hatching revealed triploids (3n) and tetraploids (4n), as well as progeny at other ploidy levels which are not included in this study. Comparable proportions of triploid and tetraploid offspring were observed across all genome compositions (EEN, EET and ETN) and water temperatures tested (18 °C, 22 °C and 28 °C), although considerable variability was observed among individual crosses. Moreover, no consistent differences were observed between groups. No statistically significant differences were detected (p > 0.05; Table 2).

Table 2.
Triploid (3n) and tetraploid (4n) progeny (in%) according to genome composition (EEN, EET, ETN) and water temperature (18 °C, 22 °C, 28 °C). It was observed that the mean proportions of triploid and tetraploid offspring were comparable across all genome compositions and temperatures, with no statistically significant differences detected.
The developmental patterns of triploid and tetraploid offspring were highly similar (Figure 2). At 1 dph, numerous melanophores appeared across the larval body, including around the eyes. Blood circulation, the intestine and an elongated yolk sac were clearly visible, and a continuous fin fold had formed, connecting the dorsal, caudal and pelvic regions. By 2 dph, four pairs of filamentous external gills and small pectoral fins were present; the eyes had darkened, and the yolk sac had become smaller. By 3 dph, the external gills were still visible and the mouth had opened, while the pectoral fins had become more prominent and enlarged (Figure 2).

Figure 2.
Properly developed triploid (3n) and tetraploid (4n) Cobitis larvae during the first three days post hatching. Early post-hatch development proceeded similarly in both ploidy levels, showing comparable timing of yolk sac absorption, fin fold formation, and pigmentation.
Physical deformities, which sometimes occurred in high numbers, were detected in both triploid and tetraploid offspring. A comparison of the percentage of abnormalities in 3n and 4n individuals, regardless of genome composition or water temperature, revealed a statistically significant difference: 43.0% of 4n offspring had abnormalities, compared to 25.2% of 3n offspring (p < 0.05). The most frequent abnormalities affected the heart cavity, yolk sac and body axis. Specifically, axial deformities such as kyphosis and lordosis, as well as lateral deformities including scoliosis and C-shaped curvature, were observed. Numerous oedemas developed within the heart cavity. Yolk sac malformations most often manifested as enlargement of the anterior region, giving it a pear-shaped appearance. Additionally, some larvae exhibited abnormally developed external gills (Figure 3).

Figure 3.
Examples of external morphological abnormalities in tetraploid (4n) Cobitis progeny of females with different genomic compositions: EEN (a–c), EET (d–f), and ETN (g–i). The most frequent malformations included cardiac edema, yolk sac edema, and axial deformities such as kyphosis, lordosis, and C-shaped body curvature. Yolk sac malformations often appeared as anterior enlargement, while some larvae also exhibited abnormal development of the external gills. Marks: arrowhead—cardiac edema; arrow yolk sac edema; dotted arrow—kyphosis, lordosis and axial curvature.
Significant differences in the frequency of abnormalities were observed among triploid progeny in relation to the genome composition of the triploid female and the water temperature (see Figure 4). A significantly higher proportion of abnormal progeny were observed in females with the EET genome (41.7%) than in those with the EEN genome (0%) at a temperature of 28 °C (p < 0.05). More abnormalities were also observed at 18 °C (45.6%) than at 22 °C (15%) within the ETN genome (p < 0.05) (Figure 4A). Among tetraploid individuals, the EEN genome was characterized by the lowest percentage of abnormal individuals at both 18 °C (14.0%) and 28 °C (0.0%) (Figure 5). A statistically significant increase in the number of abnormal individuals was observed in the progeny of females with the ETN genome (49.0%) compared to the EEN genome (13.0%) at 18 °C (p < 0.05). At 28 °C, a statistically significant increase in abnormal individuals was observed among the progeny of females with the EET (75.6%) and ETN (57.9%) genomes, compared to the EEN genome (0.0%) (Figure 4B).

Figure 4.
(A,C) Proportion of normal and abnormal larvae in triploid (3n; (A)) and tetraploid (4n; (C)) Cobitis progeny according to female genome composition (EEN, EET or ETN) and incubation water temperature (18 °C, 22 °C or 28 °C). Bars represent mean ± SD. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test (p < 0.05). Different lowercase letters indicate significant differences (p < 0.05) in the percentage of abnormal larvae between genotypes within a given temperature. Different uppercase letters indicate significant differences (p < 0.05) in the percentage of abnormal larvae among individuals between temperatures (18 °C, 22 °C and 28 °C) within each genotype. (B,D) Heatmaps showing the frequency of abnormalities in triploid (3n; (B)) and tetraploid (4n; (D)) progeny across genotypes and incubation temperatures. Color intensity represents the mean frequency of abnormalities for each genome x temperature combination.

Figure 5.
(A) Standard body length (mean ± SE) of Cobitis progeny (SL) according to female genome composition (EEN, EET and ETN) and incubation water temperature (18 °C, 22 °C and 28 °C). Different lowercase letters indicate significant differences (p ≤ 0.05) among temperatures within a given genome, and different uppercase letters indicate significant differences (p ≤ 0.05) among genomes (EEN, EET and ETN) within a given temperature. (B) Heatmap summarizing mean body length of Cobitis progeny according to maternal genome composition (EEN, EET, ETN) and incubation water temperature (18 °C, 22 °C and 28 °C). Color intensity represents the mean value for each genome x temperature combination.
2.4. Body Length of Progeny
Measurements were taken on the third day post hatching. Larvae originating from females with the EEN genome had body lengths ranging from 5.0 to 7.8 mm at 18 °C (mean 6.8 mm), from 6.2 to 7.9 mm at 22 °C (mean 7.3 mm) and from 7.9 to 8.4 mm at 28 °C (mean 8.1 mm). Larval lengths differed significantly among all tested temperatures in this genomic composition (p < 0.05).
In the EET genome, larval body length ranged from 5.3 to 7.3 mm (mean 6.6 mm) at 18 °C, from 5.1 to 7.3 mm (mean 6.5 mm) at 22 °C, and from 4.9 to 7.7 mm (mean 6.3 mm) at 28 °C, with no significant differences between temperatures (p > 0.05).
Larvae originating from females with the ETN genome measured between 5.0 and 7.9 mm (mean 6.6 mm) at 18 °C, between 4.8 and 7.2 mm (mean 6.6 mm) at 22 °C, and between 4.2 and 8.2 mm (mean 6.8 mm) at 28 °C. Again, there were no significant differences between temperatures (p > 0.05).
A comparative analysis revealed that, at 22 °C, the larval body length of the EEN genomic composition was significantly greater than that of the EET and ETN compositions (p < 0.05). However, at 28 °C, significant differences were observed among all genomic compositions (p < 0.05) (see Figure 5).
3. Discussion
This research contributes to a broader understanding of how environmental factors, particularly water temperature, influence fish reproduction and early development. The study is especially relevant in the context of progressively rising water temperatures, a trend observed worldwide since the late 19th century [34]. Variations in temperature have been shown to affect not only the rate of embryonic and larval development, but also growth efficiency and survival in many fish species [36,37,38,39]. By focusing on triploid hybrid females, which dominate Cobitis populations in inland waters across Central Europe [19,21], the present study provides an important model for examining how genomic architecture interacts with environmental temperature to shape reproductive potential and developmental stability in polyploid fishes.
This study demonstrates that both the genomic composition of triploid Cobitis females and the temperature of egg incubation determine the early developmental success of their offspring. A consistent relationship between the quantity and quality of progeny was observed across all three genomic groups (EEN, EET, ETN). Offspring of EET and ETN females were characterized by higher hatching success but also by a greater frequency of developmental abnormalities, whereas progeny of EEN females showed lower hatching success combined with fewer abnormalities and greater body length during the first days after hatching. Temperature heterogeneously influences these outcomes. The intermediate temperature of 22 °C supported the most favorable developmental performance and the lowest incidence of deformities, while both lower (18 °C) and higher (28 °C) temperatures increased the proportion of malformed larvae, particularly among EET and ETN progeny. In contrast, offspring of EEN females were comparatively resistant to temperature variation, exhibiting consistently low deformity rates and a clear positive dependence of body length on temperature (28 °C > 22 °C > 18 °C at 3 dph).
3.1. Female Genome Structure
In natural diploid–polyploid Cobitis populations in Poland, triploid females can make up to 95% of the population. The remainder of the fraction consists of diploid C. taenia (TT), C. elongatoides (EE), their diploid hybrids (which sometimes may dominate locally; [21]), and small number of allotetraploids of both sexes [29].
In this study, we induced the reproduction of triploid females of hybrid origin, whose genomic composition indicated ancestry from two (EEN, EET) or three (ETN) different Cobitis species. The analyzed females were assigned to three distinct genomic groups: EEN, EET and ETN, with corresponding karyotypes of 3n = 75, 3n = 74* and 3n = 74, respectively. The genome composition of the analyzed females was reliably determined through the combined karyotype and microsatellite analyzes, an approach previously demonstrated to provide high accuracy in Cobitis hybrids [14,19,20].
3.2. Hatching Success
The hatching success of offspring differed markedly among genomic groups. The mean hatching success of triploid Cobitis females ranged between approximately 10% in EEN, 31% in EET, and 32% in ETN, with overall success rates declining at the highest temperature (28 °C). Although these values are lower than those reported for many teleost hybrids, they are broadly comparable to previous findings in Cobitis [40], where the mean hatching rate of allotriploid females ranged from 20 to 35% under similar experimental conditions. Such consistency indicates that the relatively low reproductive efficiency of triploid Cobitis reflects inherent genomic and developmental constraints rather than experimental conditions.
However, larvae of EEN origin—both triploid and tetraploid—displayed markedly fewer developmental abnormalities across all temperatures, and at 22 °C they exhibited the lowest proportion of abnormalities relative to EET and ETN offspring. Thus, despite producing fewer hatchlings, EEN females yielded higher-quality progeny, consistent with a strategy that prioritizes offspring viability over quantity. Such an adaptive strategy may enhance larval survival under natural conditions despite reduced reproductive output and may help explain why the oldest triploid clone, EEN, maintains higher phenotypic performance and fertility compared with the younger triploid forms, EET and ETN [41].
The present findings are consistent with earlier experimental data in Cobitis [40], where crosses between allotriploid females and diploid C. taenia males produced offspring with hatching success and 14-day survival comparable to those of diploid C. taenia and diploid C. taenia crosses, but with a higher incidence of skeletal deformities. Importantly, that study examined only differences between ploidy levels and did not assess the influence of specific genome composition. Our results extend this evidence by demonstrating that the genomic configuration (EEN, EET, ETN)—not only ploidy—determines the balance between hatching success and progeny quality in Cobitis.
Comparative data from other fish taxa provide broader context for interpreting these results. In hybrids of closely related species, reproductive success is often high despite genomic divergence. Crosses between white crucian carp (Carassius cuvieri) and red crucian carp C. auratus achieved hatching rates of around 80% [42], while hybrids produced between the Russian sturgeon (Acipenser gueldenstaedtii) and American paddlefish (Polyodon spathula), the so-called sturddlefish, exhibited remarkably high fertilization (86–93%) and hatching success (78–85%), with many larvae developing into viable triploids or pentaploids [43]. Despite their complex genomic background, the early survival of these interfamily hybrids remained relatively high (62–74%), illustrating that polyploidization can sometimes facilitate the success of otherwise improbable distant crosses.
In contrast, other hybrid combinations show little or no reduction in early developmental performance. For example, in Far Eastern dace (Tribolodon), F1 hybrids hatched at rates (~76%) comparable to those of purebred offspring (~86%), indicating that moderate genomic divergence does not necessarily impair embryo viability [44]. However, polyploidization often introduces developmental costs, even when hatching success is maintained. In crucian carp × zebrafish crosses, hatching rates were moderate, but hybrid larvae exhibited altered hatching enzyme activity and chorion properties associated with higher abnormality rates [45]. Likewise, induction of gynogenetic tetraploidy in Japanese flounder (Paralichthys olivaceus) led to a sharp decrease in hatching success (~47% compared with ~90% in controls) and an increase in deformities, confirming that additional genome duplication imposes significant developmental constraints [46].
Taken together, these examples demonstrate that hybridization and polyploidization exert variable effects on reproductive success. In some taxa, genome duplication reduces embryonic viability but enhances post-hatch robustness; in others, hatch rates remain stable but malformation rates increase; and in rare cases, even distant crosses can yield unexpectedly viable hybrids. Our results suggest that, in Cobitis, reproductive potential depends not only on ploidy level but also on the specific genomic composition of triploid females. The EEN lineage appears to follow a “quality-over-quantity” strategy, producing fewer but more viable offspring, whereas EET and ETN clones achieve higher hatching success at the cost of increased malformations.
Mechanistic studies conducted so far in Cobitis and other cyprinids do not yet allow for a clear explanation why EEN, EET, and ETN differ in reproductive outcomes. In triploid hybrid Cobitis, transcriptomic analyses have shown that the duplicated genome often dominates gene expression, generating dosage-dependent phenotypic biases [15]. However, dosage asymmetry alone cannot explain our findings, because both EEN and EET share a duplicated C. elongatoides genome but produce offspring of contrasting quality. It is possible that the difference arises from interactions between the duplicated genome and the third, non-duplicated genome. The N subgenome (from C. tanaitica) appears to be more compatible with the duplicated E genome than the T subgenome (from C. taenia), although this idea is not strongly supported by existing data. Current phylogenetic and genetic studies (mtDNA and nuclear DNA) indicate a similar degree of relatedness between E-T and E-N pairs [27]. Cytogenetic studies reveal that E and N share greater karyotype similarity than E and T [27], although T and N share a similar rDNA distribution pattern [20]. Gene expression and regulatory element analyses to date have focused on the E-T pair. Bartoš et al. [15] found that E × T hybrids exhibit mostly “intermediate” gene expression, with a bias toward the C. taenia genome in somatic tissues. The dominance of the T subgenome in gene expression and the strong role of cis-regulatory variants in this pair suggest significant regulatory differences between E and T. On the other hand, there are no publications on expression analysis or cis-regulatory sequences directly comparing C. elongatoides and C. tanaitica. It cannot be excluded that transcriptional homeostasis and dosage compensation between E and N subgenomes in EEN females may be more stable, thereby reducing regulatory interference during oogenesis and early embryogenesis. In contrast, the T subgenome in EET hybrids may show greater divergence in promoter structure, repetitive element content, and heterochromatin organization, potentially increasing transcriptional noise and asynchronous gene expression during development.
Research in other cyprinids may be helpful to this interpretation. Triploid and allopolyploid fishes exhibit asymmetric gene expression, partial dosage compensation, and pronounced maternal effects that influence developmental stability [47,48]. Transcriptome-wide analyses demonstrate that distinct genomic combinations produce highly variable expression profiles of homeologous genes, even when one genome is duplicated [49]. At the chromosomal level, studies of amphitriploid fish suggest that structural interactions between subgenomes are key determinants of developmental stability and evolutionary persistence [50]. In cyprinid allotetraploids, subgenomes initially evolve asymmetrically but gradually achieve a more balanced transcriptional state during rediploidization [51]. Recent studies further suggest that maternal subgenome dominance may be influenced by interactions between nuclear and mitochondrial genomes and by the distribution of transposable elements [52]. While conclusive evidence is lacking, it is plausible that variation in subgenomic compatibility and transcriptional coordination contributes to the divergent developmental outcomes among EEN, EET, and ETN hybrids. Further research is needed in this area.
The present study demonstrated a significant effect of incubation temperature on the development and hatching success of polyploid Cobitis larvae. Temperature is one of the key abiotic factors controlling proper embryogenesis in fish. Each species has a specific temperature range (thermal window) within which viable larvae can hatch successfully [53,54]. In our experiments, incubation across a wide temperature range (18–28 °C) resulted in successful offspring production. Both lower (18 °C) and higher (28 °C) temperatures increased the frequency of deformities, particularly among EET and ETN offspring. The lowest incidence of abnormalities across all genomic groups was observed at 22 °C. The observed heterogeneity in the effects of temperature on the reproductive success of hybrid Cobitis females also reflects inter-female variability. These females, being highly environmentally flexible, are likely capable of rapid adaptation to new thermal conditions.
Fish eggs are highly sensitive to ambient temperature; increased temperatures lead to shorter incubation periods [55]. Loaches of the genus Cobitis are known to reproduce within a temperature range of approximately 18–28 °C [35]. In our study, despite identical fertilization times, the duration of embryo incubation differed markedly: hatching occurred after three days at 28 °C, four days at 22 °C, and five days at 18 °C. Similar relationships between an increase in water temperature and a higher frequency of larval deformities have been reported in other fish species, including Atlantic cod (Gadus morhua; [56]), common carp (Cyprinus carpio; [57]), Senegalese sole (Solea senegalensis) [58]) and zebrafish (Danio rerio) [59]). Moreover, temperature-dependent effects on hatching and larval development have been documented in several hybrid and polyploid fishes. In African catfish hybrids (female Clarias gariepinus × male C. macrocephalus), elevated incubation temperatures accelerated yolk-sac resorption but reduced larval viability due to desynchronization between metabolic and morphological development [60]. In channel × blue catfish hybrids (female Ictalurus punctatus × male I. furcatus), temperature was identified as a primary influence on hatching success and early survival, with intermediate regimes being most favorable [61]. Similarly, in hybrid groupers (female Epinephelus fuscoguttatus × male E. polyphekadion), water temperature higher than the optimum range resulted in a sharp increase in larval abnormalities and a remarkable decrease in hatching rates [62]. Hybrid pufferfish (female Takifugu obscurus × male T. rubripes) grew and survived best at moderate temperatures, while both lower and higher temperatures impaired embryogenesis and increased mortality [63]. Both our and previous studies highlight a universal pattern among teleosts: while moderate warming can promote faster embryogenesis, further temperature increases push developmental systems beyond their physiological limits, leading to decreased survival and higher rates of abnormalities.
The temperature optimum revealed in the present study, between 18 °C and 28 °C, is also ecologically realistic for Cobitis reproduction. Field observations from Polish populations of C. taenia and C. elongatoides indicate that spawning takes place when the water temperature is above 16–18 °C and continues from May to July, when shallow marginal habitats usually range from 19 to 25 °C [25]. Spined loaches exhibit portion spawning and actively select warmer microhabitats, often several degrees above the ambient temperature, that are 20–26 °C [64,65]. Since this fish reproduces in shallow areas where water heats rapidly, spawning at about 22 °C closely matches natural conditions during the breeding season in Central Europe. However, progressive climate warming and the increasing frequency of extreme heat events may periodically push temperatures in these habitats beyond the optimal thermal window, to which hybrid Cobitis females are adapted. Water temperatures near 28 °C, shown here to cause increased mortality and deformities, are already recorded in Polish rivers and lakes during summer heatwaves. Rising temperatures are also predicted to lower dissolved oxygen levels [66], further threatening embryo viability. Broader evidence supports these ecological implications: temperature, more than flow, predicts reproductive timing in fishes [67]; spawning phenology is shifting earlier with warming [68]; and endocrine regulation along the brain–pituitary–gonad axis is highly temperature-sensitive [69,70,71]. Thus, while moderate warming may initially extend spawning opportunities, recurring heatwaves and oxygen depletion will likely reduce recruitment success and long-term population stability [72].
3.3. Ploidy and Phenotype of Progeny
Analysis of ploidy revealed no significant differences in the proportion of triploid and tetraploid progeny among genomic groups or incubation temperatures, a pattern consistent with earlier observations in Cobitis [29]. In our experiment, only male C. taenia served as sperm donors. When fertilization occurred, incorporation of paternal genetic material resulted in tetraploid offspring of both sexes [21,73], whereas during gynogenesis, sperm only activated embryogenesis without genetic contribution, leading to clonal triploid offspring. This dual reproductive mode—sexual and gynogenetic—is characteristic for Cobitis hybrids and represents an intermediate stage of hybrid speciation, where premeiotic endoreplication compensates for meiotic incompatibility between parental genomes [26]. Similar reproductive strategies have been described in other cyprinids, such as Carassius gibelio and Misgurnus loaches [74,75,76,77]. In both the present and earlier studies [29], triploid and tetraploid larvae initially occurred in similar proportions, but the frequency of tetraploids declined during post-hatching development, approaching ratios observed in natural populations. This pattern suggests that tetraploids exhibit higher developmental mortality, which corresponds with their increased incidence of morphological abnormalities (43.0% vs. 25.2% in triploids). Similar effects have been documented in other teleosts. Induction of gynogenetic tetraploidy in Japanese flounder (Paralichthys olivaceus) reduced hatching success (~47% compared to ~90% in controls) and increased deformity frequency, confirming the developmental costs of polyploidization [46].
In Cobitis, the frequency and type of deformities among tetraploids depended on the maternal genomic composition. Among tetraploid offspring, those with the EETT genome showed the highest proportion of abnormalities, ENTT were intermediate, and EENT the lowest, with differences most pronounced at 28 °C. This gradient indicates that simply doubling the C. taenia genome amplifies latent genomic incompatibilities, leading to reduced developmental stability at higher ploidy levels.
Mechanistically, these outcomes likely stem from processes previously discussed for Cobitis, including dosage imbalance, irregular chromosome pairing, incomplete rediploidization, rDNA instability, and variable in the efficiency of premeiotic genome duplication [15,20,28,78]. The genomic composition of tetraploids further influences the interactions among these mechanisms. Fertilization of triploid eggs by C. taenia males results in the formation of tetraploids with diverse combinations of C. elongatoides, C. taenia, and C. tanaitica genomes. Lines such as EENT, despite comprising three different copies of the C. elongatoides, C. tanaitica, and C. taenia genomes, appear to maintain more compatible regulatory interactions and, consequently, exhibit greater developmental stability. In contrast, EETT lines, which contain duplicate genomes from two species differing in chromosome number and regulatory architecture, may display a higher incidence of developmental defects in these tetraploids.
The high incidence of abnormalities in tetraploids, particularly those with less compatible genomic combinations, likely limits their viability in natural populations. Most tetraploid individuals represent transient or ephemeral lineages, while the more stable EENT configuration may occasionally persist long enough to act as a genetic bridge between clonal triploid forms. Nevertheless, the overall contribution of tetraploids to gene flow and recruitment appears minimal. This pronounced asymmetry in developmental success between triploid and tetraploid offspring may help explain why gynogenetic reproduction dominates in natural Cobitis populations, ensuring the long-term persistence of triploid clonal lineages under diverse environmental conditions.
3.4. Body Length of Progeny
In addition to differences in hatching success and developmental stability, our results showed that both incubation temperature and maternal genomic composition influenced the early growth of Cobitis larvae. Temperature exerts a major control over fish growth and body size, although its effects vary depending on species, life stage, and ecological context. In general, lower temperatures tend to produce larger size at hatching [79,80] because embryogenesis proceeds more slowly and yolk resources are utilized more completely, whereas higher temperatures accelerate metabolic rate and promote faster post-hatch growth within the species’ thermal tolerance [81,82]. In the present study, larval length was measured three days post-hatching, reflecting early post-hatch growth rather than size at emergence.
Against this background, offspring of EEN females exhibited a clear positive temperature response (28 °C > 22 °C > 18 °C), showing enhanced early growth under warmer conditions. In contrast, temperature had little effect on the body length of EET and ETN offspring, suggesting either a narrower thermal growth window or a physiological trade-off in which energy was diverted from somatic growth toward processes maintaining homeostasis during thermal stress.
Similar relationships between early thermal environment and larval growth have been described in other fish species. In hybrid yellow catfish (female Tachysurus fulvidraco × male Pseudobagrus vachellii), temperature and dissolved oxygen jointly shaped growth, survival, and oxidative capacity of newly hatched larvae [83], indicating that benefits of faster growth at higher temperatures are constrained by oxygen availability and metabolic capacity. Furthermore, in polyploid sterlet (Acipenser ruthenus), departures from the optimal thermal range reduced larval survival and condition, indicating that polyploid fishes may be particularly sensitive to suboptimal temperature regimes [84].
Our previous studies [40] showed that the average total length of progeny from triploid Cobitis females was statistically higher than that of diploid C. taenia offspring during the first ten days after hatching, although these differences disappeared after two weeks. This pattern is consistent with the well-known principle that elevated water temperature promotes faster initial growth but results in smaller adult body size [85]. Similar inverse relationships between temperature and growth have been observed in other teleosts: larval walleye pollock (Gadus chalcogrammus) exhibited growth rates inversely proportional to water temperature [86,87], and the developmental period of northern pike (Esox lucius) was approximately three times longer at the lowest tested temperature than at the highest [88].
Taken together, these observations agree with our results and indicate that temperature promotes early post-hatch growth only within a limited thermal window, beyond which developmental or metabolic constraints offset potential benefits. In our study, EEN larvae attained greater length at higher temperatures, consistent with accelerated growth within a favorable thermal range, whereas EET and ETN larvae showed little variation across temperatures, reflecting reduced thermal plasticity and tighter physiological regulation.
4. Materials and Methods
Twenty triploid Cobitis females, representing three different genomic compositions (EEN, EET and ETN) depending on the species with which they were hybridized, were crossed with diploid C. taenia males. Diploid males of C. taenia used as sperm donors came from two populations consisting exclusively of diploid individuals, all had 2n = 48 chromosomes. Their eggs and larvae were then incubated under three temperature conditions (18 °C, 22 °C and 28 °C) for three days after hatching.
4.1. Fish Sampling and Genomic Identification
The fish used in the crossing experiment were caught in June, which coincided with their natural spawning period, and the experiment was conducted over three consecutive years (2022–2024). Twenty allotriploid (3n) Cobitis females were collected from two diploid-polyploid populations: the Pilica River (51°34′29.6″ N, 20°20′16″ E) in the Vistula River drainage basin of the Baltic Sea, and twenty diploid (2n) C. taenia males were obtained from an exclusively diploid population in Lake Legińskie (53°58′40.9″ N, 21°08′28.0″ E) in the Pregoła River basin of the Baltic Sea drainage system. Mature males were identified by the presence of a lamina circularis at the base of the pectoral fins [25].
The ploidy levels of the parental individuals were determined before induced spawning by flow cytometry using fin clips, as previously described [89], and this was confirmed post-experimentally via chromosome counts. The genome of the 3n females was analyzed using species-specific microsatellite markers, sequencing of the nuclear S7 gene intron and karyotyping after crossing experiments, as previously described by Boroń et al. [20]. DNA was extracted from the dorsal fin or muscle tissue using a Genomic Mini Kit (A&A Biotechnology, Gdansk, Poland). Microsatellite genotyping was performed using an ABI PRISM 3130 Genetic Analyzer (Applied Biosystems, Waltham, MA, USA), with allele scoring performed with GeneMapper v3.7 (Applied Biosystems, Waltham, MA, USA) [90]. The S7 intron was amplified according to the method of Janko et al. [19,21] and sequenced commercially (Genomed, Warsaw, Poland). Sequence alignments were carried out manually in BioEdit v.7.2.5 (Ibis Biosciences, Carlsbad, CA, USA).
Karyotyping was performed to confirm ploidy and genomes. Live fish were injected with 1 mL of 0.05% colchicine per 100 g of body weight. After 1.5 h, the fish were euthanized with an overdose of MS222 (100 mg/L). The kidney cells were then hypotonized in 0.075 M KCl for 50 min and fixed in methanol:acetic acid (3:1). Chromosome spreads were prepared using the splash method and stained with 4% Giemsa [20]. The chromosomes were examined using a Nikon Eclipse 80i microscope (Nikon Corporation, Tokyo, Japan). A minimum of 12 metaphase spreads per individual were analyzed using MultiScan v14.02 software (Computer Scanning Systems, Warsaw, Poland; multiscan.idsl.pl). The chromosomes were classified according to Levan et al. [91]. The karyotypes of triploid hybrid parental females were analyzed as previously described in detail by Boroń et al. [20].
4.2. Crossing Experiments
Hormonal stimulation of the parent fish was carried out using Ovopel (a GnRH analog and metoclopramide) (Interfish Ltd. Budapest, Hungary), as described by Juchno et al. [29]. The eggs collected from each triploid female were divided into three portions and fertilized with sperm from C. taenia in Petri dishes at the following temperatures: 18 °C, 22 °C and 28 °C. The fertilized eggs were then transferred to 50 L aquaria within closed water circulation systems, each of which was maintained at the corresponding temperature. The eggs were checked daily and any dead eggs, identified by whitening, were removed using a Pasteur pipette. The larvae were reared under controlled conditions with a light–dark cycle to mimic natural conditions. Subsets of the larvae were sampled at 1, 2 and 3 days post hatching (dph) for further analysis (see Figure 6). Offspring were examined during the first three days post-hatching since previous studies have demonstrated a rapid decline in the number of tetraploid offspring after this timeframe [29].
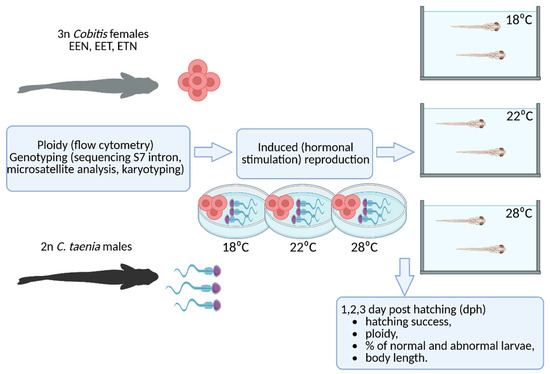
Figure 6.
Experimental design scheme (created with BioRender.com). Cobitis females were examined for ploidy using flow cytometry. Genotyping was performed using S7 intron sequencing, microsatellite analysis, and karyotyping. Crossing experiments were conducted between triploid Cobitis females with three different genomes (EEN, EET, and ETN) and diploid C. taenia males. Artificial spawning and subsequent larval development took place at three temperatures: 18 °C, 22 °C, and 28 °C. The offspring obtained were examined for hatching success, ploidy, percentage of abnormalities, and body length in the first three days after hatching.
The following parameters were determined for larvae obtained from females with three genomes (EEN, EET and ETN) that were reared at three different temperatures:
4.3. Hatching Success
Hatching success was calculated as a percentage of the total number of eggs obtained, relative to the number of larvae hatched at 1 dph.
4.4. Ploidy Analysis of Progeny
The ploidy status of approximately 10 randomly selected offspring per each cross was determined by flow cytometry (Table 3), following the method of Jablonska et al. [89] with slight modifications. Larvae collected at 1, 2 and 3 dph were used for nuclei isolation. The nuclei were stained using the CyStain UV Precise T kit (Sysmex Partec GmbH, Görlitz, Saxony, Germany) and analyzed using a CyFlow Ploidy Analyzer (Partec GmbH, Görlitz, Saxony, Germany). The relative DNA content was then compared against a diploid reference from C. taenia × C. taenia progeny.

Table 3.
Sample size for ploidy and phenotype analysis according to genome composition (EEN, EET, ETN) and water temperature (18 °C, 22 °C, 28 °C).
4.5. Phenotype Analysis of Progeny
To assess normal and abnormal development, the external morphology of approximately 10 randomly selected larvae from each cross was examined up to three days post hatching (dph) (Table 3). Larvae deformities were categorized according to the descriptions provided by Jezierska et al. [92] and Alix et al. [93]. We classified a larva as abnormal if it exhibited any developmental malformation. Representative images of normal and abnormal progeny across different ploidy levels and genomes were captured using NIS-Elements F 4.60.00 64-bit imaging software and a Nikon SMZ1270 microscope (Nikon Corporation, Tokyo, Japan).
4.6. Measurement of Body Length in Progeny
Larval body measurements were taken using NIS-Elements F imaging software to determine the standard length (SL in mm), which was measured from the tip of the snout to the end of the caudal peduncle. Approximately 10 individuals were randomly selected from each cross and measured at 3 dph.
4.7. Statistical Analysis
For statistical analysis, we pooled data from three years (spawning seasons) because the experimental conditions were identical in each year. Percentage data were arcsine-transformed prior to statistical analysis. The normality of the distribution was checked using the Shapiro–Wilk test. One-way ANOVA was performed using Statistica v13.3 software (TIBCO Software Inc., Palo Alto, CA, USA) to determine the effects of the 3n Cobitis female genome composition, water temperature, and ploidy distribution on hatching success and the external morphology of the larvae. Differences between the studied groups were assessed using the Tukey HSD post hoc test.
5. Conclusions
This study provides the first experimental evidence that early developmental success in Cobitis hybrids is shaped by genomic composition and incubation temperature, reflecting complex interactions between inherited genomic architecture and environmental conditions. The contrasting reproductive strategies of the triploid lineages—quantity-biased in EET and ETN, and quality-biased in EEN—illustrate how polyploid systems balance offspring number and viability in response to both genetic and thermal conditions. Furthermore, the elevated abnormality rates observed in tetraploids highlight the developmental costs of increased ploidy and help explain why triploid gynogenesis dominates in natural populations.
These findings provide a solid foundation for further research into the mechanisms underlying genome–environment interactions in polyploid fishes, particularly how female genomic composition and rising water temperatures influence reproductive success, developmental stability, and the evolution of Cobitis hybrid lineages. Understanding these processes will be essential for predicting how hybrid and clonal fish systems respond to ongoing climatic change and for identifying the genetic and ecological factors that support their long-term stability.
Author Contributions
S.D., O.J., A.B. and D.J. conceived the study and designed the experiments. S.D., O.J., D.J. and R.K. performed crossing experiments and reared progeny. O.J., A.B. and K.J. performed species identification. S.D. and O.J. performed tissue collection. S.D. wrote the first draft of the manuscript, which was further improved by A.B., D.J., O.J., K.J. and R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NATIONAL SCIENCE CENTRE (NCN) in Poland (grant UMO-2021/41/B/NZ8/03231. This study was also supported by the Czech Science Foundation project No. GACR_24-12217S. Institute of Animal Physiology and Genetics receives support from project by the Czech Academy of Sciences of the Czech Republic RVO67985904.
Institutional Review Board Statement
Fish sampling and experimental animal procedures performed in these studies were performed with the approval of the Polish Ministry of Environment (permit no. DZP-WG.6401.85.2022TŁ2) and in accordance with the ethical guidelines of the Animal Ethics Committee at the University of Warmia and Mazury in Olsztyn, Poland (approval no. 51/2022 the approval date: 20 July 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to Sławomir Boroń for collecting the fish used in this study and to Małgorzata Tanajewska for her valuable assistance in their care and maintenance.
Conflicts of Interest
The authors declare no conflicts of interest. The authors declare that the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Alix, M.; Kjesbu, O.S.; Anderson, K.C. From gametogenesis to spawning: How climate-driven warming affects teleost reproductive biology. J. Fish Biol. 2020, 97, 607–632. [Google Scholar] [CrossRef]
- Qiang, J.; Duan, X.-J.; Zhu, H.-J.; He, J.; Tao, Y.-F.; Bao, J.-W.; Zhu, X.-W.; Xu, P. Some ‘White’ Oocytes Undergo Atresia and Fail to Mature during the Reproductive Cycle in Female Genetically Improved Farmed Tilapia (Oreochromis niloticus). Aquaculture 2021, 534, 736278. [Google Scholar] [CrossRef]
- Pino, M.E.; Braanaas, M.F.; Balseiro, P.; Kraugerud, M.; Pedrosa, C.; Imsland, A.K.D.; Handeland, S.O. Constant High Temperature Promotes Early Changes in Testis Development Associated with Sexual Maturation in Male Atlantic Salmon (Salmo salar L.) Post-Smolts. Fishes 2022, 7, 341. [Google Scholar] [CrossRef]
- Calvo-Rodríguez, L.; Ortiz-Delgado, J.B.; Cañón, L.; de Paz, P.; Fernández, I.; Riesco, M.F. Current summer heat waves impair rainbow trout (Oncorhynchus mykiss) spermatogenesis: Implications for future fish farming management practices in South Europe. Aquaculture 2025, 596, 741716. [Google Scholar] [CrossRef]
- Tinkir, M.; Memiş, D.; Cheng, Y.; Xin, M.; Rodina, M.; Gela, D.; Tučková, V.; Linhart, O. Level of in Vitro Storage of the European Catfish (Silurus glanis L.) Eggs at Different Temperatures. Fish Physiol. Biochem. 2021, 47, 163–171. [Google Scholar] [CrossRef]
- Qiang, J.; Tao, Y.F.; Zhu, J.H.; Lu, S.Q.; Zao, Z.M.; Ma, J.L.; He, J.; Xu, P. Effects of heat stress on follicular development and atresia in Nile tilapia (Oreochromis niloticus) during one reproductive cycle and its potential regulation by autophagy and apoptosis. Aquaculture 2022, 555, 738171. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Li, G.-L.; Zhu, C.-H.; Deng, S.-P. Effects of Fish Oil on Ovarian Development in Spotted Scat (Scatophagus argus). Anim. Reprod. Sci. 2013, 141, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.; Li, J.; Wen, H.; Likang, L. Effects of temperature on gonadal differentiation of black rockfish (Sebastes schlegelii) and its mechanism. J. Fish. China 2019, 43, 1569–1580. [Google Scholar] [CrossRef]
- Nakatani, Y.; Shingate, P.; Ravi, V.; Pillai, N.; Prasad, A.; McLysaght, A.; Venkatesh, B. Reconstruction of proto-vertebrate, proto-cyclostome and proto-gnathostome genomes provides new insights into early vertebrate evolution. Nat. Commun. 2021, 12, 4489. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Ren, Y.; Uesaka, M.; Beavan, A.; Muffato, M.; Shen, J.; Li, Y.; Sato, I.; Wan, W.; Clark, J.; et al. Hagfish genome elucidates vertebrate whole-genome duplication events and their evolutionary consequences. Nat. Ecol. Evol. 2024, 8, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Naylor, G.; Mayden, R. Deciphering Reticulate Evolution of the Largest Group of Polyploid Vertebrates, the Subfamily Cyprininae (Teleostei: Cypriniformes). Mol Phylogenet Evol. 2021, 166, 107323. [Google Scholar] [CrossRef]
- Mable, B.; Alexandrou, M.; Taylor, M. Genome duplication in amphibians and fish: An extended synthesis. J. Zool. 2011, 284, 151–182. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Brunelli, E.; Odierna, G.; Guarino, F.M. Evolutionary and Genomic Diversity of True Polyploidy in Tetrapods. Animals 2023, 13, 1033. [Google Scholar] [CrossRef]
- Majtánová, Z.; Choleva, L.; Symonová, R.; Ráb, P.; Kotusz, J.; Pekárik, L.; Janko, K. Asexual Reproduction Does Not Apparently Increase the Rate of Chromosomal Evolution: Karyotype Stability in Diploid and Triploid Clonal Hybrid Fish (Cobitis, Cypriniformes, Teleostei). PLoS ONE 2016, 11, e0146872. [Google Scholar] [CrossRef] [PubMed]
- Bartoš, O.; Röslein, J.; Kotusz, J.; Paces, J.; Pekárik, L.; Petrtýl, M.; Halačka, K.; Štefková Kašparová, E.; Mendel, J.; Boroń, A.; et al. The Legacy of Sexual Ancestors in Phenotypic Variability, Gene Expression, and Homoeolog Regulation of Asexual Hybrids and Polyploids. Mol. Biol. Evol. 2019, 36, 1902–1920. [Google Scholar] [CrossRef]
- Mezhzherin, S.V.; Tsyba, A.O. Genomic structure and diversity of European polyploid spined loaches (Cypriniformes, Cobitidae) in a situation of genetic instability. J. Fish. Biol. 2025, 1–12. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Zou, L.; Luo, Y.; Tan, H.; Yao, J.; Zhang, M.; Liu, S. Hox Genes Reveal Variations in the Genomic DNA of Allotetraploid Hybrids Derived from Carassius auratus Red Var. (Female) × Cyprinus carpio L. (Male). BMC Genet. 2020, 21, 24. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. 2025. Available online: www.fishbase.org (accessed on 1 August 2025).
- Janko, K.; Flajšhans, M.; Choleva, L.; Bohlen, J.; Šlechtová, V.; Rábová, M.; Lajbner, Z.; Šlechta, V.; Ivanova, P.; Dobrovolov, I.; et al. Diversity of European Spined Loaches (Genus Cobitis L.): An Update of the Geographic Distribution of the Cobitis taenia Hybrid Complex with a Description of New Molecular Tools for Species and Hybrid Determination. J. Fish Biol. 2007, 71, 387–408. [Google Scholar] [CrossRef]
- Boroń, A.; Grabowska, A.; Jablonska, O.; Kirtiklis, L.; Duda, S.; Juchno, D. Chromosomal rDNA Distribution Patterns in Clonal Cobitis Triploid Hybrids (Teleostei, Cobitidae): Insights into Parental Genomic Contributions. Genes 2025, 16, 68. [Google Scholar] [CrossRef]
- Janko, K.; Kotusz, J.; De Gelas, K.; Šlechtová, V.; Opoldusová, Z.; Drozd, P.; Choleva, L.; Popiołek, M.; Baláž, M. Dynamic Formation of Asexual Diploid and Polyploid Lineages: Multilocus Analysis of Cobitis Reveals the Mechanisms Maintaining the Diversity of Clones. PLoS ONE 2012, 7, e45384. [Google Scholar] [CrossRef] [PubMed]
- Juchno, D.; Pecio, A.; Boroń, A.; Leska, A.; Jablonska, O.; Cejko, B.I.; Kowalski, R.K.; Judycka, S.; Przybylski, M. Evidence of the Sterility of Allotetraploid Cobitis Loaches (Teleostei, Cobitidae) Using Testes Ultrastructure. J. Exp. Zool. Pt. A 2017, 327, 66–74. [Google Scholar] [CrossRef]
- Mezhzherin, S.V.; Tsyba, A.A.; Kryvokhyzha, D. Cryptic expansion of hybrid polyploid spined loaches Cobitis in the rivers of Eastern Europe. Hydrobiologia 2022, 849, 1689–1700. [Google Scholar] [CrossRef]
- Choleva, L.; Musilova, Z.; Kohoutova-Sediva, A.; Paces, J.; Rab, P.; Janko, K. Distinguishing between Incomplete Lineage Sorting and Genomic Introgressions: Complete Fixation of Allospecific Mitochondrial DNA in a Sexually Reproducing Fish (Cobitis; Teleostei), despite Clonal Reproduction of Hybrids. PLoS ONE 2014, 9, e80641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Juchno, D.; Boroń, A.; Gołaszewski, J. Comparative Morphology and Histology of the Ovaries of the Spined Loach Cobitis taenia L. and Natural Allopolyploids of Cobitis (Cobitidae). J. Fish Biol. 2007, 70, 1392–1411. [Google Scholar] [CrossRef]
- Dedukh, D.; Majtánová, Z.; Marta, A.; Pšenička, M.; Kotusz, J.; Klíma, J.; Juchno, D.; Boron, A.; Janko, K. Parthenogenesis as a Solution to Hybrid Sterility: The Mechanistic Basis of Meiotic Distortions in Clonal and Sterile Hybrids. Genetics 2020, 215, 975–987. [Google Scholar] [CrossRef]
- Marta, A.; Tichopád, T.; Bartoš, O.; Klíma, J.; Shah, M.A.; Bohlen, V.Š.; Bohlen, J.; Halačka, K.; Choleva, L.; Stöck, M.; et al. Genetic and Karyotype Divergence between Parents Affect Clonality and Sterility in Hybrids. eLife 2023, 12, RP88366. [Google Scholar] [CrossRef]
- Dedukh, D.; Marta, A.; Janko, K. Challenges and Costs of Asexuality: Variation in Premeiotic Genome Duplication in Gynogenetic Hybrids from Cobitis taenia Complex. Int. J. Mol. Sci. 2021, 22, 12117. [Google Scholar] [CrossRef]
- Juchno, D.; Jabłońska, O.; Boroń, A.; Kujawa, R.; Leska, A.; Grabowska, A.; Nynca, A.; Świgońska, S.; Król, M.; Spóz, A.; et al. Ploidy-Dependent Survival of Progeny Arising from Crosses between Natural Allotriploid Cobitis Females and Diploid C. taenia Males (Pisces, Cobitidae). Genetica 2014, 142, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, S.; Hu, J.; He, G.; Liu, Y.; Chen, X.; Lei, T.; Li, Q.; Yang, L.; Li, W.; et al. Polyploidization of Plumbago auriculata Lam. in vitro and its characterization including cold tolerance. Plant Cell Tissue Organ Cult. 2020, 140, 315–325. [Google Scholar] [CrossRef]
- Glennon, K.L.; Niemann, H.J.; Archibald, S. Fire-mediated effects on polyploid biology. Trends Ecol. Evol. 2024, 39, 424–426. [Google Scholar] [CrossRef]
- Hermaniuk, A.; Rybacki, M.; Taylor, J.R.E. Low Temperature and Polyploidy Result in Larger Cell and Body Size in an Ectothermic Vertebrate. Physiol. Biochem. Zool. 2016, 89, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Maciak, S.; Janko, K.; Kotusz, J.; Choleva, L.; Boroń, A.; Juchno, D.; Kujawa, R.; Kozłowski, J.; Konarzewski, M. Standard metabolic rate (SMR) is inversely related to erythrocyte and genome size in allopolyploid fish of the Cobitis taenia hybrid complex. Funct. Ecol. 2011, 25, 1072–1078. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Yuan, X.; Jiang, Y. Stage-specific Influence of Temperature on the Growth Rate of Japanese Spanish Mackerel (Scomberomorus niphonius) in Early Life. J. Fish Biol. 2022, 100, 498–506. [Google Scholar] [CrossRef]
- Bohlen, J. Reproduction of Spined Loach, Cobitis taenia, (Cypriniformes; Cobitidae) under Laboratory Conditions. J. Appl. Ichthyol. 1999, 15, 49–53. [Google Scholar] [CrossRef]
- Niu, J.; Huss, M.; Vasemägi, A.; Gårdmark, A. Decades of Warming Alters Maturation and Reproductive Investment in Fish. Ecosphere 2023, 14, e4381. [Google Scholar] [CrossRef]
- Fraz, S.; Laframboise, L.; Manzon, R.; Somers, C.M.; Wilson, J.Y. Embryonic and Larval Development of Yellow Perch (Perca flavescens) and Its Sensitivity to Incubation Temperature. J. Fish Biol. 2024, 105, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Sardi, A.E.; Bégout, M.L.; Lalles, A.L.; Cousin, X.; Budzinski, H. Temperature and feeding frequency impact the survival, growth, and metamorphosis success of Solea solea larvae. PLoS ONE 2023, 18, e0281193. [Google Scholar] [CrossRef]
- Howard, C.; Taylor, J.F.; Migaud, H.; Gutierrez, A.P.; Bekaert, M. Comparison of Diploid and Triploid Atlantic Salmon (Salmo salar) Physiological Embryonic Development. Animals 2023, 13, 3352. [Google Scholar] [CrossRef]
- Juchno, D.; Boroń, A.; Szlachciak, J.; Kujawa, R. Early Development and Post Embryonic Skeletal Morphology of the Progeny of Spined Loach Cobitis taenia L. (Teleostei, Cobitidae) and its Naturally Occurring Allotriploids. Folia Biol. 2016, 64, 153–162. [Google Scholar] [CrossRef]
- Kočí, J.; Röslein, J.; Pačes, J.; Kotusz, J.; Halačka, K.; Koščo, J.; Fedorčák, J.; Iakovenko, N.; Janko, K. No evidence for accumulation of deleterious mutations and fitness degradation in clonal fish hybrids: Abandoning sex without regrets. Mol. Ecol. 2020, 29, 3038–3055. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, J.; Zeng, M.; Xu, K.; Tao, M.; Zhang, C.; Duan, W.; Liu, W.; Luo, K.; Liu, Y.; et al. Genomic Variation in the Hybrids of White Crucian Carp and Red Crucian Carp: Evidence from Ribosomal DNA. Sci. China Life Sci. 2015, 58, 590–601. [Google Scholar] [CrossRef]
- Káldy, J.; Mozsár, A.; Fazekas, G.; Farkas, M.; Fazekas, D.L.; Fazekas, G.L.; Goda, K.; Gyöngy, Z.; Kovács, B.; Semmens, K.; et al. Hybridization of Russian Sturgeon (Acipenser gueldenstaedtii, Brandt and Ratzeberg, 1833) and American Paddlefish (Polyodon spathula, Walbaum 1792) and Evaluation of Their Progeny. Genes 2020, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, K.; Nomoto, K.; Machida, Y.; Ichimura, M.; Koizumi, I. No reduction of hatching rates among F1hybrids of naturally hybridizing three Far Eastern daces, genus Tribolodon (Cypriniformes, Cyprinidae). Ichthyol. Res. 2017, 65, 165–167. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, J.; Xu, W.; He, S.; Long, M.; Liao, Q.; Liu, J.; Peng, L.; Liu, W.; Xiao, Y. Characteristics of hatching enzymes and egg envelope in cross progenies from crucian carp (Carassius auratus var.) and zebrafish (Barchydanio rerio var.). Reprod. Breed. 2021, 1, 81–88. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Sun, Z.; Zhao, Y.; Du, W.; Cui, J.; Hou, J.; Wang, Y. Induction of gyno-tetraploidy in Japanese flounder Paralichthys olivaceus. J. Oceanol. Limnol. 2019, 38, 288–293. [Google Scholar] [CrossRef]
- Ren, L.; Tang, C.; Li, W.; Cui, J.; Tan, X.; Xiong, Y.; Chen, J.; Wang, J.; Xiao, J.; Zhou, Y.; et al. Determination of dosage compensation and comparison of gene expression in a triploid hybrid fish. BMC Genom. 2017, 18, 38. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Tan, H.; Luo, L.; Cui, J.; Hu, J.; Wang, S.; Liu, Q.; Hu, F.; Tang, C.; et al. Asymmetric expression patterns reveal a strong maternal effect and dosage compensation in polyploid hybrid fish. BMC Genom. 2018, 19, 517. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, X.; Li, J.; Yan, X.; Gao, X.; Cui, J.; Tang, C.; Liu, S. Diverse transcriptional patterns of homoeologous recombinant transcripts in triploid fish (Cyprinidae). Sci. China Life Sci. 2021, 64, 1491–1501. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Xu, W.; Wang, K.; Wu, B.; Xu, M.; Chen, Y.; Miao, L.; Wang, Z.; Li, Z.; et al. Comparative genome anatomy reveals evolutionary insights into a unique amphitriploid fish. Nat Ecol Evol. 2022, 6, 1354–1366. [Google Scholar] [CrossRef]
- Luo, J.; Chai, J.; Wen, Y.; Tao, M.; Lin, G.; Liu, X.; Ren, L.; Chen, Z.; Wu, S.; Li, S.; et al. From asymmetrical to balanced genomic diversification during rediploidization: Subgenomic evolution in allotetraploid fish. Sci. Adv. 2020, 6, eaaz7677. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liao, Z.; Brock, J.; Du, K.; Li, G.; Chen, Z.; Wang, Y.; Gao, Z.; Agarwal, G.; Wei, K.; et al. Maternal dominance contributes to subgenome differentiation in allopolyploid fishes. Nat. Commun. 2023, 14, 8357. [Google Scholar] [CrossRef]
- Hasan, M.; Sultana Mely, S.; Faruk, A.; Nayeem Hossain, M. Climate Change Effects on Hatching Success, Embryonic Development and Larvae Survival of Freshwater Fish: A Critical Review. Int. J. Ecotoxicol. Ecobiol. 2023, 16, 24. [Google Scholar] [CrossRef]
- Ashaf-Ud-Doulah, M.; Islam, S.M.M.; Zahangir, M.M.; Islam, M.S.; Brown, C.; Shahjahan, M. Increased water temperature interrupts embryonic and larval development of Indian major carp rohu Labeo rohita. Aquacult Int. 2021, 29, 711–722. [Google Scholar] [CrossRef]
- Schmitz, A.M.; Sepulveda Villet, O.J. Short term temperature fluctuations affect embryonic and larval development of yellow perch (Perca flavescens). J. Aquac. Mar. Biol. 2021, 10, 168–176. [Google Scholar] [CrossRef]
- Pepin, P.; Orr, D.C.; Anderson, J.T. Time to Hatch and Larval Size in Relation to Temperature and Egg Size in Atlantic Cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 1997, 54, 2–10. [Google Scholar] [CrossRef]
- El-Gamal, A.E.-H.E. Effect of Temperature on Hatching and Larval Development and Mucin Secretion in Common Carp, Cyprinus carpio (Linnaeus, 1758). Glob. Vet. 2009, 3, 80–90. [Google Scholar]
- Dionísio, G.; Campos, C.; Valente, L.M.P.; Conceição, L.E.C.; Cancela, M.L.; Gavaia, P.J. Effect of Egg Incubation Temperature on the Occurrence of Skeletal Deformities in Solea senegalensis: Effect of Incubation Temperature on Skeletal Deformities of Sole. J. Appl. Ichthyol. 2012, 28, 471–476. [Google Scholar] [CrossRef]
- De Souza, A.M.; da Silva Junior, F.C.; Dantas, É.D.; Galvão-Pereira, M.C.; de Medeiros, S.R.B.; Luchiari, A.C. Temperature effects on development and lifelong behavior in zebrafish. Sci. Total Environ. 2025, 973, 179172. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.; Nguyen, T.; Singchat, W.; Punthum, T.; Kraichak, E.; Panochit, P.; Maneeaphai, W.; Phuonnim, A.; Sengtrakool, S.; Sriphairoj, K.; et al. Impact of higher temperatures on yolk sac absorption and early development in hybrid catfish between Clarias gariepinus and C. macrocephalus. J. World Aquac. Soc. 2025, 56, e13119. [Google Scholar] [CrossRef]
- Myers, J.; Chatakondi, N.; Dunham, R.; Butts, I. Genetic architecture of early life history traits for channel catfish, Ictalurus punctatus ♀ × blue catfish, I. furcatus ♂ hybrid production. Aquaculture 2020, 514, 734436. [Google Scholar] [CrossRef]
- Huang, J.; Chen, G.; Wang, Z.; Zhang, J. Use of response surface methodology to study the combined effects of temperature and salinity on hatching and deformity of the hybrid grouper, Epinephelus fuscoguttatus (♀)× Epinephelus polyphekadion (♂). Aquac. Res. 2018, 49, 1997–2005. [Google Scholar] [CrossRef]
- Lee, J.; Han, K.; Choi, W.; Yu, T.; Kim, H.; Lee, S. Effects of Water Temperature and Salinity on the Growth and Survival of Hybrid Pufferfish Larvae (Takifugu obscurus ♀ × T. rubripes ♂). Aquac. Res. 2024, 2024, 8511980. [Google Scholar] [CrossRef]
- Bohlen, J. Spawning habitat in the spined loach, Cobitis taenia (Cypriniformes: Cobitidae). Ichthyol Res. 2003, 50, 0098–0101. [Google Scholar] [CrossRef]
- Bohlen, J.; Ritterbusch, D. Which factors affect sex ratio of spined loach (genus Cobitis) in Lake Müggelsee? Environ. Biol. Fishes 2000, 59, 347–352. [Google Scholar] [CrossRef]
- Aristarkhova, E.; Fedoniuk, T.; Romanchuk, L.; Latushynskyi, S.; Kot, I. Features of the surface water oxygen regime in the Ukrainian Polesie Region. J. Water Land Dev. 2023, 49, 104–110. [Google Scholar] [CrossRef]
- Fraser, G.; Bestgen, K.; Winkelman, D.; Thompson, K. Temperature—Not Flow—Predicts Native Fish Reproduction with Implications for Climate Change. Trans. Am. Fish. Soc. 2019, 148, 509–527. [Google Scholar] [CrossRef]
- Koenigbauer, S.; Cubbage, M.; Warren, L.; Tellier, J.; Selz, O.; Sass, G.; Höök, T. Fish reproductive phenology shifts with increasing temperature and year. Biol. Lett. 2025, 21, 20240240. [Google Scholar] [CrossRef]
- Pankhurst, N.; Munday, P. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef]
- Servili, A.; Canário, A.; Mouchel, O.; Muñoz-Cueto, J. Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocrinol. 2020, 291, 113439. [Google Scholar] [CrossRef]
- Lema, S.; Luckenbach, J.; Yamamoto, Y.; Housh, M. Fish reproduction in a warming world: Vulnerable points in hormone regulation from sex determination to spawning. Philos. Trans. R. Soc. B 2024, 379, 20220516. [Google Scholar] [CrossRef]
- Mitra, A.; Abdel-Gawad, F.; Bassem, S.; Barua, P.; Assisi, L.; Parisi, C.; Temraz, T.; Vangone, R.; Kajbaf, K.; Kumar, V.; et al. Climate Change and Reproductive Biocomplexity in Fishes: Innovative Management Approaches towards Sustainability of Fisheries and Aquaculture. Water 2023, 15, 725. [Google Scholar] [CrossRef]
- Choleva, L.; Janko, K.; De Gelas, K.; Bohlen, J.; Šlechtová, V.; Rábová, M.; Ráb, P. Synthesis of clonality and polyploidy in vertebrate animals by hybridization between two sexual species. Evolution 2012, 66, 2191–2203. [Google Scholar] [CrossRef]
- Luo, K.; Xiao, J.; Liu, S.; Wang, J.; He, W.; Hu, J.; Qin, Q.; Zhang, C.; Tao, M.; Liu, Y. Massive Production of All-Female Diploids and Triploids in the Crucian Carp. Int. J. Biol. Sci. 2011, 7, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Y.; Gui, J.-F. Genetic Evidence for Gonochoristic Reproduction in Gynogenetic Silver Crucian Carp (Carassius auratus gibelio Bloch) as Revealed by RAPD Assays. J. Mol. Evol. 2000, 51, 498–506. [Google Scholar] [CrossRef]
- Arai, K.; Fujimoto, T. Genomic Constitution and Atypical Reproduction in Polyploid and Unisexual Lineages of the Misgurnus Loach, a Teleost Fish. Cytogenet. Genome Res. 2013, 140, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Arai, K. Meiosis and gametogenesis in hybrid, polyploid, and clonal fishes: Case studies in the dojo loach Misgurnus anguillicaudatus. Fish. Sci. 2023, 89, 537–570. [Google Scholar] [CrossRef]
- Janko, K.; Bartoš, O.; Kočí, J.; Roslein, J.; Janková Drdová, E.; Kotusz, J.; Eisner, J.; Mokrejš, M.; Štefková-Kašparová, E. Genome Fractionation and Loss of Heterozygosity in Hybrids and Polyploids: Mechanisms, Consequences for Selection, and Link to Gene Function. Mol. Biol. Evol. 2021, 38, 5255–5274. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, A.R.; Blanchard, J.L.; Wotherspoon, S.; Stuart-Smith, R.D.; Edgar, G.J.; Barrett, N.; Audzijonyte, A. Mean Reef Fish Body Size Decreases towards Warmer Waters. Ecol. Lett. 2024, 27, e14375. [Google Scholar] [CrossRef]
- Lindmark, M.; Audzijonyte, A.; Blanchard, J.L.; Gårdmark, A. Temperature Impacts on Fish Physiology and Resource Abundance Lead to Faster Growth but Smaller Fish Sizes and Yields under Warming. Glob. Chang. Biol. 2022, 28, 6239–6253. [Google Scholar] [CrossRef]
- Park, J.W.; Yoo, H.; Jung, H.; Park, H.J.; Bae, K.; Kang, C.K.; Lee, C. Effects of water temperature changes on the early life stages (egg and larvae) of walleye Pollock (Gadus chalcogrammus)—Laboratory experiments and field applications. J. Exp. Mar. Biol. Ecol. 2024, 571, 151980. [Google Scholar] [CrossRef]
- Miller, N.A.; Stillman, J.H. Physiological Optima and Critical Limits. Nat. Educ. Knowl. 2012, 3, 1. [Google Scholar]
- Qiang, J.; Zhong, C.; Bao, J.; Liang, M.; Liang, C.; Li, H.; He, J.; Xu, P. The effects of temperature and dissolved oxygen on the growth, survival and oxidative capacity of newly hatched hybrid yellow catfish larvae (Tachysurus fulvidraco ♀ × Pseudobagrus vachellii ♂). J. Therm. Biol. 2019, 86, 102436. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, M.; Kašpar, V.; Tichopád, T.; Rodina, M.; Flajšhans, M. How do suboptimal temperatures affect polyploid sterlet Acipenser ruthenus during early development? J. Fish Biol. 2022, 101, 77–91. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and organism size—A biological law for ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar]
- Ohlberger, J. Climate Warming and Ectotherm Body Size—From Individual Physiology to Community Ecology. Funct. Ecol. 2013, 27, 991–1001. [Google Scholar] [CrossRef]
- Spies, I.; Canino, M.; Dorn, M.; Jimenez-Hidalgo, I.; Hauser, L. Growth Patterns of Larval Walleye Pollock Gadus chalcogrammus from Core and Peripheral Habitat Differ in Response to Temperature. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2022, 199, 105083. [Google Scholar] [CrossRef]
- Réalis-Doyelle, E.; Pasquet, A.; Fontaine, P.; Teletchea, F. Effects of Temperature on the Survival and Development of the Early Life Stages of Northern Pike (Esox lucius). Knowl. Manag. Aquat. Ecosyst. 2022, 423, 10. [Google Scholar] [CrossRef]
- Jablonska, O.; Duda, S.; Gajowniczek, S.; Nitkiewicz, A.; Fopp-Bayat, D. Toll-like Receptor Type 2 and 13 Gene Expression and Immune Cell Profiles in Diploid and Triploid Sterlets (Acipenser ruthenus): Insights into Immune Competence in Polyploid Fish. Int. J. Mol. Sci. 2025, 26, 3986. [Google Scholar] [CrossRef]
- De Gelas, K.; Janko, K.; Volckaert, F.A.M.; De Charleroy, D.; Van Houdt, J.K.J. Development of Nine Polymorphic Microsatellite Loci in the Spined Loach, Cobitis taenia, and Cross-species Amplification in the Related Species C. elongatoides, C. taurica and C. tanaitica. Mol. Ecol. Resour. 2008, 8, 1001–1003. [Google Scholar] [CrossRef] [PubMed]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for Centromeric Position on Chromosomes. Hereditas 2009, 52, 201–220. [Google Scholar] [CrossRef]
- Jezierska, B.; Lugowska, K.; Witeska, M.; Sarnowski, P. Malformations of newly hatched common carp larvae. Electron. J. Pol. Agric. Univ. 2000, 3, 1. [Google Scholar]
- Alix, M.; Zarski, D.; Chardard, D.; Fontaine, P.; Schaerlinger, B. Deformities in Newly Hatched Embryos of Eurasian Perch Populations Originating from Two Different Rearing Systems. J. Zool. 2017, 302, 126–137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).