α-Bisabolol, a Dietary Bioactive Terpene Attenuates Oxidative Stress and Inflammation in Colonic Mucosa of Acetic Acid-Induced Colitis in Rats
Abstract
1. Introduction
2. Results
2.1. Effect of α-Bisabolol on Body Weight
2.2. Effect of α-Bisabolol on Macroscopic Ulcer Score (MaUS)
2.3. Effect of α-Bisabolol on Microscopic Ulcers and Score (MiUS)
2.4. Effect of α-Bisabolol on Myeloperoxidase in Colon
2.5. Effect of α-Bisabolol on Calprotectin Levels in the Colon
2.6. Effect of α-Bisabolol on Interleukin-1 (IL-1) Levels in Colon
2.7. Effect of α-Bisabolol on Interleukin-23 Levels in Colon
2.8. Effect of α-Bisabolol on the Glutathione (GSH) Levels in Colon
2.9. Effect of α-Bisabolol on Superoxide Dismutase Activity in the Colon
2.10. Effect of α-Bisabolol on Catalase Activity in Colon
2.11. Effect of α-Bisabolol on Malondialdehyde (MDA) Levels in Colon
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Chemicals and Kits
4.3. Experimental Design
4.4. Assessment of Macroscopic Ulcer Score
4.5. Assessment of Microscopic Ulcer Score
4.6. Preparation of Colon Tissue Homogenate
4.7. Determination of Myeloperoxidase (MPO)
4.8. Determination of Calprotectin
4.9. Measurements of Reduced Glutathione (GSH), Superoxide Dismutase (SOD) and Catalase
4.10. Measurement of Malondialdehyde (MDA)
4.11. Determination of Pro-Inflammatory Factors IL-1β and IL-23
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stepaniuk, P.; Bernstein, C.N.; Targownik, L.E.; Singh, H. Characterization of inflammatory bowel disease in elderly patients: A review of epidemiology, current practices and outcomes of current management strategies. Can. J. Gastroenterol. Hepatol. 2015, 29, 327–333. [Google Scholar] [CrossRef]
- Malik, T.A. Inflammatory Bowel Disease: Historical Perspective, Epidemiology, and Risk Factors. Surg. Clin. 2015, 95, 1105–1122. [Google Scholar]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Inflammatory bowel disease as a disorder of an imbalance between pro- and anti-inflammatory molecules and deficiency of resolution bioactive lipids. Lipids Health Dis. 2016, 15, 11. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.R. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Exp. Biol. Med. 2012, 237, 474–480. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- de Mattos, B.R.; Garcia, M.P.; Nogueira, J.B.; Paiatto, L.N.; Albuquerque, C.G.; Souza, C.L.; Fernandes, L.G.; Tamashiro, W.M.; Simioni, P.U. Inflammatory bowel disease: An overview of immune mechanisms and biological treatments. Mediat. Inflamm. 2015, 2015, 493012. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Basso, P.J.; Alves, V.B.; Fonseca, M.T.; Bonfá, G.; Nardini, V.; Cardoso, C.R. Classical and recent advances in the treatment of inflammatory bowel diseases. Braz. J. Med. Biol. Res. 2015, 48, 96–107. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Algieri, F.; Rodriguez-Nogales, A.; Rodriguez-Cabezas, M.E.; Risco, S.; Ocete, M.A.; Galvez, J. Botanical Drugs as an Emerging Strategy in Inflammatory Bowel Disease: A Review. Mediat. Inflamm. 2015, 2015, 179616. [Google Scholar] [CrossRef]
- Santana, M.T.; Cercato, L.M.; Oliveira, J.P.; Camargo, E.A. Medicinal Plants in the Treatment of Colitis: Evidence from Preclinical Studies. Planta Med. 2017, 83, 588–614. [Google Scholar] [CrossRef]
- Hung, A.; Kang, N.; Bollom, A.; Wolf, J.L.; Lembo, A. Complementary and alternative medicine use is prevalent among patients with gastrointestinal diseases. Dig. Dis. Sci. 2015, 60, 1883–1888. [Google Scholar] [CrossRef]
- Cheifetz, A.S.; Gianotti, R.; Luber, R.; Gibson, P.R. Complementary and Alternative Medicines Used by Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 415–429. [Google Scholar] [CrossRef]
- Venkataraman, B.; Ojha, S.; Belur, P.D.; Bhongade, B.; Raj, V.; Collin, P.D.; Adrian, T.E. Phytochemical drug candidates for the modulation of peroxisome proliferator-activated receptor γ in inflammatory bowel diseases. Phytother. Res. 2020, 34, 1530–1549. [Google Scholar] [CrossRef]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Phytotherapies in inflammatory bowel disease. J. Res. Med. Sci. 2019, 24, 42. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, S.M.A.; Adeghate, E.; Amir, N.; Ojha, S.; Oz, M. Menthol inhibits oxidative stress and inflammation in acetic acid-induced colitis in rat colonic mucosa. Am. J. Transl. Res. 2018, 10, 4210–4222. [Google Scholar] [PubMed]
- O’Hara, M.; Kiefer, D.; Farrell, K.; Kemper, K. A review of 12 commonly used medicinal herbs. Arch. Fam. Med. 1998, 7, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.F.N.; Azimullah, S.; Laham, F.; Tariq, S.; Goyal, S.N.; Adeghate, E.; Ojha, S. α-Bisabolol protects against β-adrenergic agonist-induced myocardial infarction in rats by attenuating inflammation, lysosomal dysfunction, NLRP3 inflammasome activation and modulating autophagic flux. Food Funct. 2020, 11, 965–976. [Google Scholar] [CrossRef]
- Zhang, Y.; Fein, E.B.; Fein, S.B. Feeding of dietary botanical supplements and teas to infants in the United States. Pediatrics 2011, 127, 1060–1066. [Google Scholar] [CrossRef]
- Nayeri, S.D.; Kheirkhah, M.; Janani, L. The effect of chamomile ointment on the healing of breastfeeding mothers’ nipple sore-a randomized controlled clinical trial. J. Evol. Med. Dent. Sci. 2019, 8, 1399–1404. [Google Scholar] [CrossRef]
- Bhatia, S.P.; McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on alpha-bisabolol. Food Chem. Toxicol. 2008, 46 (Suppl. S11), S72–S76. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M. Bisabolol. Int. J. Toxicol. 2017, 36 (Suppl. S2), 24S–25S. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.F.N.; Laham, F.; Al-Taee, H.; Azimullah, S.; Ojha, S. Protective effects of α-bisabolol on altered hemodynamics, lipid peroxidation, and nonenzymatic antioxidants in isoproterenol-induced myocardial infarction: In vivo and in vitro evidence. J. Biochem. Mol. Toxicol. 2018, 32, e22200. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, H.A.O.; Silva-Filho, S.E.; Wiirzler, L.A.M.; Cardia, G.F.E.; Uchida, N.S.; de Souza Silva-Comar, F.M.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of (-)-α-Bisabolol on the Inflammatory Response in Systemic Infection Experimental Model in C57BL/6 Mice. Inflammation 2020, 43, 193–203. [Google Scholar] [CrossRef]
- Guesmi, F.; Tyagi, A.K.; Prasad, S.; Landoulsi, A. Terpenes from essential oils and hydrolate of Teucrium alopecurus triggered apoptotic events dependent on caspases activation and PARP cleavage in human colon cancer cells through decreased protein expressions. Oncotarget 2018, 9, 32305–32320. [Google Scholar] [CrossRef]
- Arunachalam, S.; Nagoor Meeran, M.F.; Azimullah, S.; Kumar Jha, N.; Saraswathiamma, D.; Albawardi, A.; Beiram, R.; Ojha, S. α-Bisabolol Attenuates NF-κB/MAPK Signaling Activation and ER-Stress-Mediated Apoptosis by Invoking Nrf2-Mediated Antioxidant Defense Systems against Doxorubicin-Induced Testicular Toxicity in Rats. Nutrients 2022, 14, 4648. [Google Scholar] [CrossRef]
- Rocha, N.F.M.; de Oliveira, G.V.; de Araújo, F.Y.R.; Rios, E.R.V.; Carvalho, A.M.R.; Vasconcelos, L.F.; Macêdo, D.S.; Soares, P.M.G.; De Sousa, D.P.; de Sousa, F.C.F. (-)-α-Bisabolol-induced gastroprotection is associated with reduction in lipid peroxidation, superoxide dismutase activity and neutrophil migration. Eur. J. Pharm. Sci. 2011, 44, 455–461. [Google Scholar] [CrossRef]
- De, O. Leite, G.; Leite, L.H.; de S. Sampaio, R.; Araruna, M.K.A.; de Menezes, I.R.A.; da Costa, J.G.M.; Campos, A.R. (-)-α-Bisabolol attenuates visceral nociception and inflammation in mice. Fitoterapia. 2011, 82, 208–211. [Google Scholar] [CrossRef]
- Solovăstru, L.G.; Stîncanu, A.; De Ascentii, A.; Capparé, G.; Mattana, P.; Vâţă, D. Randomized, controlled study of innovative spray formulation containing ozonated oil and α-bisabolol in the topical treatment of chronic venous leg ulcers. Adv. Skin. Wound Care. 2015, 28, 406–409. [Google Scholar] [CrossRef]
- de Siqueira, R.J.; Freire, W.B.; Vasconcelos-Silva, A.A.; Fonseca-Magalhães, P.A.; Lima, F.J.; Brito, T.S.; Mourão, L.T.; Ribeiro, R.A.; Lahlou, S.; Magalhães, P.J. In-vitro characterization of the pharmacological effects induced by (-)-α-bisabolol in rat smooth muscle preparations. Can. J. Physiol. Pharmacol. 2012, 90, 23–35. [Google Scholar] [CrossRef] [PubMed]
- D’almeida, A.P.L.; de Oliveira, M.T.P.; de Souza, É.T.; Coutinho, D.d.S.; Ciambarella, B.T.; Gomes, C.R.; Terroso, T.; Guterres, S.S.; Pohlmann, A.R.; Silva, P.M.; et al. α-bisabolol-loaded lipid-core nanocapsules reduce lipopolysaccharide-induced pulmonary inflammation in mice. Int. J. Nanomed. 2017, 12, 4479–4491. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Pérez, V.M.; Ortiz, M.I.; Ponce-Monter, H.A.; Monter-Pérez, V.; Barragán-Ramírez, G. Anti-inflammatory and utero-relaxant effect of α-bisabolol on the pregnant human uterus. Korean J. Physiol. Pharmacol. 2018, 22, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Singh, M.; Dubey, V.; Srivastava, S.; Luqman, S.; Bawankule, D.U. α-(-)-bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Curr. Pharm. Biotechnol. 2014, 15, 173–181. [Google Scholar] [CrossRef]
- Teixeira, G.F.; Costa, F.N.; Campos, A.R. Corneal antinociceptive effect of (-)-α-bisabolol. Pharm. Biol. 2017, 55, 1089–1092. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Cariño-Cortés, R.; Ponce-Monter, H.A.; Castañeda-Hernández, G.; Chávez-Piña, A.E. Pharmacological interaction of α-bisabolol and diclofenac on nociception, inflammation, and gastric integrity in rats. Drug. Dev. Res. 2018, 79, 29–37. [Google Scholar] [CrossRef]
- Bezerra, S.B.; Leal, L.K.; Nogueira, N.A.; Campos, A.R. Bisabolol-induced gastroprotection against acute gastric lesions: Role of prostaglandins, nitric oxide, and KATP+ channels. J. Med. Food. 2010, 13, 231. [Google Scholar] [CrossRef]
- Braga, P.C.; Dal Sasso, M.; Fonti, E.; Culici, M. Antioxidant activity of bisabolol: Inhibitory effects on chemiluminescence of human neutrophil bursts and cell-free systems. Pharmacology 2009, 83, 110–115. [Google Scholar] [CrossRef]
- Rocha, N.F.M.; Venâncio, E.T.; Moura, B.A.; Silva, M.I.G.; Neto, M.R.A.; Rios, E.R.V.; De Sousa, D.P.; Vasconcelos, S.M.M.; Fonteles, M.M.D.F.; De Sousa, F.C.F. Gastroprotection of (-)-alpha-bisabolol on acute gastric mucosal lesions in mice: The possible involved pharmacological mechanisms. Fundam. Clin. Pharmacol. 2010, 24, 63–71. [Google Scholar] [CrossRef]
- Torrado, S.; Torrado, S.; Agis, A.; Jimenez, M.E.; Cadórniga, R. Effect of dissolution profile and (-)-alpha-bisabolol on the gastrotoxicity of acetylsalicylic acid. Die Pharmazie 1995, 50, 141–143. [Google Scholar]
- Venkataraman, B.; Almarzooqi, S.; Raj, V.; Dudeja, P.K.; Bhongade, B.A.; Patil, R.B.; Ojha, S.K.; Attoub, S.; Adrian, T.E.; Subramanya, S.B. α-Bisabolol Mitigates Colon Inflammation by Stimulating Colon PPAR-γ Transcription Factor: In Vivo and In Vitro Study. PPAR Res. 2022, 2022, 5498115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gopalsamy, R.G.; dos Santos Barreto, M.; Ummalyma, S.B.; Edwin Hillary, V.; Hariharan, G.; Athesh, K.; Krishnakumar, N.M.; de Novaes, A.M.; Pereira, M.M.; Montalvao, M.M.; et al. Dietary Essential Oil Components Enhance Gut Function by Modulating Interleukins and Mitigating Inflammatory Markers: A Systematic Review. Food Rev. Int. 2025, 18, 1–30. [Google Scholar] [CrossRef]
- Bastaki, S.M.A.; Amir, N.; Adeghate, E.; Ojha, S. Nerolidol, a sesquiterpene attenuate oxidative stress and inflammation in acetic acid-induced colitis in rats. Mol. Cell Biochem. 2021, 476, 3497–3512. [Google Scholar] [CrossRef] [PubMed]
- Kalra, J.; Lingaraju, M.C.; Mathesh, K.; Kumar, D.; Parida, S.; Singh, T.U.; Sharma, A.K.; Kumar, D.; Tandan, S.K. Betulinic acid alleviates dextran sulfate sodium-induced colitis and visceral pain in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 285–297. [Google Scholar] [CrossRef]
- de Santana Souza, M.T.; Teixeira, D.F.; de Oliveira, J.P.; Oliveira, A.S.; Quintans-Junior, L.J.; Correa, C.B.; Camargo, E.A. Protective effect of carvacrol on acetic acid-induced colitis. Biomed. Pharmacother. 2017, 96, 313–319. [Google Scholar] [CrossRef]
- Medicherla, K.; Sahu, B.D.; Kuncha, M.; Kumar, J.M.; Sudhakar, G.; Sistla, R. Oral administration of geraniol ameliorates acute experimental murine colitis by inhibiting pro-inflammatory cytokines and NF-κB signaling. Food Funct. 2015, 6, 2984–2995. [Google Scholar] [CrossRef]
- Jia, Z.; Xu, C.; Shen, J.; Xia, T.; Yang, J.; He, Y. The natural compound celastrol inhibits necroptosis and alleviates ulcerative colitis in mice. Int. Immunopharmacol. 2015, 29, 552–559. [Google Scholar] [CrossRef]
- Santos, F.A.; Silva, R.M.; Campos, A.R.; De Araújo, R.P.; Lima Júnior, R.C.; Rao, V.S. 1,8-cineole (eucalyptol), a monoterpene oxide attenuates the colonic damage in rats on acute TNBS-colitis. Food Chem. Toxicol. 2004, 42, 579–584. [Google Scholar] [CrossRef]
- MacPherson, B.R.; Pfeiffer, C.J. Experimental production of diffuse colitis in rats. Digestion 1978, 17, 135–150. [Google Scholar] [CrossRef]
- Warren, B.F. Another model of acetic acid-induced colitis in the rat. Aliment. Pharmacol. Ther. 1994, 8, 659–660. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, G.; Mahajan, U.B.; Goyal, S.N.; Ojha, S.; Patil, C.R.; Subramanya, S.B. Protective effect of Lagerstroemia speciosa against dextran sulfate sodium induced ulcerative colitis in C57BL/6 mice. Am. J. Transl. Res. 2017, 9, 1792–1800. [Google Scholar]
- Zhang, Y.; Wang, L.; Ocansey, D.K.W.; Wang, B.; Wang, L.; Xu, Z. Mucin-Type O-Glycans: Barrier, Microbiota, and Immune Anchors in Inflammatory Bowel Disease. J. Inflamm. Res. 2021, 14, 5939–5953. [Google Scholar] [CrossRef] [PubMed]
- Sørbye, H.; Svanes, K. The role of blood flow in gastric mucosal defense, damage and healing. Dig. Dis. 1994, 12, 305–317. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Tüzün, A.; Erdil, A.; Inal, V.; Aydin, A.; Bağci, S.; Yeşilova, Z.; Sayal, A.; Karaeren, N.; Dağalp, K. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin. Biochem. 2002, 35, 569–572. [Google Scholar] [CrossRef]
- Bastaki, S.M.; Al Ahmed, M.M.; Al Zaabi, A.; Amir, N.; Adeghate, E. Effect of turmeric on colon histology, body weight, ulcer, IL-23, MPO and glutathione in acetic-acid-induced inflammatory bowel disease in rats. BMC Complement. Altern. Med. 2016, 16, 72. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A.; Al Mofleh, I.A.; Al-Jebreen, A.M. Lipid peroxides in patients with inflammatory bowel disease. Saudi J. Gastroenterol. 2007, 13, 187–190. [Google Scholar] [CrossRef]
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef]
- Sartor, R.B. Cytokines in intestinal inflammation: Pathophysiological and clinical considerations. Gastroenterology 1994, 106, 533–539. [Google Scholar] [CrossRef]
- Kim, S.; Jung, E.; Kim, J.H.; Park, Y.H.; Lee, J.; Park, D. Inhibitory effects of (-)-α-bisabolol on LPS-induced inflammatory response in RAW264.7 macrophages. Food Chem. Toxicol. 2011, 49, 2580–2585. [Google Scholar] [CrossRef]
- Fernandes, M.Y.D.; Carmo, M.R.S.D.; Fonteles, A.A.; Neves, J.C.d.S.; da Silva, A.T.A.; Pereira, J.F.; Ferreira, E.d.O.; de Lima, N.M.R.; Neves, K.R.T.; de Andrade, G.M. (-)-α-bisabolol prevents neuronal damage and memory deficits through reduction of proinflammatory markers induced by permanent focal cerebral ischemia in mice. Eur. J. Pharmacol. 2019, 842, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Mullane, K.M.; Kraemer, R.; Smith, B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J. Pharmacol. Methods 1985, 14, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Chatzikonstantinou, M.; Konstantopoulos, P.; Stergiopoulos, S.; Kontzoglou, K.; Verikokos, C.; Perrea, D.; Dimitroulis, D. Calprotectin as a diagnostic tool for inflammatory bowel diseases. Biomed. Rep. 2016, 5, 403–407. [Google Scholar] [CrossRef]
- AbuTaha, N.; Nasr, F.A.; Al-Zharani, M.; Alqahtani, A.S.; Noman, O.M.; Mubarak, M.; Abdelhabib, S.; Wadaan, M.A. Effects of Hexane Root Extract of Ferule hermonis Boiss. on Human Breast and Colon Cancer Cells: An In Vitro and In Vivo Study. Biomed. Res. Int. 2019, 2019, 3079895. [Google Scholar] [CrossRef]
- Wallace, J.L.; Keenan, C.M. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am. J. Physiol. 1990, 258, G527–G534. [Google Scholar] [CrossRef]
- Adeghate, E.; Donáth, T. Morphological findings in long-term pancreatic tissue transplants in the anterior eye chamber of rats. Pancreas 1990, 5, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Fuss, I.; Kelsall, B.L.; Stüber, E.; Strober, W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 1995, 182, 1281–1290. [Google Scholar] [CrossRef]
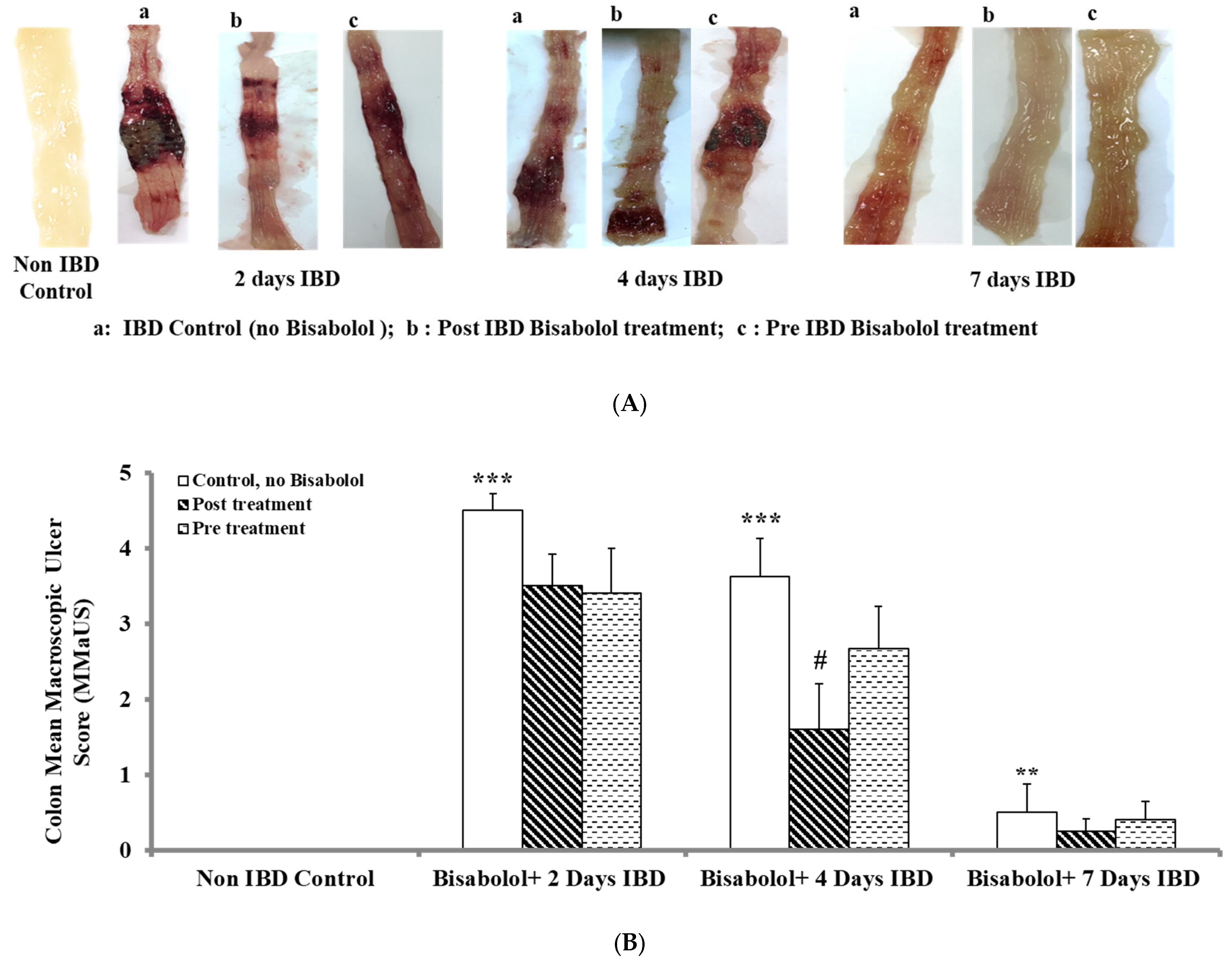
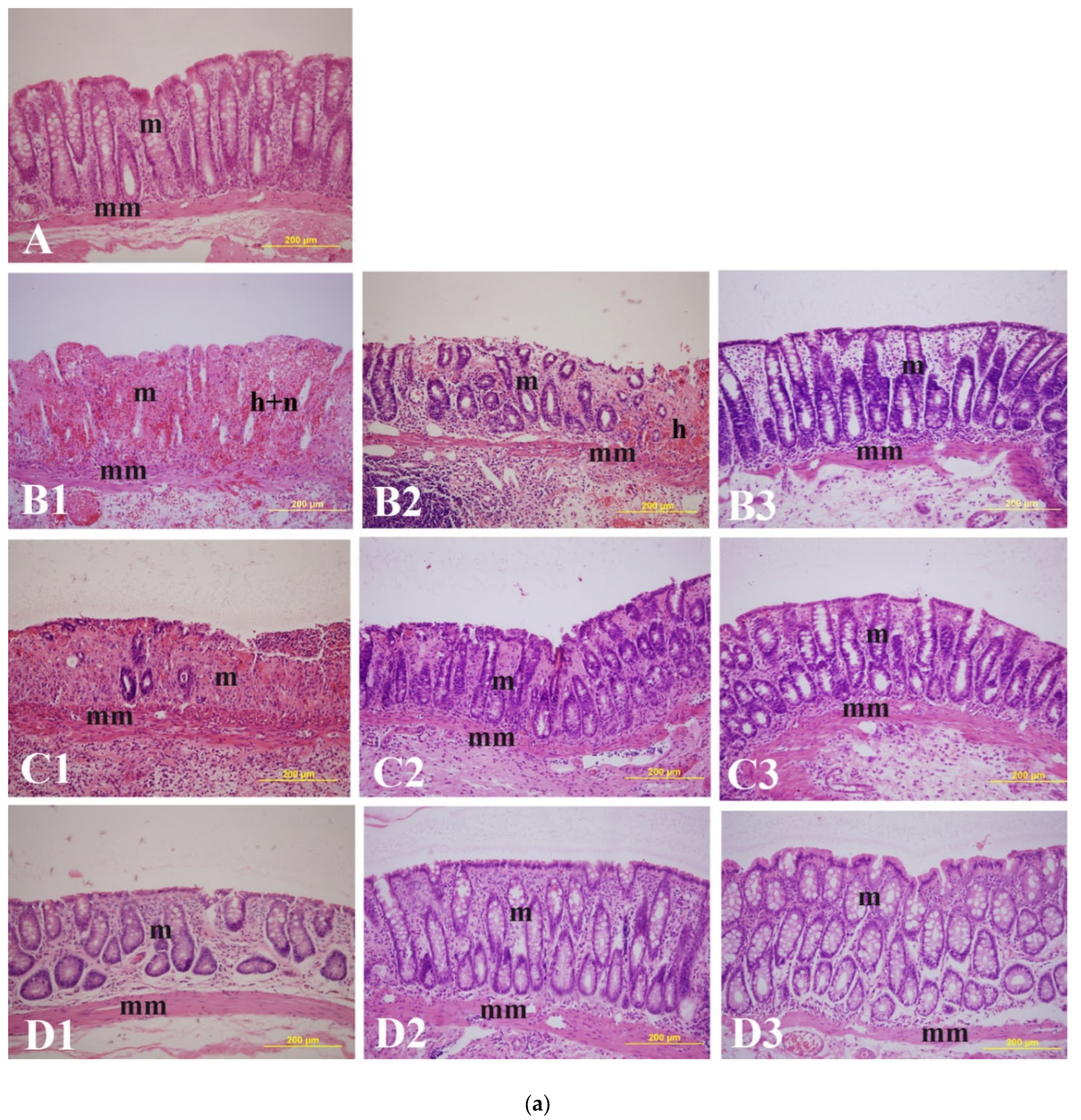
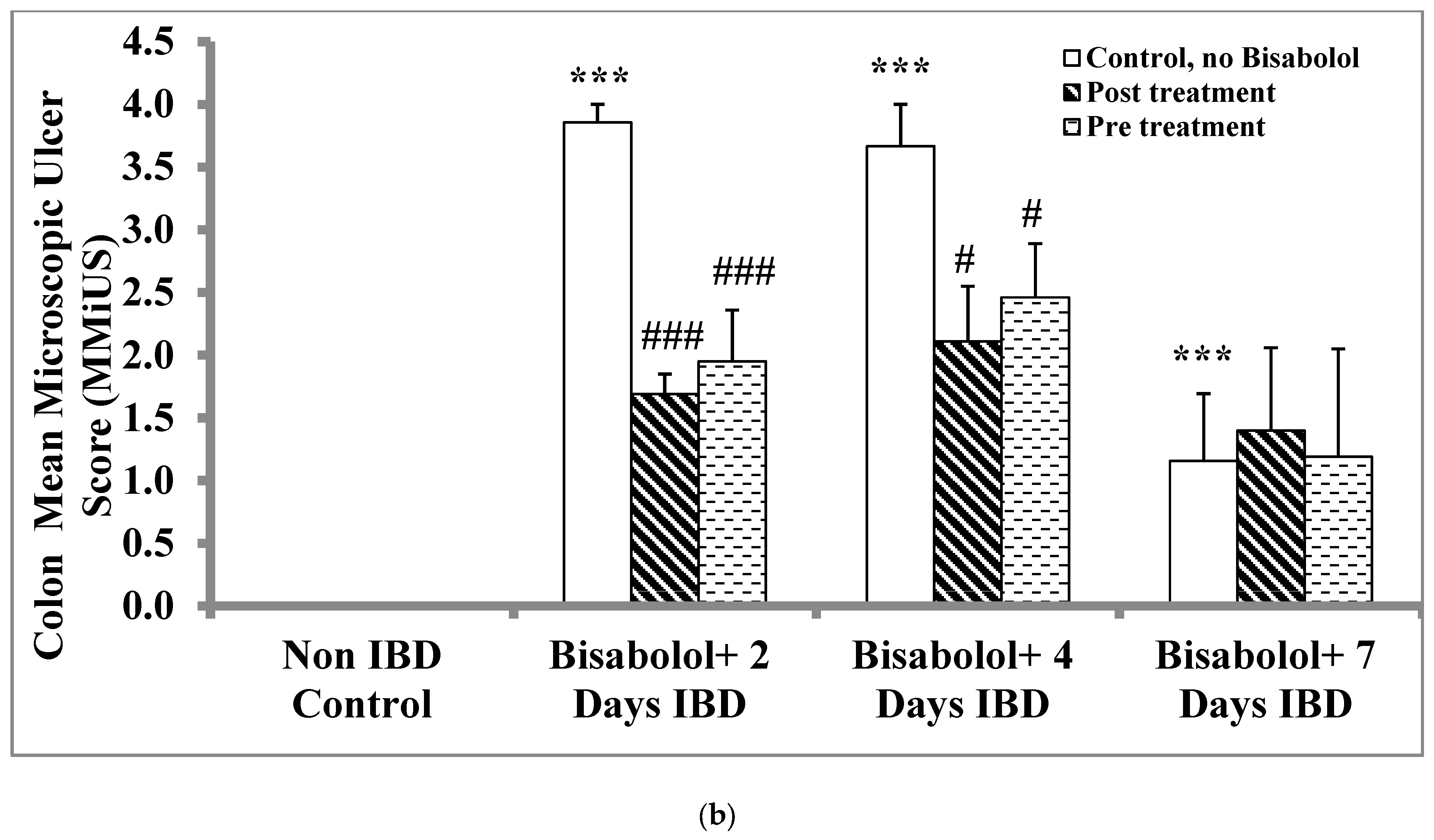
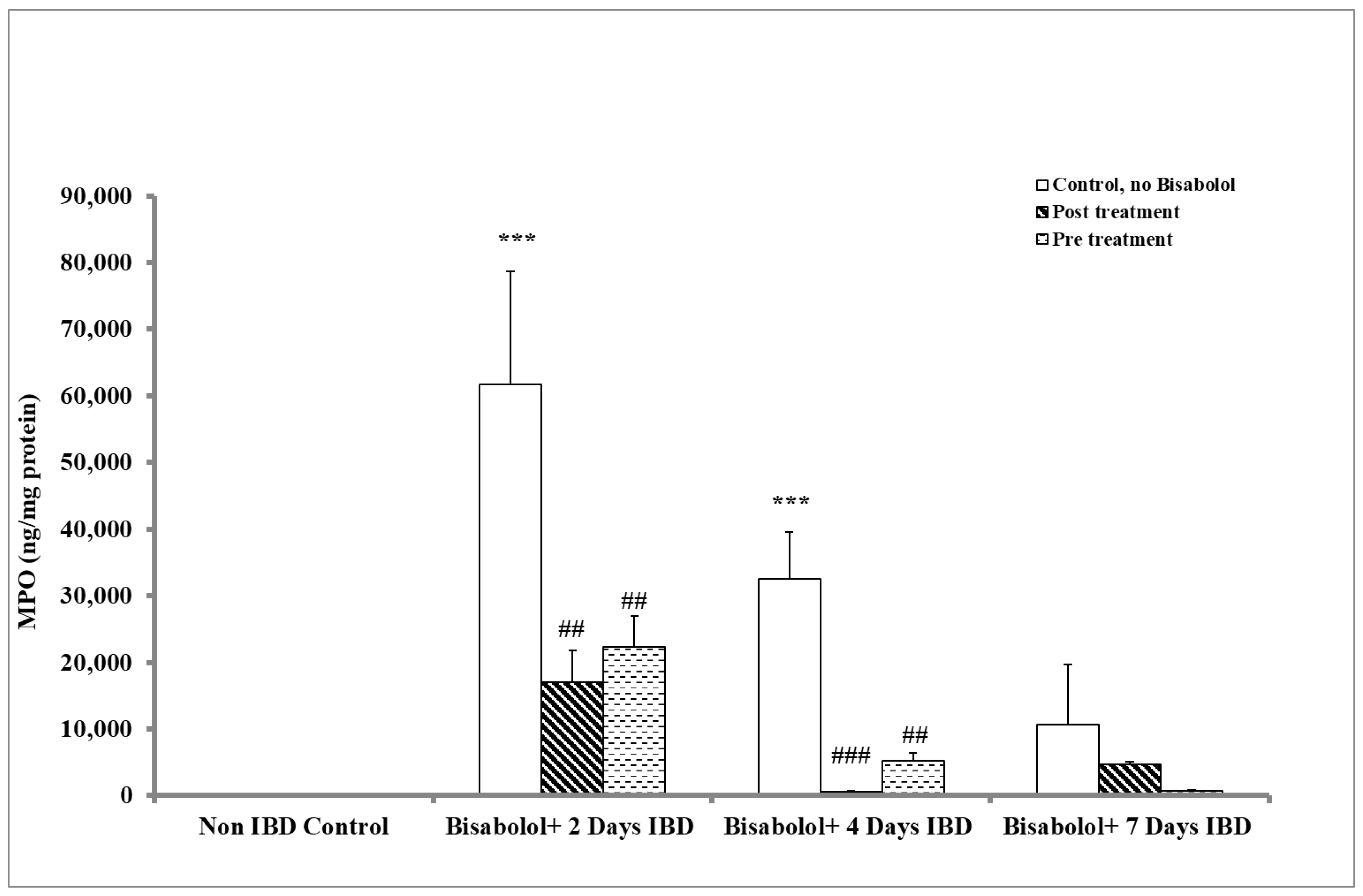
 Non-IBD control.
Non-IBD control.
 Non-IBD control.
Non-IBD control.
 Non-IBD control.
Non-IBD control.
 Non-IBD control.
Non-IBD control.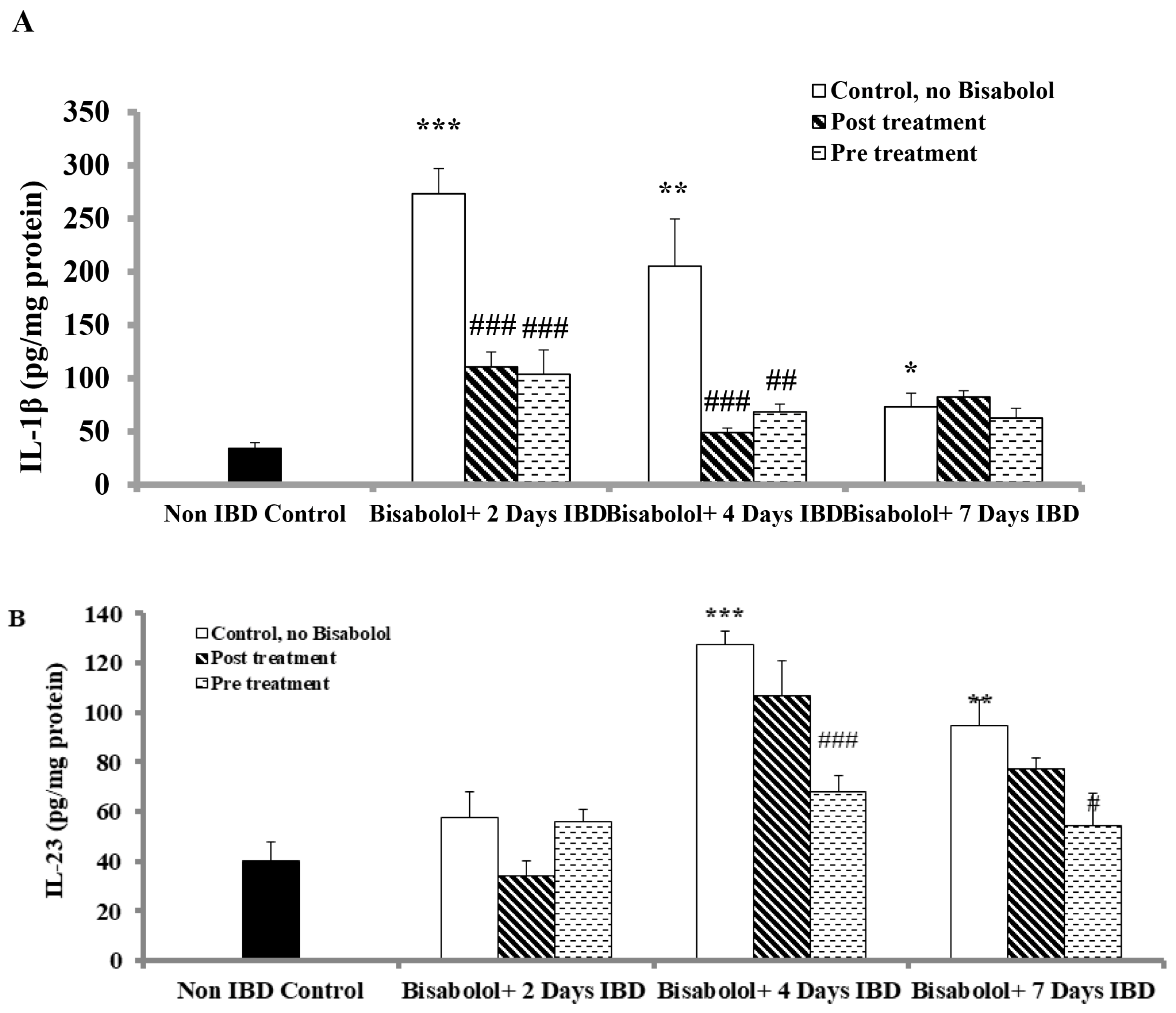
 Non-IBD control.
Non-IBD control.
 Non-IBD control.
Non-IBD control.
| Groups | Day 0 | Day 2 | Day 4 | Day 7 |
|---|---|---|---|---|
| Non-IBD Control | 205.17 ± 3.70 | 228.80 ± 3.46 | 244.50 ± 2.45 | 255.60 ± 1.96 |
| IBD control | 207.75 ± 2.34 | 206.83 ± 2.99 *** | 210.00 ± 4.66 *** | 211.60 ± 2.71 *** |
| IBD post-treated | 201.86 ± 3.08 | 213.50 ± 4.10 | 213.38 ± 3.98 | 243.75 ± 1.75 ### |
| IBD pretreated | 206.43 ± 3.41 | 209.13 ± 4.73 | 214.50 ± 4.86 | 241.25 ± 4.76 ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastaki, S.M.A.; Amir, N.; Ojha, S.; Adeghate, E. α-Bisabolol, a Dietary Bioactive Terpene Attenuates Oxidative Stress and Inflammation in Colonic Mucosa of Acetic Acid-Induced Colitis in Rats. Int. J. Mol. Sci. 2025, 26, 8168. https://doi.org/10.3390/ijms26178168
Bastaki SMA, Amir N, Ojha S, Adeghate E. α-Bisabolol, a Dietary Bioactive Terpene Attenuates Oxidative Stress and Inflammation in Colonic Mucosa of Acetic Acid-Induced Colitis in Rats. International Journal of Molecular Sciences. 2025; 26(17):8168. https://doi.org/10.3390/ijms26178168
Chicago/Turabian StyleBastaki, Salim M. A., Naheed Amir, Shreesh Ojha, and Ernest Adeghate. 2025. "α-Bisabolol, a Dietary Bioactive Terpene Attenuates Oxidative Stress and Inflammation in Colonic Mucosa of Acetic Acid-Induced Colitis in Rats" International Journal of Molecular Sciences 26, no. 17: 8168. https://doi.org/10.3390/ijms26178168
APA StyleBastaki, S. M. A., Amir, N., Ojha, S., & Adeghate, E. (2025). α-Bisabolol, a Dietary Bioactive Terpene Attenuates Oxidative Stress and Inflammation in Colonic Mucosa of Acetic Acid-Induced Colitis in Rats. International Journal of Molecular Sciences, 26(17), 8168. https://doi.org/10.3390/ijms26178168








