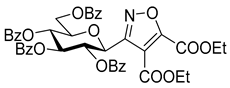
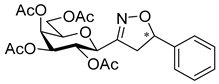
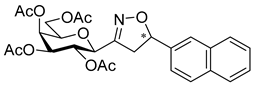
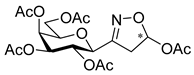
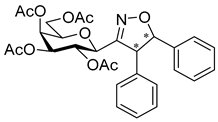
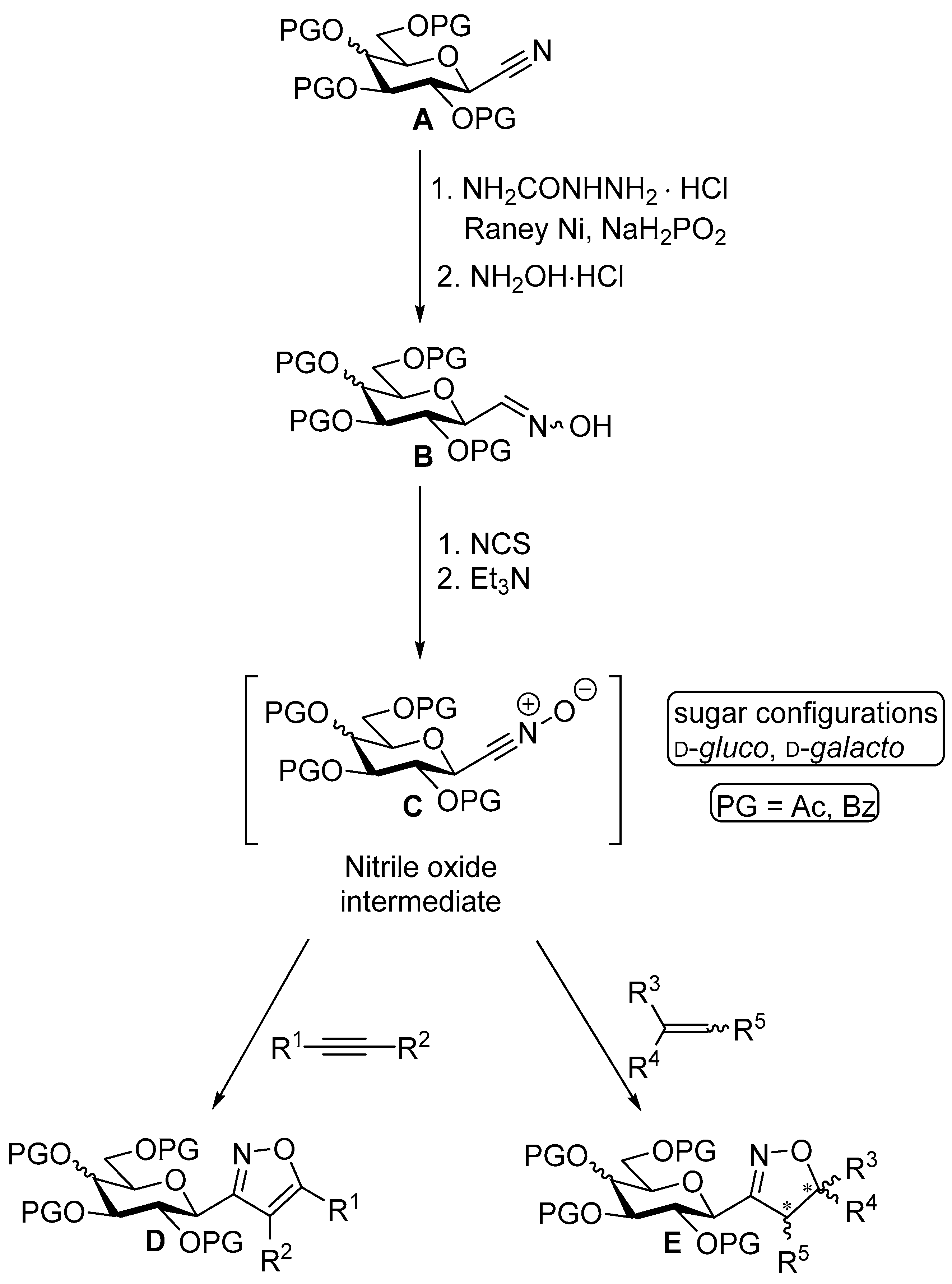
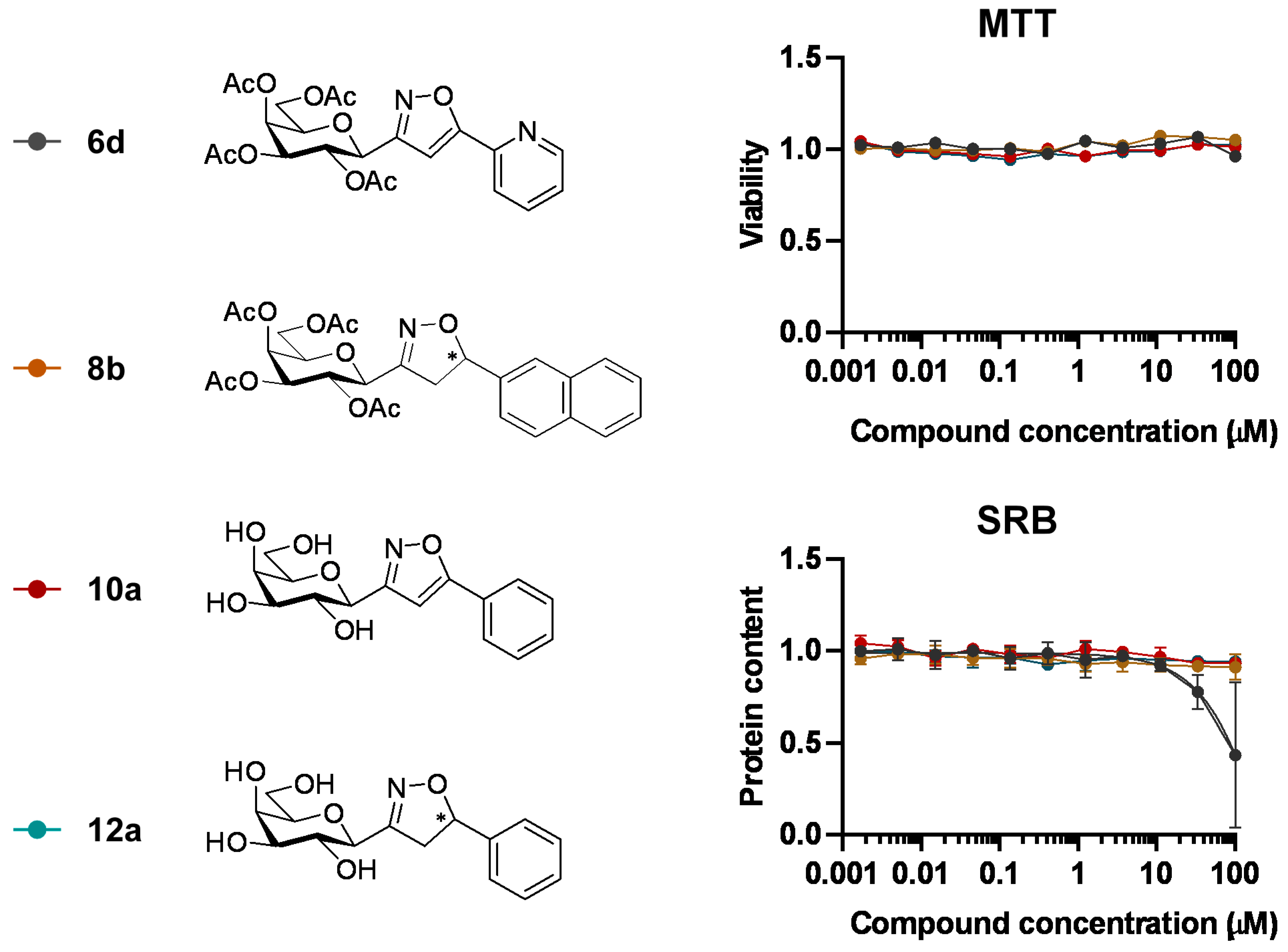
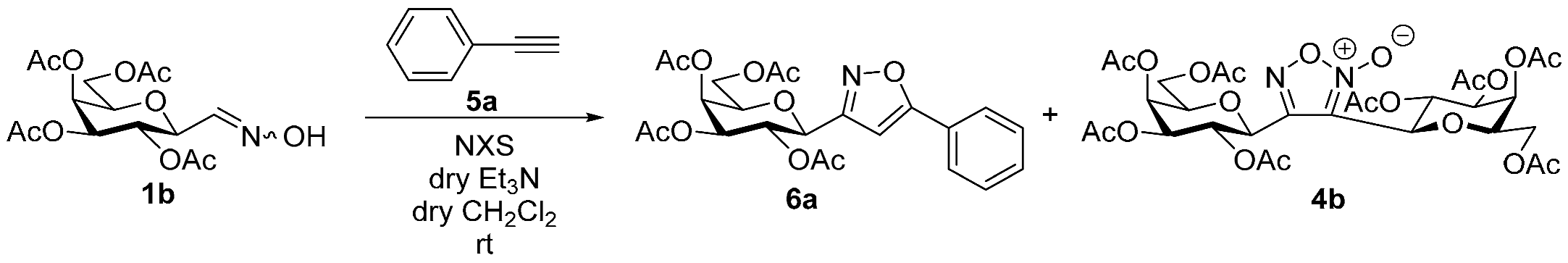
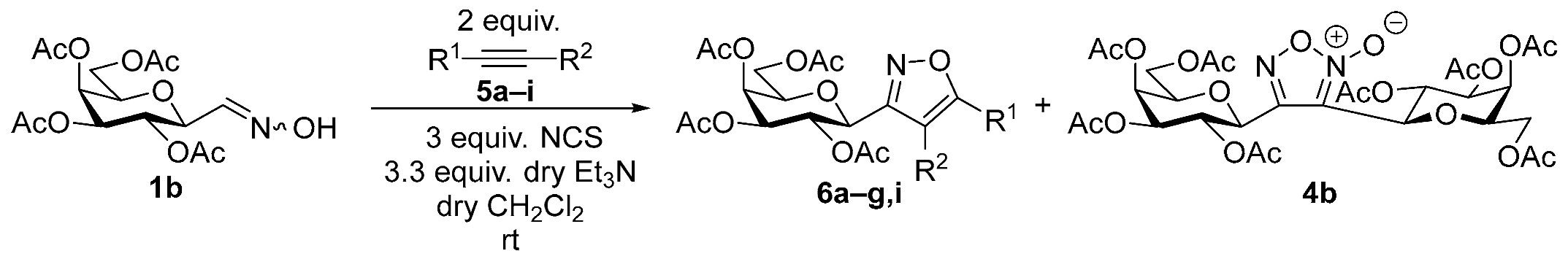
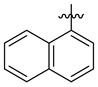
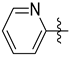
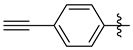
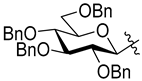
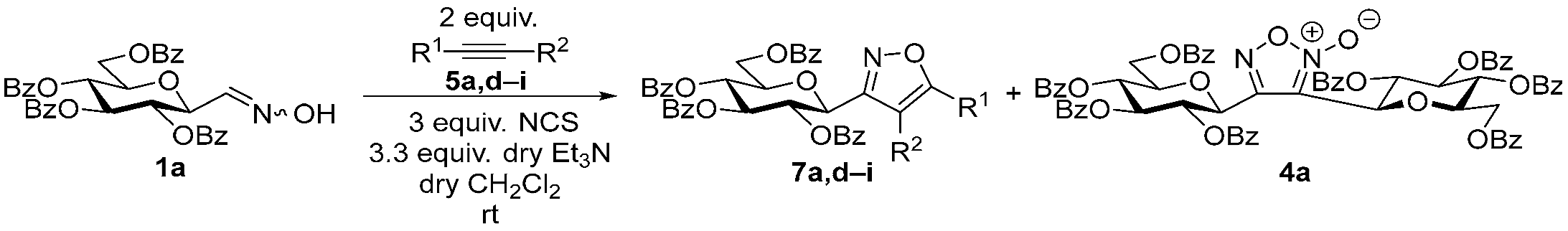
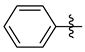
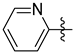
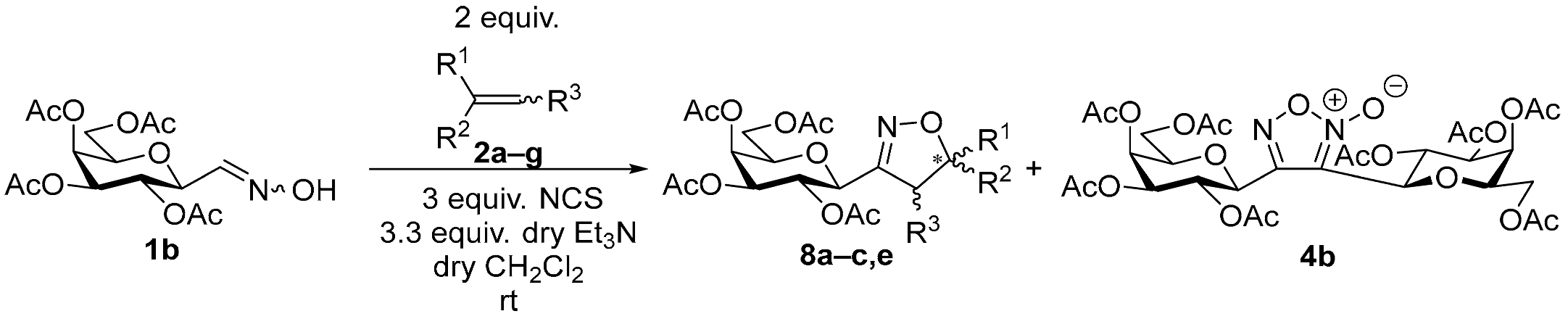
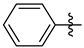
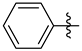
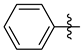
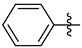
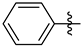
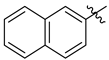
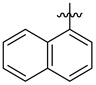
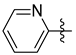
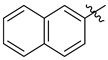
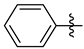
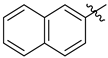
3.1.2. General Procedure I for Synthesis of 5-Substituted and 4,5-Disubstituted 3-(2′,3′,4′,6′-Tetra-O-acyl-β-d-glycopyranosyl)isoxazoles and -isoxazolines (3, 6, 7, 8)
A C-(2,3,4,6-tetra-O-acyl-β-d-glycopyranosyl)formaldehyde oxime (3,4,5,7-tetra-O-acyl-2,6-anhydro-heptose oxime) (1a and 1b 1 mmol), N-chlorosuccinimide (3 mmol), and dipolarophile (alkene or alkyne (2 mmol)) were added to dry dichloromethane (17 mL). The suspension was stirred for 30 min at room temperature, and then a solution of dry trimethylamine (3.3 mmol) in dry dichloromethane (40 mL) was added dropwise with a syringe pump in 16 h. When TLC (1:2 EtOAc–hexane for 1a, 1:1 EtOAc–hexane for 1b) indicated complete consumption of the starting compound (~16 h), the solvent was removed under reduced pressure, and the residue was purified by silica gel column chromatography with eluents indicated for the particular compounds to give 5-substituted and 4,5-disubstituted 3-(2′,3′,4′,6′-tetra-O-acyl-β-d-glycopyranosyl)isoxazoles 6 and 7 and -isoxazolines 3 and 8.
![Ijms 26 08167 i037 Ijms 26 08167 i037]()
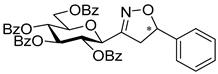
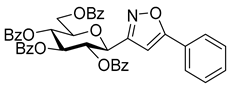
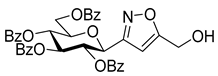
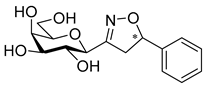
3-(2′,3′,4′,6′-Tetra-O-benzoyl-β-d-glucopyranosyl)-5-phenylisoxazolines (3a)
Prepared from oxime 1a (0.10 g, 0.16 mmol) and ethenylbenzene 2a (2 equiv., 36.7 µL, 0.03 g, 0.32 mmol) according to the General Procedure I. Purified by column chromatography (1:3 EtOAc–hexane) to yield 89 mg (77%) of (diastereomeric ratio: 1.3:1) as a white amorphous product. Rf: 0.26 (1:2 EtOAc–hexane). 3a-I 1H NMR (500 MHz, CDCl3) δ (ppm) 8.14–7.75 (8H, m, Ar), 7.64–7.14 (17H, m, Ar), 6.03 (1H, pseudo t, J3′,4′ 9.5 Hz, H-3′), 5.72 (1H, pseudo t, J4′,5′ 9.4 Hz, H-4′), 5.63–5.52 (2H, m, H-5, H-2′), 4.78 (1H, d, J1′,2′ 9.9 Hz, H-1′), 4.61 (1H, dd, J6a′,6b′ 12.3 Hz, H-6a′), 4.47 (1H, dd, H-6b′), 4.23 (1H, ddd, J5′,6a′ 2.3, J5′,6b′ 5.2 Hz, H-5′), 3.68 (1H, dd, J4a,4b 17.0, J4a,5 10.9 Hz, H-4a), 3.13 (1H, dd, J4b,5 9.2 Hz, H-4b). 13C NMR (125 MHz, CDCl3) δ (ppm) 166.2, 165.9, 165.8, 165.4 (4 × CO), 155.2 (C-3), 140.4–125.9 (Ar), 83.1 (C-5), 76.6 (C-5′), 74.6 (C-1′), 73.7 (C-3′), 70.1 (C-2′), 69.5 (C-4′), 63.2 (C-6′), 40.7 (C-4). 3a-II 1H NMR (500 MHz, CDCl3) δ (ppm) 8.14–7.75 (8H, m, Ar), 7.64–7.14 (17H, m, Ar), 6.00 (1H, pseudo t, J3′,4′ 9.5 Hz, H-3′), 5.70 (1H, pseudo t, J4′,5′ 9.4 Hz, H-4′), 5.63–5.52 (1H, m, H-5), 5.55 (1H, pseudo t, J2′,3′ 9.7 Hz, H-2′), 4.82 (1H, d, J1′,2′ 10.0 Hz, H-1′), 4.65 (1H, dd, J6a′,6b′ 12.3 Hz, H-6a′), 4.47 (1H, dd, H-6b′), 4.23 (1H, ddd, J5′,6a′ 1.9, J5′,6b′ 5.2 Hz, H-5′), 3.55 (1H, dd, J4a,4b 17.2, J4a,5 11.3 Hz, H-4a), 3.26 (1H, dd, J4b,5 9.2 Hz, H-4b). 13C NMR (125 MHz, CDCl3) δ (ppm) 166.2, 165.9, 165.8, 165.4 (4 × CO), 154.8 (C-3), 140.4–125.9 (Ar), 83.1 (C-5), 76.7 (C-5′), 74.6 (C-1′), 73.9 (C-3′), 69.6 (C-2′), 69.4 (C-4′), 63.2 (C-6′), 40.7 (C-4). HR-ESI-MS positive mode (m/z): calcd. for C43H35NO10 (725.23) [M + Na]+ = 748.2153, found: [M + Na]+ = 748.2152.
![Ijms 26 08167 i038 Ijms 26 08167 i038]()
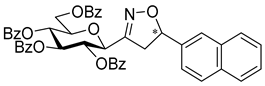
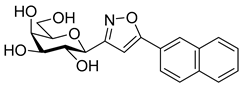
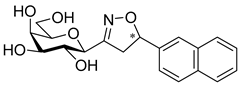
3-(2′,3′,4′,6′-Tetra-O-benzoyl-β-d-glucopyranosyl)-5-(naphth-2-yl)isoxazolines (3b)
Prepared from oxime 1a (0.10 g, 0.16 mmol) and 2-ethenylnaphthalene 2b (2 equiv., 0.05 g, 0.32 mmol) according to the General Procedure I. Purified by column chromatography (1:3 EtOAc–hexane) to yield 116 mg (93%) of 3b (diastereomeric ratio: 1:1) as a yellow amorphous product. Rf: 0.23 (1:2 EtOAc–hexane). 3b-I 1H NMR (500 MHz, CDCl3) δ (ppm) 8.15–7.63 (12H, m, Ar), 7.62–7.16 (15H, m, Ar), 6.05 (1H, pseudo t, J3′,4′ 9.6 Hz, H-3′), 5.81–5.73 (1H, m, H-5), 5.73 (1H, pseudo t, J4′,5′ 10.0 Hz, H-4′), 5.62 (1H, pseudo t, J2′,3′ 9.9 Hz, H-2′), 4.81 (1H, d, J1′,2′ 9.9 Hz, H-1′), 4.61 (1H, dd, J6a′,6b′ 12.3 Hz, H-6a′), 4.48 (1H, dd, H-6b′), 4.25 (1H, ddd, J5′,6a′ 2.4, J5′,6b′ 4.9 Hz, H-5′), 3.75 (1H, dd, J4a,4b 17.1, J4a,5 11.0 Hz, H-4a), 3.21 (1H, dd, J4b,5 9.2 Hz, H-4b). 13C NMR (125 MHz, CDCl3) δ (ppm) 166.2, 165.9, 165.8, 165.4 (4 × CO), 155.3 (C-3), 137.6–123.7 (Ar), 83.2 (C-5), 76.6 (C-5′), 74.6 (C-1′), 73.7 (C-3′), 70.2 (C-2′), 69.5 (C-4′), 63.1 (C-6′), 40.8 (C-4). 3b-II 1H NMR (500 MHz, CDCl3) δ (ppm) 8.15–7.63 (12H, m, Ar), 7.62–7.16 (15H, m, Ar), 6.01 (1H, pseudo t, J3′,4′ 9.6 Hz, H-3′), 5.81–5.73 (1H, m, H-5), 5.71 (1H, pseudo t, J4′,5′ 10.0 Hz, H-4′), 5.57 (1H, pseudo t, J2′,3′ 9.7 Hz, H-2′), 4.85 (1H, d, J1′,2′ 10.0 Hz, H-1′), 4.66 (1H, dd, J6a′,6b′ 12.2 Hz, H-6a′), 4.46 (1H, dd, H-6b′), 4.25 (1H, ddd, J5′,6a′ 2.1, J5′,6b′ 4.9 Hz, H-5′), 3.63 (1H, dd, J4a,4b 17.1, J4a,5 11.2 Hz, H-4a), 3.37 (1H, dd, J4b,5 9.0 Hz, H-4b). 13C NMR (125 MHz, CDCl3) δ (ppm) 166.3, 165.9, 165.4, 165.3 (4 × CO), 154.9 (C-3), 137.6–123.7 (Ar), 83.1 (C-5), 76.7 (C-5′), 74.6 (C-1′), 73.8 (C-3′), 69.6 (C-2′), 69.4 (C-4′), 63.1 (C-6′), 40.8 (C-4). HR-ESI-MS positive mode (m/z): calcd. for C47H37NO10 (775.24) [M + Na]+ = 798.2310, found: [M + Na]+ = 798.2310.
![Ijms 26 08167 i039 Ijms 26 08167 i039]()
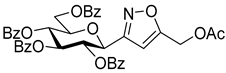
[3-(2′,3′,4′,6′-Tetra-O-benzoyl-β-d-glucopyranosyl)isoxazol-5-yl] acetates (3c)
Prepared from oxime 1a (0.10 g, 0.16 mmol) and ethenyl acetate 2c (2 equiv., 29.6 µL, 0.03 g, 0.32 mmol) according to the General Procedure I. Purified by column chromatography (1:3 EtOAc–hexane) to yield 67 mg (59%) of 3c (diastereomeric ratio: 1.1:1) as a white amorphous product. Rf: 0.36 (1:1 EtOAc–hexane). 3c-I 1H NMR (500 MHz, CDCl3) δ (ppm) 8.08–7.74 (8H, m, Ar), 7.62–7.12 (12H, m, Ar), 6.72 (1H, d, H-5), 6.03 (1H, pseudo t, J3′,4′ 9.6 Hz, H-3′), 5.76 (1H, pseudo t, J4′,5′ 9.9 Hz, H-4′), 5.49 (1H, pseudo t, J2′,3′ 9.7 Hz, H-2′), 4.81 (1H, d, J1′,2′ 9.9 Hz, H-1′), 4.66 (1H, dd, H-6a′), 4.51 (1H, dd, J6a′,6b′ 12.3 Hz, H-6b′), 4.25 (1H, ddd, J5′,6a′ 2.4, J5′,6b′ 4.8 Hz, H-5′), 3.56 (1H, dd, J4a,4b 18.3, J4a,5 7.2 Hz, H-4a), 3.13 (1H, dd, J4b,5 < 1.0 Hz, H-4b), 2.03 (3H, s, CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 169.7 (OCOCH3), 166.2, 165.8, 165.3 (4 × CO), 156.3 (C-3), 134.1–128.1 (Ar), 95.8 (C-5), 76.7 (C-5′), 74.2 (C-1′), 73.5 (C-3′), 69.9 (C-2′), 69.3 (C-4′), 63.0 (C-6′), 39.3 (C-4), 21.1 (CH3). 3c-II 1H NMR (500 MHz, CDCl3) δ (ppm) 8.08–7.74 (8H, m, Ar), 7.62–7.12 (12H, m, Ar), 6.63 (1H, d, H-5), 5.99 (1H, pseudo t, J3′,4′ 9.6 Hz, H-3′), 5.74 (1H, pseudo t, J4′,5′ 9.9 Hz, H-4′), 5.67 (1H, pseudo t, J2′,3′ 9.7 Hz, H-2′), 4.87 (1H, d, J1′,2′ 10.0 Hz, H-1′), 4.66 (1H, dd, H-6a′), 4.46 (1H, dd, J6a′,6b′ 12.3 Hz, H-6b′), 4.25 (1H, ddd, J5′,6a′ 2.4, J5′,6b′ 5.2 Hz, H-5′), 3.35 (1H, dd, J4a,4b 18.2, J4a,5 6.7 Hz, H-4a), 3.20 (1H, dd, J4b,5 < 1.0 Hz, H-4b), 1.82 (3H, s, CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 169.7 (OCOCH3), 166.2, 165.8, 165.3 (4 × CO), 155.7 (C-3), 134.1–128.1 (Ar), 95.7 (C-5), 76.9 (C-5′), 74.0 (C-1′), 73.8 (C-3′), 69.7 (C-2′), 69.4 (C-4′), 63.0 (C-6′), 39.3 (C-4), 20.7 (CH3). HR-ESI-MS positive mode (m/z): calcd. for C39H33NO12 (707.20) [M + Na]+ = 730.1895, found: [M + Na]+ = 730.1895.
![Ijms 26 08167 i040 Ijms 26 08167 i040]()
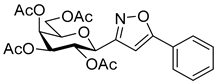
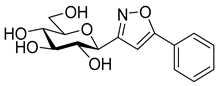
3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)-5-phenylisoxazole (6a)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and ethynylbenzene 5a (2 equiv., 59.7 µL, 0.06 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (1:2 EtOAc–hexane) to yield 86 mg (68%) of 6a as a yellow amorphous product. Rf: 0.59 (1:1 EtOAc–hexane); [α]D−16 (c 0.18, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 7.86–7.73 (2H, m, Ar), 7.52–7.39 (3H, m, Ar), 6.68 (1H, s, H-4), 5.55 (1H, dd, J4′,5′ 0.9 Hz, H-4′), 5.47 (1H, pseudo t, J2′,3′ 10.1 Hz, H-2′), 5.21 (1H, dd, J3′,4′ 3.4 Hz, H-3′), 4.69 (1H, d, J1′,2′ 9.9 Hz, H-1′), 4.24–4.14 (2H, m, H-6a′, H-6b′), 4.12 (1H, ddd, J5′,6a′ 6.1, J5′,6b′ 6.7 Hz, H-5′), 2.21, 2.05, 2.01, 1.97 (12H, 4s, 4 × CH3). 13C NMR (90 MHz, CDCl3) δ (ppm) 170.7, 170.3, 170.2, 169.6 (4 × CO), 170.6 (C-5), 161.5 (C-3), 130.9–125.6 (Ar), 97.9 (C-4), 75.1 (C-5′), 73.5 (C-1′), 71.9 (C-3′), 67.8 (C-2′), 67.7 (C-4′), 61.8 (C-6′), 20.82, 20.78, 20.72, 20.68 (4 × CH3). HR-ESI-MS positive mode (m/z): calcd. for C23H25NO10 (475.15) [M + Na]+ = 498.1371, found: [M + Na]+ = 498.1370.
![Ijms 26 08167 i041 Ijms 26 08167 i041]()
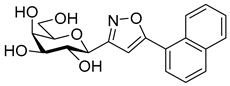
3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)-5-(naphth-2-yl)isoxazole (6b)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and 2-ethynylnaphthalene 5b (2 equiv., 0.08 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (1:1 EtOAc–hexane) to yield 78 mg (56%) of 6b as a yellow amorphous product. Rf: 0.35 (1:1 EtOAc–hexane); [α]D−23 (c 0.23, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 8.31 (1H, bs, Ar), 7.91 (2H, d, J 8.1 Hz, Ar), 7.88–7.79 (2H, m, Ar), 7.57–7.51 (2H, m, Ar), 6.80 (1H, s, H-4), 5.57 (1H, dd, J4′,5′ 0.5 Hz, H-4′), 5.50 (1H, pseudo t, J2′,3′ 10.1 Hz, H-2′), 5.23 (1H, dd, J3′,4′ 3.3 Hz, H-3′), 4.72 (1H, d, J1′,2′ 9.9 Hz, H-1′), 4.25–4.07 (3H, m, H-5′, H-6a′, H-6b′), 2.23, 2.06, 2.01, 1.99 (12H, 4s, 4 × CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 170.7, 170.5, 170.3, 170.2, 169.6 (4 × CO, C-5), 161.6 (C-3), 134.2–122.8 (Ar), 98.3 (C-4), 75.1 (C-5′), 73.5 (C-1′), 71.9 (C-3′), 67.8 (C-2′), 67.7 (C-4′), 61.8 (C-6′), 20.82, 20.81, 20.80, 20.71 (4 × CH3). HR-ESI-MS positive mode (m/z): calcd. for C27H27NO10 (525.16) [M + H]+ = 526.1708, found: [M + H]+ = 526.1702.
![Ijms 26 08167 i042 Ijms 26 08167 i042]()
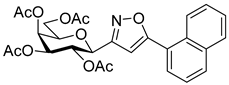
3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)-5-(naphth-1-yl)isoxazole (6c)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and 2-ethynylnaphthalene 5c (2 equiv., 0.08 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (1:2 EtOAc–hexane) to yield 80 mg (57%) of 6c as a pale yellow amorphous product. Rf: 0.41 (1:1 EtOAc–hexane); [α]D−17 (c 0.11, CH2Cl2). 1H NMR (700 MHz, CDCl3) δ (ppm) 8.28 (1H, d, J 8.4 Hz, Ar), 7.97 (1H, d, J 8.2 Hz, Ar), 7.92 (1H, d, J 8.1 Hz, Ar), 7.81 (1H, dd, J 1.0, 7.1 Hz, Ar), 7.61 (1H, ddd, J 1.2, 6.8, 8.3 Hz, Ar), 7.59–7.48 (2H, m, Ar), 6.77 (1H, s, H-4), 5.57 (1H, dd, J4′,5′ 0.8 Hz, H-4′), 5.55 (1H, pseudo t, J2′,3′ 10.1 Hz, H-2′), 5.25 (1H, dd, J3′,4′ 3.5 Hz, H-3′), 4.76 (1H, d, J1′,2′ 9.9 Hz, H-1′), 4.23–4.17 (2H, m, H-6a′, H-6b′), 4.17–4.13 (1H, m, H-5′), 2.21, 2.06, 2.02, 2.01 (12H, 4s, 4 × CH3). 13C NMR (175 MHz, CDCl3) δ (ppm) 170.7, 170.6, 170.4, 170.2, 169.7 (4 × CO, C-5), 161.1 (C-3), 134.0–124.9 (Ar), 102.1 (C-4), 75.2 (C-5′), 73.6 (C-1′), 71.9 (C-3′), 67.9 (C-2′), 67.7 (C-4′), 61.8 (C-6′), 20.87, 20.84, 20.83, 20.75 (4 × CH3). HR-ESI-MS positive mode (m/z): calcd. for C27H27NO10 (525.16) [M + H]+ = 526.1708, found: [M + H]+ = 526.1713.
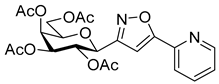
![Ijms 26 08167 i043 Ijms 26 08167 i043]()
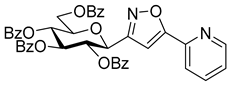
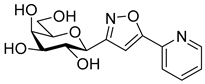
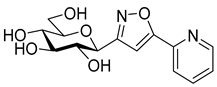
3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)-5-(pyridin-2-yl)isoxazole (6d)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and 2-ethynylpyridine 5d (2 equiv., 56.1 µL, 0.05 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (1:1 EtOAc–hexane) to yield 80 mg (63%) of 6d as a pale orange amorphous product. Rf: 0.57 (1:1 EtOAc–hexane); [α]D−21 (c 0.19, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ (ppm) 8.75–8.64 (1H, m, Ar), 7.93–7.77 (2H, m, Ar), 7.41–7.30 (1H, m, Ar), 7.09 (1H, s, H-4), 5.54 (1H, dd, J4′,5′ 0.9 Hz, H-4′), 5.46 (1H, pseudo t, J2′,3′ 10.1 Hz, H-2′), 5.21 (1H, dd, J3′,4′ 3.3 Hz, H-3′), 4.71 (1H, d, J1′,2′ 9.9 Hz, H-1′), 4.21–4.07 (3H, m, H-5′, H-6a′, H-6b′), 2.20, 2.05, 2.01, 1.98 (12H, 4s, 4 × CH3). 13C NMR (100 MHz, CDCl3) δ (ppm) 170.6, 170.4, 170.2, 169.5 (4 × CO), 169.7 (C-5), 162.0 (C-3), 177.9–120.6 (Ar), 101.0 (C-4), 74.9 (C-5′), 73.2 (C-1′), 71.8 (C-3′), 67.8 (C-2′), 67.6 (C-4′), 61.9 (C-6′), 20.77, 20.76, 20.73, 20.69 (4 × CH3). HR-ESI-MS positive mode (m/z): calcd. for C22H24N2O10 (476.14) [M + H]+ = 477.1504, found: [M + H]+ = 477.1504.
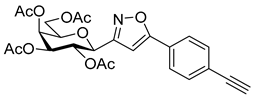
![Ijms 26 08167 i044 Ijms 26 08167 i044]()
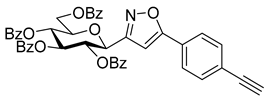
3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)-5-(4-ethynylphenyl)isoxazole (6e)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and 1,4-diethynylbenzene 5e (2 equiv., 0.07 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (1.2:1 EtOAc–hexane) to yield 55 mg (41%) of 6e as a yellow amorphous product. Rf: 0.58 (1:1 EtOAc–hexane); [α]D−37 (c 0.18, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 7.75 (2H, d, J 8.3 Hz, Ar), 7.58 (2H, d, J 8.4 Hz, Ar), 6.70 (1H, s, H-4), 5.55 (1H, dd, J4′,5′ 0.9 Hz, H-4′), 5.45 (1H, pseudo t, J2′,3′ 10.1 Hz, H-2′), 5.20 (1H, dd, J3′,4′ 3.4 Hz, H-3′), 4.68 (1H, d, J1′,2′ 10.0 Hz, H-1′), 4.21–4.15 (2H, m, H-6a′, H-6b′), 4.11 (1H, ddd, J5′,6a′ 6.7, J5′,6b′ 6.6 Hz, H-5′), 3.21 (1H, s, CCH), 2.21, 2.05, 2.01, 1.98 (12H, 4s, 4 × CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 170.6, 170.3, 170.2, 169.7 (4 × CO), 169.7 (C-5), 161.7 (C-3), 133.0–124.2 (Ar), 98.7 (C-4), 83.0 (CCH), 79.6 (CCH), 75.1 (C-5′), 73.5 (C-1′), 71.8 (C-3′), 67.7 (C-2′, C-4′), 61.8 (C-6′), 20.85 (2), 20.81, 20.75 (4 × CH3). HR-ESI-MS positive mode (m/z): calcd. for C25H25NO10 (499.47). [M + H]+ = 500.1551, found: [M + H]+ = 500.1552.
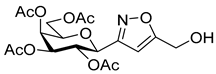
![Ijms 26 08167 i045 Ijms 26 08167 i045]()
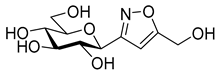
[3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)isoxazol-5-yl]methanol (6f)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and prop-2-yn-1-ol 5f (2 equiv., 28.8 µL, 0.03 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (from 1:3 to 1:1 EtOAc–hexane) to yield 54 mg (47%) of 6f as a pale orange amorphous product. Rf: 0.15 (1:1 EtOAc–hexane); [α]D−0.1 (c 0.15, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 6.42 (1H, s, H-4), 5.52 (1H, dd, J4′,5′ 0.6 Hz, H-4′), 5.38 (1H, pseudo t, J2′,3′ 10.2 Hz, H-2′), 5.18 (1H, dd, J3′,4′ 2.8 Hz, H-3′), 4.76 (2H, s, CH2OH), 4.63 (1H, d, J1′,2′ 9.9 Hz, H-1′), 4.18–4.12 (2H, m, H-6a′, H-6b′), 4.08 (1H, ddd, J5′,6a′ 6.1, J5′,6b′ 6.2 Hz, H-5′), 2.38 (1H, bs, CH2OH), 2.19, 2.05, 2.00, 1.96 (12H, 4s, 4 × CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 172.1 (C-5), 170.6, 170.3, 170.2, 169.8 (4 × CO), 161.0 (C-3), 100.5 (C-4), 75.0 (C-5′), 73.3 (C-1′), 71.8 (C-3′), 67.8 (C-2′), 67.6 (C-4′), 61.8 (C-6′), 56.7 (CH2OH), 20.83, 20.81, 20.79, 20.73 (4 × CH3). HR-ESI-MS positive mode (m/z): calcd. for C18H23NO11 (429.13) [M + H]+ = 430.1344, found: [M + H]+ = 430.1343.
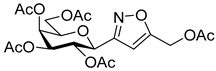
![Ijms 26 08167 i046 Ijms 26 08167 i046]()
[3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)isoxazol-5-yl]methyl acetate (6g)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and prop-2-yn-1-yl acetate 5g (2 equiv., 52.9 µL, 0.05 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (from 1:2 to 1:0 EtOAc–hexane) to yield 58 mg (46%) of 6g as a pale yellow amorphous product. Rf: 0.55 (2:1 EtOAc–hexane); [α]D−5 (c 0.22, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 6.46 (1H, s, H-4), 5.52 (1H, dd, J4′,5′ 0.4 Hz, H-4′), 5.37 (1H, pseudo t, J2′,3′ 10.1 Hz, H-2′), 5.19 (1H, d, JCHa,CHb 13.3 Hz, CHaOAc), 5.18 (1H, dd, J3′,4′ 3.2 Hz, H-3′), 5.15 (1H, d, CHbOAc), 4.64 (1H, d, J1′,2′ 9.9 Hz, H-1′), 4.19–4.11 (2H, m, H-6a′, H-6b′), 4.08 (1H, ddd, J5′,6a′ 6.1, J5′,6b′ 6.2 Hz, H-5′), 2.14 (3H, s, CH2OCOCH3), 2.19, 2.05, 2.00, 1.96 (12H, 4s, 4 × CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 170.3 (CH2OCOCH3), 170.5, 170.2, 170.1, 169.9 (4 × CO), 167.4 (C-5), 161.1 (C-3), 102.6 (C-4), 75.0 (C-5′), 73.2 (C-1′), 71.7 (C-3′), 67.7 (C-2′), 67.5 (C-4′), 61.7 (C-6′), 56.4 (CH2OAc), 20.78, 20.77, 20.72, 20.69, 20.68 (5×CH3). HR-ESI-MS positive mode (m/z): calcd. for C20H25NO12 (471.42) [M + Na]+ = 494.1269, found: [M + Na]+ = 494.1268.
![Ijms 26 08167 i047 Ijms 26 08167 i047]()
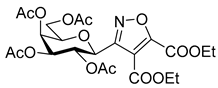
Diethyl 3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)isoxazole-4,5-dicarboxylate (6i)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and diethyl but-2-ynedioate 5i (2 equiv., 85.3 µL, 0.09 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (from 1:2 to 2:1 EtOAc–hexane) to yield 64 mg (44%) of 6i as a pale yellow amorphous product. Rf: 0.30 (1:1 EtOAc–hexane); [α]D+2 (c 0.33, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 5.77 (1H, pseudo t, J2′,3′ 10.2 Hz, H-2′), 5.50 (1H, dd, J4′,5′ 0.6 Hz, H-4′), 5.17 (1H, dd, J3′,4′ 3.3 Hz, H-3′), 4.88 (1H, d, J1′,2′ 10.0 Hz, H-1′), 4.49–4.32 (4H, m, 2 × CH2CH3) 4.15–4.04 (3H, m, H-5′, H-6a′, H-6b′), 2.19, 2.04, 2.01, 1.96 (12H, 4s, 4 × CH3), 1.40 (6H, 2 × t, J 7.1 Hz, 2 × CH2CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 170.5, 170.4, 170.3, 169.2 (4 × CO), 160.1 (C-5, COOEt), 159.0 (C-3), 156.0 (COOEt), 115.7 (C-4), 75.2 (C-5′), 72.7 (C-1′), 72.0 (C-3′), 67.5 (C-4′), 67.2 (C-2′), 63.1, 62.2 (2 × CH2CH3), 61.6 (C-6′), 20.78, 20.75, 20.74 (2) (4 × CH3), 14.2, 14.1 (2 × CH2CH3). HR-ESI-MS positive mode (m/z): calcd. for C23H29NO14 (543.16) [M + H]+ = 544.1661, found: [M + H]+ = 544.1664.
![Ijms 26 08167 i048 Ijms 26 08167 i048]()
3-(2′,3′,4′,6′-Tetra-O-benzoyl-β-d-glucopyranosyl)-5-phenylisoxazole (7a)
Prepared from oxime
1a (0.10 g, 0.16 mmol) and ethynylbenzene
5a (2 equiv., 35.2 µL, 0.03 g, 0.32 mmol) according to the General Procedure I. Purified by column chromatography (from 1:3 to 1:2 EtOAc–hexane) to yield 67 mg (57%) of
7a as a yellow amorphous product. R
f: 0.34 (1:2 EtOAc–hexane).
1H NMR (500 MHz, CDCl
3) δ (ppm) 8.14–7.68 (10H, m, Ar), 7.64–7.20 (15H, m, Ar), 6.72 (1H, s, H-4), 6.05 (1H, pseudo t,
J3′,4′ 9.5 Hz, H-3′), 5.84 (1H, pseudo t,
J4′,5′ 9.5 Hz, H-4′), 5.82 (1H, pseudo t,
J2′,3′ 9.4 Hz, H-2′), 5.10 (1H, d,
J1′,2′ 10.0 Hz, H-1′), 4.69 (1H, dd,
J6a′,6b′ 12.3 Hz, H-6
a′), 4.53 (1H, dd, H-6
b′), 4.35 (1H, ddd,
J5′,6a′ 2.6,
J5′,6b′ 5.1 Hz, H-5′).
13C NMR (125 MHz, CDCl
3) δ (ppm) 170.8 (C-5), 166.3, 165.9, 165.4, 165.2 (4 × CO), 161.2 (C-3), 134.9–125.6 (Ar), 97.8 (C-4), 76.9 (C-5′), 74.3 (C-3′), 73.5 (C-1′), 71.3 (C-2′), 69.6 (C-4′), 63.3 (C-6′). C
43H
33NO
10 (723.21). NMR spectra are identical with those reported [
16].
![Ijms 26 08167 i049 Ijms 26 08167 i049]()
3-(2′,3′,4′,6′-Tetra-O-benzoyl-β-d-glucopyranosyl)-5-(pyridin-2-yl)isoxazole (7d)
Prepared from oxime 1a (0.10 g, 0.16 mmol) and 2-ethynylpyridine 5d (2 equiv., 32.4 µL, 0.03 g, 0.32 mmol) according to the General Procedure I. Purified by column chromatography (1:3 EtOAc–hexane) to yield 46 mg (39%) of 7d as a pale orange amorphous product. Rf: 0.16 (1:2 EtOAc–hexane); [α]D−57 (c 0.22, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 8.67 (1H, d, J 4.8 Hz, Ar), 8.08–7.71 (10H, m, Ar), 7.59–7.22 (13H, m, Ar), 7.12 (1H, s, H-4), 6.05 (1H, pseudo t, J3′,4′ 9.5 Hz, H-3′), 5.84 (1H, pseudo t, J4′,5′ 9.4 Hz, H-4′), 5.82 (1H, pseudo t, J2′,3′ 9.6 Hz, H-2′), 5.12 (1H, d, J1′,2′ 10.0 Hz, H-1′), 4.67 (1H, dd, J6a′,6b′ 12.3 Hz, H-6a′), 4.53 (1H, dd, H-6b′), 4.35 (1H, ddd, J5′,6a′ 2.5, J5′,6b′ 5.1 Hz, H-5′). 13C NMR (125 MHz, CDCl3) δ (ppm) 170.0 (C-5), 166.3, 166.0, 165.4, 165.1 (4 × CO), 161.6 (C-3), 150.3–120.7 (Ar), 100.8 (C-4), 76.9 (C-5′), 74.3 (C-3′), 73.5 (C-1′), 71.3 (C-2′), 69.6 (C-4′), 63.4 (C-6′). HR-ESI-MS positive mode (m/z): calcd. for C42H32N2O10 (724.21) [M + Na]+ = 747.1949, found: [M + Na]+ = 747.1946.
![Ijms 26 08167 i050 Ijms 26 08167 i050]()
3-(2′,3′,4′,6′-Tetra-O-benzoyl-β-d-glucopyranosyl)-5-(4-ethynylphenyl)isoxazole (7e)
Prepared from oxime 1a (0.10 g, 0.16 mmol) and 1,4-diethynylbenzene 5e (2 equiv., 0.04 g, 0.32 mmol) according to the General Procedure I. Purified by column chromatography (1:3 EtOAc–hexane) to yield 29 mg (24%) of 7e as a yellow amorphous product. Rf: 0.36 (1:2 EtOAc–hexane); [α]D−96 (c 0.13, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 8.08–7.78 (8H, m, Ar), 7.68 (1H, d, J 8.3 Hz, Ar), 7.60–7.24 (14H, m, Ar), 6.73 (1H, s, H-4), 6.04 (1H, pseudo t, J3′,4′ 9.7 Hz, H-3′), 5.83 (1H, pseudo t, J4′,5′ 9.6 Hz, H-4′), 5.80 (1H, pseudo t, J2′,3′ 9.7 Hz, H-2′), 5.09 (1H, d, J1′,2′ 10.0 Hz, H-1′), 4.69 (1H, dd, J6a′,6b′ 12.3 Hz, H-6a′), 4.52 (1H, dd, H-6b′), 4.34 (1H, ddd, J5′,6a′ 2.8, J5′,6b′ 5.1 Hz, H-5′), 3.19 (1H, s, CCH). 13C NMR (125 MHz, CDCl3) δ (ppm) 169.8 (C-5), 166.3, 165.9, 165.4, 165.2 (4 × CO), 161.3 (C-3), 135.5–124.0 (Ar), 98.5 (C-4), 83.0 (CCH), 79.5 (CCH), 77.0 (C-5′), 74.2 (C-3′), 73.4 (C-1′), 71.2 (C-2′), 69.6 (C-4′), 63.3 (C-6′). HR-ESI-MS positive mode (m/z): calcd. for C42H32N2O10 (747.21) [M + Na]+ = 770.1997, found: [M + Na]+ = 770.1998.
![Ijms 26 08167 i051 Ijms 26 08167 i051]()
[3-(2′,3′,4′,6′-Tetra-O-benzoyl-β-d-glucopyranosyl)isoxazol-5-yl]methanol (7f)
Prepared from oxime 1a (0.10 g, 0.16 mmol) and prop-2-yn-1-ol 5f (2 equiv., 18.5 µL, 0.02 g, 0.32 mmol) according to the General Procedure I. Purified by column chromatography (1:2 EtOAc–hexane) to yield 73 mg (67%) of 7f as a white amorphous product. Rf: 0.18 (1:2 EtOAc–hexane); [α]D−3 (c 0.34, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 8.10–7.77 (8H, m, Ar), 7.59–7.22 (12H, m, Ar), 6.45 (1H, s, H-4), 6.03 (1H, pseudo t, J3′,4′ 9.5 Hz, H-3′), 5.81 (1H, pseudo t, J4′,5′ 9.8 Hz, H-4′), 5.74 (1H, pseudo t, J2′,3′ 9.6 Hz, H-2′), 5.05 (1H, d, J1′,2′ 10.0 Hz, H-1′), 4.67 (2H, s, CH2OH), 4.66 (1H, dd, J6a′,6b′ 12.3 Hz, H-6a′), 4.50 (1H, dd, H-6b′), 4.32 (1H, ddd, J5′,6a′ 2.7, J5′,6b′ 5.1 Hz, H-5′), 2.57 (1H, bs, CH2OH). 13C NMR (125 MHz, CDCl3) δ (ppm) 172.4 (C-5), 166.3, 165.9, 165.4, 165.3 (4 × CO), 160.6 (C-3), 133.9–128.0 (Ar), 100.3 (C-4), 76.9 (C-5′), 74.2 (C-3′), 73.3 (C-1′), 71.3 (C-2′), 69.5 (C-4′), 63.2 (C-6′), 56.6 (CH2OH). HR-ESI-MS positive mode (m/z): calcd. for C38H31NO11 (677.19) [M + Na]+ = 700.1789, found: [M + Na]+ = 700.1789.
![Ijms 26 08167 i052 Ijms 26 08167 i052]()
[3-(2′,3′,4′,6′-Tetra-O-benzoyl-β-d-glucopyranosyl)isoxazol-5-yl]methyl acetate (7g)
Prepared from oxime 1a (0.10 g, 0.16 mmol) and prop-2-yn-1-yl acetate 5g (2 equiv., 31.8 µL, 0.03 g, 0.32 mmol) according to the General Procedure I. Purified by column chromatography (1:3 EtOAc–hexane) to yield 54 mg (47%) of 7g as a white amorphous product. Rf: 0.18 (1:2 EtOAc–hexane); [α]D−11 (c 0.20, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 8.10–7.74 (8H, m, Ar), 7.60–7.13 (12H, m, Ar), 6.51 (1H, s, H-4), 6.02 (1H, pseudo t, J3′,4′ 9.5 Hz, H-3′), 5.80 (1H, pseudo t, J4′,5′ 9.8 Hz, H-4′), 5.73 (1H, pseudo t, J2′,3′ 9.6 Hz, H-2′), 5.12 (2H, s, CH2OAc), 5.05 (1H, d, J1′,2′ 10.0 Hz, H-1′), 4.66 (1H, dd, J6a′,6b′ 12.3 Hz, H-6a′), 4.50 (1H, dd, H-6b′), 4.32 (1H, ddd, J5′,6a′ 2.8, J5′,6b′ 5.1 Hz, H-5′), 2.08 (3H, s, CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 170.2 (CH2OCOCH3), 167.6 (C-5), 166.3, 165.9, 165.3, 165.1 (4 × CO), 160.8 (C-3), 134.0–128.0 (Ar), 102.4 (C-4), 76.9 (C-5′), 74.2 (C-3′), 73.3 (C-1′), 71.2 (C-2′), 69.5 (C-4′), 63.2 (C-6′), 56.4 (CH2OAc), 20.7 (CH3). HR-ESI-MS positive mode (m/z): calcd. for C40H33NO12 (719.20) [M + Na]+ = 742.1895, found: [M + Na]+ = 742.1892.
![Ijms 26 08167 i053 Ijms 26 08167 i053]()
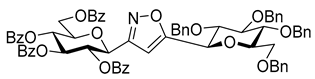
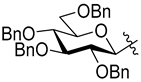
3-(2′,3′,4′,6′-Tetra-O-benzoyl-β-d-glucopyranosyl)-5-(2″,3″,4″,6″-tetra-O-benzyl-β-d-glucopyranosyl)isoxazole (7h)
Prepared from oxime
1a (0.05 g, 0.08 mmol) and 2-
C-(2′,3′,4′,6′-tetra-
O-benzyl-β-
d-glucopyranosyl)ethyne (3,7-anhydro-4,5,6,8-tetra-
O-benzyl-1,2-dideoxy-
d-
glycero-
d-
gulo-oct-1-ynitol)
5h [
20] (2 equiv., 0.09 g, 0.16 mmol) according to the General Procedure I. Purified by column chromatography (from 1:4 to 1:2 EtOAc–hexane) to yield 15 mg (16%) of
7h as a white amorphous product. R
f: 0.35 (1:2 EtOAc–hexane); [α]
D−5 (
c 0.10, CH
2Cl
2).
1H NMR (500 MHz, CDCl
3) δ (ppm) 8.09–7.69 (8H, m, Ar), 7.61–6.99 (32H, m, Ar), 6.57 (1H, s, H-4), 6.02 (1H, pseudo t,
J3′,4′ 9.6 Hz, H-3′), 5.79 (1H, pseudo t,
J4′,5′ 9.7 Hz, H-4′), 5.73 (1H, pseudo t,
J2′,3′ 9.7 Hz, H-2′), 5.07 (1H, d,
J1′,2′ 10.0 Hz, H-1′), 4.87–4.77 (3H, m, CH
2), 4.65 (1H, dd,
J6a′,6b′ 12.3 Hz, H-6
a′), 4.61–4.47 (3H, m, CH
2), 4.51 (1H, dd, H-6
b′), 4.44 (1H, d,
J1″,2″ 8.9 Hz, H-1″), 4.32 (1H, ddd,
J5′,6a′ 2.7,
J5′,6b′ 4.6 Hz, H-5′), 4.31 (1H, d,
J 10.5 Hz, CH
2), 4.14 (1H, d,
J 10.5 Hz, CH
2), 3.79–3.60 (5H, m, H-2″, H-3″, H-4″, H-6
a″, H-6
b″), 3.54 (1H, ddd,
J4′,5′ 9.6,
J5″,6a″ 2.0,
J5″,6b″ 3.6 Hz, H-5″).
13C NMR (125 MHz, CDCl
3) δ (ppm) 170.3 (C-5), 166.3, 165.9, 165.4, 165.0 (4 × CO), 160.7 (C-3), 138.7–127.4 (Ar), 102.1 (C-4), 86.4 (C-3″), 81.2 (C-2″), 79.9 (C-5″), 77.8 (C-4″), 76.7 (C-5′), 75.7, 75.3, 75.0 (3×CH
2), 74.3 (C-3′), 73.7 (C-1″, CH
2), 73.5 (C-1′), 71.3 (C-2′), 69.6 (C-4′), 68.9 (C-6″), 63.4 (C-6′). HR-ESI-MS positive mode (
m/
z): calcd. for C
71H
63NO
15 (1169.42) [M + Na]
+ = 1192.4090, found: [M + Na]
+ = 1192.4093.
![Ijms 26 08167 i054 Ijms 26 08167 i054]()
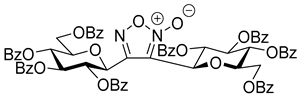
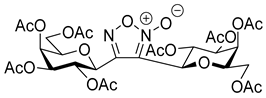
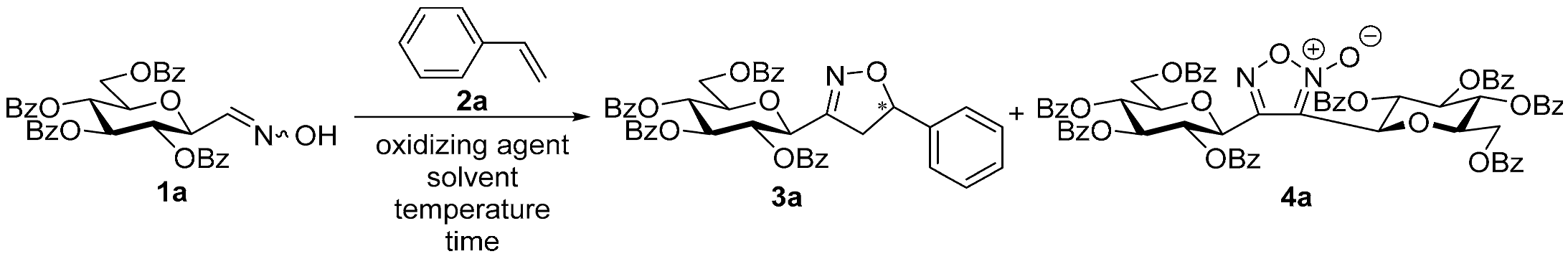
3,4-Di(2,3,4,6-Tetra-O-benzoyl-β-d-glucopyranosyl)-1,2,5-oxadiazole-2-oxide (4a)
Prepared from the previous reaction mixture from oxime
1a (0.05 g, 0.08 mmol) and 2-
C-(2′,3′,4′,6′-tetra-
O-benzyl-β-
d-glucopyranosyl)ethyne (3,7-anhydro-4,5,6,8-tetra-
O-benzyl-1,2-dideoxy-
d-
glycero-
d-
gulo-oct-1-ynitol)
5h [
20] (2 equiv., 0.09 g, 0.16 mmol) according to the General Procedure I. Purified by column chromatography (from 1:4 to 1:2 EtOAc–hexane) to yield 15 mg (31%) of
4a as a white amorphous product. R
f: 0.29 (1:2 EtOAc–hexane); [α]
D−18 (
c 0.14, CH
2Cl
2).
1H NMR (500 MHz, CDCl
3) δ (ppm) 8.12–7.89 (8H, m, Ar), 7.87–7.70 (8H, m, Ar), 7.59–7.09 (24H, m, Ar), 6.18–6.09 (3H, m, H-2′, H-3′, H-3″), 6.07 (1H, pseudo t,
J2″,3″ 9.5 Hz, H-2″), 5.79 (1H, pseudo t,
J3′,4′ 9.7,
J4′,5′ 10.2 Hz, H-4′), 5.77 (1H, pseudo t,
J3″,4″ 9.7,
J4″,5″ 9.9 Hz, H-4″), 5.26 (1H, d,
J1′,2′ 10.1 Hz, H-1′), 5.17 (1H, d,
J1″,2″ 9.4 Hz, H-1″), 4.87–4.81 (2H, m, H-6
a′, H-6
a″), 4.81 (1H, dd, H-6
b′), 4.75 (1H, dd,
J6a″,6b″ 12.8 Hz, H-6
b″), 4.47 (1H, ddd,
J5′,6a′ 2.8,
J5′,6b′ 6.9 Hz, H-5′), 4.43 (1H, ddd,
J5″,6a″ 2.7,
J5″,6b″ 7.4 Hz, H-5″).
13C NMR (125 MHz, CDCl
3) δ (ppm) 166.2, 165.9, 165.8, 165.4, 165.0, 164.9 (8×CO), 153.7 (C-4), 134.0–128.1 (Ar), 112.9 (C-3), 77.6 (C-5′, C-5″), 74.4 (C-1″), 73.7 (C-3′), 73.5 (C-3″), 72.0 (C-1′), 71.2 (C-2′), 71.0 (C-2″), 70.0 (C-4′), 69.9 (C-4″), 63.8 (C-6′, C-6″). HR-ESI-MS positive mode (
m/
z): calcd. for C
70H
54N
2O
20 (1242.33). [M + Na]
+ = 1265.3162, found: [M + Na]
+ = 1265.3161.
![Ijms 26 08167 i055 Ijms 26 08167 i055]()
Diethyl 3-(2′,3′,4′,6′-Tetra-O-benzoyl-β-d-glucopyranosyl)isoxazole-4,5-dicarboxylate (7i)
Prepared from oxime 1a (0.10 g, 0.16 mmol) and diethyl but-2-ynedioate 5i (2 equiv., 51.3 µL, 0.05 g, 0.32 mmol) according to the General Procedure I. Purified by column chromatography (1:2 EtOAc–hexane) to yield 64 mg (50%) of 7i as a pale yellow amorphous product. Rf: 0.42 (1:1 EtOAc–hexane); [α]D−3 (c 0.58, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 8.11–7.74 (8H, m, Ar), 7.58–7.16 (12H, m, Ar), 6.22 (1H, pseudo t, J2′,3′ 9.7 Hz, H-2′), 6.02 (1H, pseudo t, J3′,4′ 9.5 Hz, H-3′), 5.82 (1H, pseudo t, J4′,5′ 9.7 Hz, H-4′), 5.27 (1H, d, J1′,2′ 10.0 Hz, H-1′), 4.62 (1H, dd, J6a′,6b′ 12.4 Hz, H-6a′), 4.45 (1H, dd, H-6b′), 4.41 (2H, q, J 7.2 Hz, CH2CH3), 4.30 (1H, ddd, J5′,6a′ 2.5, J5′,6b′ 5.1 Hz, H-5′), 4.29–4.18 (2H, m, CH2CH3), 1.37, 1.30 (6H, 2 × t, J 7.1 Hz, 2 × CH2CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 166.3, 166.0, 165.3, 164.9 (4 × CO), 160.8 (C-5), 160.2 (COOEt), 159.0 (C-3), 156.1 (COOEt), 133.7–128.4 (Ar), 115.1 (C-4), 77.0 (C-5′), 74.3 (C-3′), 72.9 (C-1′), 70.5 (C-2′), 69.2 (C-4′), 63.1 (C-6′), 63.1, 62.3 (2 × CH2CH3), 14.1 (2 × CH2CH3). HR-ESI-MS positive mode (m/z): calcd. for C47H37NO14 (791.22) [M + H]+ = 814.2106, found: [M + H]+ = 814.2098.
![Ijms 26 08167 i056 Ijms 26 08167 i056]()
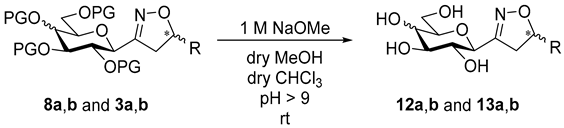
3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)-5-phenylisoxazolines (8a)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and ethenylbenzene 2a (2 equiv., 61.1 µL, 0.06 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (1:1 EtOAc–hexane) to yield 106 mg (83%) of 8a (diastereomeric ratio: 1.1:1) as a yellow amorphous product. Rf: 0.44 (1:1 EtOAc–hexane). 8a-I 1H NMR (400 MHz, CDCl3) δ (ppm) 7.43–7.25 (5H, m, Ar), 5.59 (1H, dd, H-5), 5.49 (1H, dd, J4′,5′ 0.5 Hz, H-4′), 5.27 (1H, pseudo t, J2′,3′ 10.7 Hz, H-2′), 5.17 (1H, dd, J3′,4′ 3.3 Hz, H-3′), 4.39 (1H, d, J1′,2′ 9.6 Hz, H-1′), 4.17–4.06 (2H, m, H-6a′, H-6b′), 4.05–3.96 (1H, m, H-5′), 3.57 (1H, dd, J4a,4b 17.2, J4a,5 11.0 Hz, H-4a), 3.08 (1H, dd, J4b,5 9.3 Hz, H-4b), 2.15, 2.08, 2.03, 2.01 (12H, 4s, 4 × CH3). 13C NMR (90 MHz, CDCl3) δ (ppm) 170.4, 170.1, 170.0 (4 × CO), 155.4 (C-3), 140.6–125.6 (Ar), 82.7 (C-5), 74.6 (C-5′), 74.4 (C-1′), 71.2 (C-3′), 67.4 (C-4′), 66.4 (C-2′), 61.5 (C-6′), 40.9 (C-4), 20.77, 20.68, 20.64 (2) (4 × CH3). 8a-II 1H NMR (400 MHz, CDCl3) δ (ppm) 7.43–7.25 (5H, m, Ar), 5.57 (1H, dd, H-5), 5.48 (1H, dd, J4′,5′ 0.5 Hz, H-4′), 5.22 (1H, pseudo t, J2′,3′ 10.5 Hz, H-2′), 5.14 (1H, dd, J3′,4′ 3.3 Hz, H-3′), 4.41 (1H, d, J1′,2′ 9.3 Hz, H-1′), 4.17–4.06 (2H, m, H-6a′, H-6b′), 4.05–3.96 (1H, m, H-5′), 3.49 (1H, dd, J4a,4b 17.3, J4a,5 11.2 Hz, H-4a), 3.16 (1H, dd, J4b,5 10.2 Hz, H-4b), 2.13, 2.06, 2.01, 1.98 (12H, 4s, 4 × CH3). 13C NMR (90 MHz, CDCl3) δ (ppm) 170.4, 170.1, 170.0, 169.9 (4 × CO), 155.4 (C-3), 140.6–125.6 (Ar), 83.0 (C-5), 74.6 (C-5′), 74.5 (C-1′), 71.4 (C-3′), 67.5 (C-4′), 66.0 (C-2′), 61.7 (C-6′), 40.9 (C-4), 20.73, 20.64 (2), 20.59 (4 × CH3). HR-ESI-MS positive mode (m/z): calcd. for C23H27NO10 (477.16) [M + H]+ = 478.1708, found: [M + H]+ = 478.1710.
![Ijms 26 08167 i057 Ijms 26 08167 i057]()
3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)-5-(naphth-2-yl)isoxazolines (8b)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and 2-ethenylnaphthalene 2b (2 equiv., 0.08 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (1:1 EtOAc–hexane) to yield 106 mg (83%) of 8b (diastereomeric ratio: 1.1:1) as a pale yellow amorphous product. Rf: 0.38 (1:1 EtOAc–hexane). 8b-I 1H NMR (400 MHz, CDCl3) δ (ppm) 7.92–7.76 (4H, m, Ar), 7.54–7.38 (3H, m, Ar), 5.74 (1H, dd, H-5), 5.49 (1H, dd, J4′,5′ 0.5 Hz, H-4′), 5.30 (1H, pseudo t, J2′,3′ 10.5 Hz, H-2′), 5.17 (1H, dd, J3′,4′ 3.5 Hz, H-3′), 4.41 (1H, d, J1′,2′ 9.4 Hz, H-1′), 4.17–4.05 (2H, m, H-6a′, H-6b′), 4.04–3.96 (1H, m, H-5′), 3.64 (1H, dd, J4a,4b 17.2, J4a,5 10.9 Hz, H-4a), 3.16 (1H, dd, J4b,5 9.2 Hz, H-4b), 2.15, 2.10, 2.01, 1.97 (12H, 4s, 4 × CH3). 13C NMR (90 MHz, CDCl3) δ (ppm) 170.5, 170.2, 170.0 (4 × CO), 155.6 (C-3), 137.8–123.3 (Ar), 82.9 (C-5), 74.6 (C-5′), 74.5 (C-1′), 71.3 (C-3′), 67.5 (C-4′), 66.5 (C-2′), 61.5 (C-6′), 41.0 (C-4), 20.83, 20.70, 20.67, 20.64 (4 × CH3). 8b-II 1H NMR (400 MHz, CDCl3) δ (ppm) 7.92–7.76 (4H, m, Ar), 7.54–7.38 (3H, m, Ar), 5.77 (1H, dd, H-5), 5.48 (1H, dd, J4′,5′ 0.5 Hz, H-4′), 5.24 (1H, pseudo t, J2′,3′ 10.3 Hz, H-2′), 5.14 (1H, dd, J3′,4′ 3.4 Hz, H-3′), 4.44 (1H, d, J1′,2′ 8.9 Hz, H-1′), 4.17–4.05 (2H, m, H-6a′, H-6b′), 4.04–3.96 (1H, m, H-5′), 3.56 (1H, dd, J4a,4b 17.4, J4a,5 11.2 Hz, H-4a), 3.26 (1H, dd, J4b,5 9.8 Hz, H-4b), 2.12, 2.06, 2.01, 1.97 (12H, 4s, 4 × CH3). 13C NMR (90 MHz, CDCl3) δ (ppm) 170.5, 170.4, 170.0, 169.9 (4 × CO), 155.5 (C-3), 137.8–123.3 (Ar), 83.1 (C-5), 74.7 (C-5′), 74.6 (C-1′), 71.5 (C-3′), 67.5 (C-4′), 66.1 (C-2′), 61.7 (C-6′), 40.9 (C-4), 20.77, 20.70, 20.67, 20.61 (4 × CH3). HR-ESI-MS positive mode (m/z): calcd. for C27H29NO10 (527.18) [M + H]+ = 528.1684, found: [M + H]+ = 528.1682.
![Ijms 26 08167 i058 Ijms 26 08167 i058]()
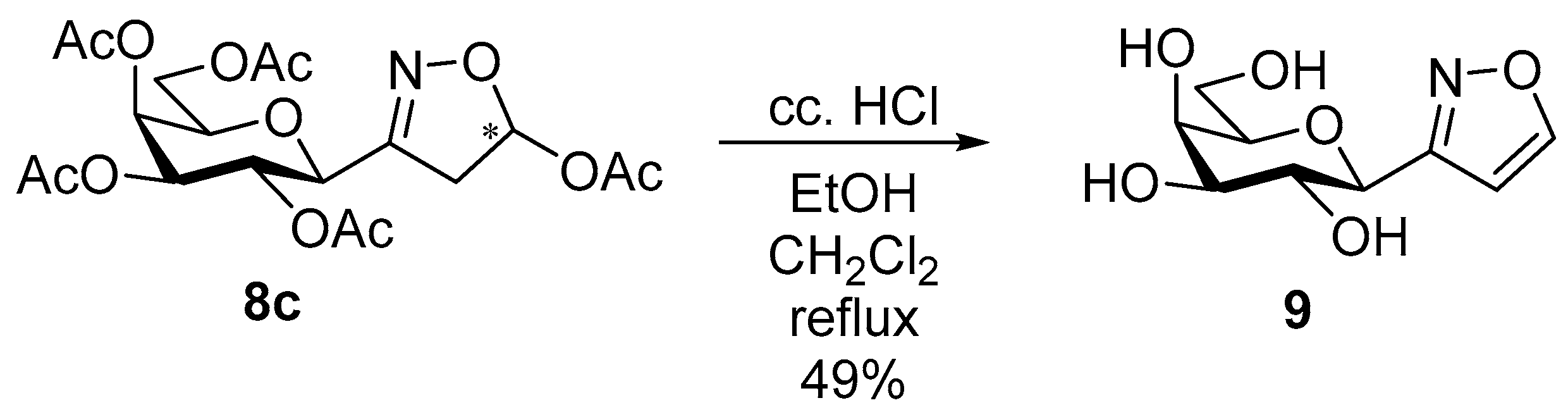
[3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)isoxazol-5-yl] acetates (8c)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and ethenyl acetate 2c (2 equiv., 49.1 µL, 0.05 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (from 1:2 to 2:1 EtOAc–hexane) to yield 65 mg (53%) of 8c (diastereomeric ratio: 1.6:1) as a yellow amorphous product. Rf: 0.42 (2:1 EtOAc–hexane). 8c-I 1H NMR (500 MHz, CDCl3) δ (ppm) 6.70 (1H, dd, H-5), 5.52–5.48 (1H, m, H-4′), 5.20–5.12 (2H, m, H-2′, H-3′), 4.45–4.39 (1H, m, H-1′), 4.19–4.08 (2H, m, H-6a′, H-6b′), 4.04 (1H, ddd, J5′,6a′ 6.1, J5′,6b′ 6.3 Hz, H-5′), 3.43 (1H, dd, J4a,4b 18.3, J4a,5 7.1 Hz, H-4a), 3.09 (1H, dd, J4b,5 < 1.0 Hz, H-4b), 2.08 (3H, s, CH2OCOCH3), 2.17, 2.06, 2.02, 2.00 (12H, 4s, 4 × CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 170.5, 170.3, 170.1, 170.0 (4 × CO), 169.7 (OCOCH3), 156.6 (C-3), 95.8 (C-5), 74.7 (C-5′), 74.0 (C-1′), 71.1 (C-3′), 67.3 (C-4′), 66.2 (C-2′), 61.4 (C-6′), 39.5 (C-4), 21.07, 20.96, 20.74, 20.69, 20.62 (5 × CH3). 8c-II 1H NMR (500 MHz, CDCl3) δ (ppm) 6.65 (1H, dd, H-5), 5.52–5.48 (1H, m, H-4′), 5.32 (1H, pseudo t, J2′,3′ 10.1 Hz, H-2′), 5.15 (1H, dd, J3′,4′ 3.3 Hz, H-3′), 4.46 (1H, d, J1′,2′ 9.8 Hz, H-1′), 4.19–4.08 (2H, m, H-6a′, H-6b′), 4.03 (1H, ddd, J5′,6a′ 6.2, J5′,6b′ 6.0 Hz, H-5′), 3.32 (1H, dd, J4a,4b 18.5, J4a,5 6.9 Hz, H-4a), 3.13 (1H, dd, J4b,5 < 1.0 Hz, H-4b), 2.08 (3H, s, CH2OCOCH3), 2.18, 2.05, 2.04, 2.00 (12H, 4s, 4 × CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 170.5, 170.1, 170.0, 169.5 (4 × CO), 169.8 (OCOCH3), 155.9 (C-3), 95.9 (C-5), 74.9 (C-5′), 73.9 (C-1′), 71.3 (C-3′), 67.5 (C-4′), 66.1 (C-2′), 61.6 (C-6′), 39.8 (C-4), 21.07, 20.96, 20.74, 20.69, 20.62 (5×CH3). HR-ESI-MS positive mode (m/z): calcd. for C19H25NO12 (459.40) [M + Na]+ = 482.1269, found: [M + Na]+ = 482.1269.
![Ijms 26 08167 i059 Ijms 26 08167 i059]()
3-(2′,3′,4′,6′-Tetra-O-acetyl-β-d-galactopyranosyl)-4,5-diphenylisoxazoline (8e)
Prepared from oxime 1b (0.10 g, 0.27 mmol) and (E)-1,2-diphenylethene 2e (2 equiv., 0.10 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (from 1:3 to 1:1 EtOAc–hexane) to yield 33 mg (23%) of 8e as a colourless amorphous product. Rf: 0.60 (2:1 EtOAc–hexane); [α]D+76 (c 0.59, CH2Cl2). 1H NMR (500 MHz, CDCl3) δ (ppm) 7.39–7.29 (6H, m, Ar), 7.29–7.23 (4H, m, Ar), 5.54 (1H, d, H-5), 5.42 (1H, pseudo t, J2′,3′ 10.0 Hz, H-2′), 5.29 (1H, dd, J4′,5′ 0.4 Hz, H-4′), 4.97 (1H, dd, J3′,4′ 3.3 Hz, H-3′), 4.46 (1H, d, J4,5 7.9 Hz, H-4), 4.27 (1H, d, J1′,2′ 10.0 Hz, H-1′), 3.76–3.67 (3H, m, H-5′, H-6a′, H-6b′), 2.05, 2.01, 1.98, 1.95 (12H, 4s, 4 × CH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 170.4, 170.2, 169.6 (4 × CO), 155.9 (C-3), 139.9–125.5 (Ar), 91.7 (C-5), 74.2 (C-5′), 73.6 (C-1′), 72.0 (C-3′), 67.4 (C-4′), 66.1 (C-2′), 62.4 (C-4), 61.4 (C-6′), 20.86, 20.78, 20.73, 20.72 (4 × CH3). HR-ESI-MS positive mode (m/z): calcd. for C29H31NO10 (553.56) [M + Na]+ = 576.1840, found: [M + Na]+ = 576.1841.
![Ijms 26 08167 i060 Ijms 26 08167 i060]()
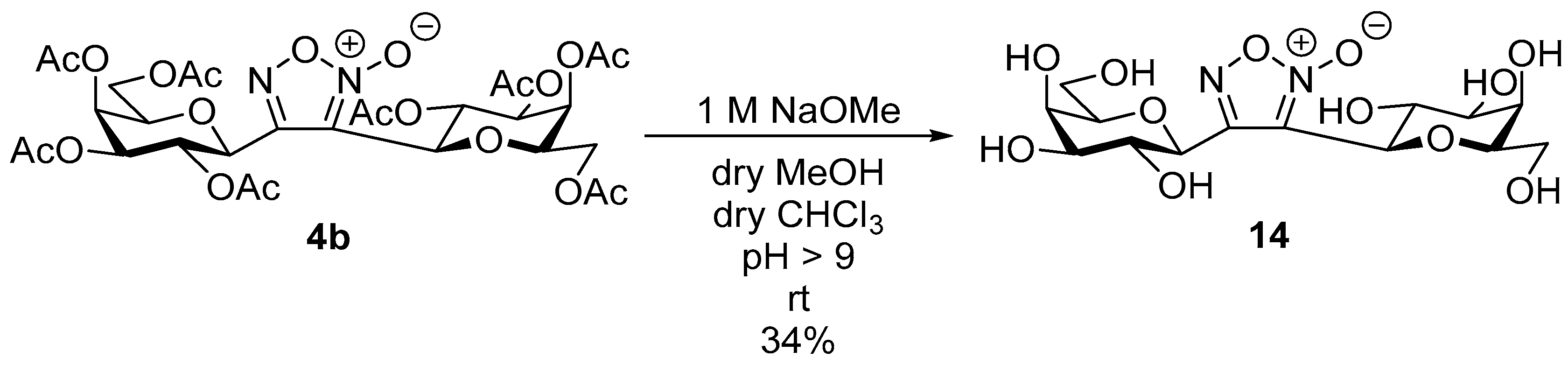
3,4-Di(2,3,4,6-Tetra-O-acetyl-β-d-galactopyranosyl)-1,2,5-oxadiazole-2-oxide (4b)
Prepared from the previous reaction mixture from oxime
1b (0.10 g, 0.27 mmol) and (
E)-1,2-diphenylethene
2e (2 equiv., 0.10 g, 0.53 mmol) according to the General Procedure I. Purified by column chromatography (from 1:3 to 1:1 EtOAc–hexane) to yield 76 mg (76%) of
4b as a yellow amorphous product. R
f: 0.47 (2:1 EtOAc–hexane).
1H NMR (500 MHz, CDCl
3) δ (ppm) 5.65 (1H, pseudo t,
J2′,3′ 10.0 Hz, H-2′), 5.58 (1H, pseudo t,
J2″,3″ 10.0 Hz, H-2″), 5.55 (1H, dd,
J4′,5′ 0.7 Hz, H-4′), 5.51 (1H, dd,
J4″,5″ 0.7 Hz, H-4″), 5.19 (1H, dd,
J3′,4′ 3.3 Hz, H-3′), 5.17 (1H, dd,
J3″,4″ 3.3 Hz, H-3″), 4.84 (1H, d,
J1′,2′ 10.1 Hz, H-1′), 4.82 (1H, d,
J1″,2″ 10.1 Hz, H-1″), 4.31–4.01 (6H, m, H-5′, H-5″, H-6
a′, H-6
a″, H-6
b′, H-6
b″), 2.24, 2.06, 2.05, 2.02, 2.00, 1.99 (24H, 8s, 8 × CH
3).
13C NMR (125 MHz, CDCl
3) δ (ppm) 170.4, 170.2, 170.1, 170.0, 169.5, 169.2 (8 × CO), 153.3 (C-4), 111.7 (C-3), 75.4 (C-5″), 75.2 (C-5′), 72.9 (C-1″), 71.7 (C-3′, C-3″), 70.8 (C-1′), 67.3 (C-4′, C-4″), 66.9 (C-2″), 65.9 (C-2′), 61.4 (C-6′, C-6″), 20.77, 20.74, 20.70 (2), 20.61 (2), 20.59, 20.49 (8 × CH
3). NMR spectra are identical with those reported [
14].
![Ijms 26 08167 i061 Ijms 26 08167 i061]()
3.1.4. General Procedure II for Removal of O-Acyl Protecting Groups (10, 11, 12, 13)
5-Substituted 3-(2′,3′,4′,6′-tetra-O-acyl-β-d-glycopyranosyl)isoxazole or -isoxazolines 3, 6, 7, 8 (100 mg) were dissolved in dry MeOH (5 mL) and dry chloroform (3 mL) then a solution of NaOMe (1 M in MeOH) was added to the solution in a catalytic amount. The reaction mixture was stirred at room temperature. When the reaction was complete (TLC, 7:3 CHCl3–MeOH) (1–3 h), the solution was neutralized with a cation exchange resin Amberlyst 15 (H+ form). The resin was filtered off with suction and the filtrate was evaporated under reduced pressure. The crude product was purified by silica gel column chromatography with eluents indicated for the particular compounds to give 5-substituted 3-(β-d-glycopyranosyl)isoxazoles 10 and 11 and -isoxazolines 12 and 13.
![Ijms 26 08167 i062 Ijms 26 08167 i062]()
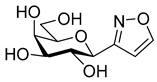
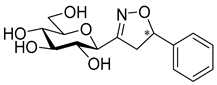
3-(β-d-Galactopyranosyl)-5-phenylisoxazole (10a)
Prepared from isoxazole 6a (0.06 g, 0.12 mmol) according to the General Procedure II. Purified by column chromatography (4:1 CHCl3–MeOH) to yield 18 mg (49%) of 10a as a pale yellow amorphous product. Rf: 0.56 (7:3 CHCl3–MeOH); [α]D+18 (c 0.12, MeOH). 1H NMR (700 MHz, CD3OD) δ (ppm) 7.83 (2H, d, J 7.2 Hz, Ar), 7.53–7.45 (3H, m, Ar), 6.96 (1H, s, H-4), 4.35 (1H, d, J1′,2′ 9.7 Hz, H-1′), 3.99 (1H, dd, J4′,5′ 0.6 Hz, H-4′), 3.87 (1H, pseudo t, J2′,3′ 9.4 Hz, H-2′), 3.80 (1H, dd, J6a′,6b′ 11.3 Hz, H-6a′), 3.73 (1H, dd, H-6b′), 3.70 (1H, ddd, J5′,6b′ 4.9, J5′,6a′ 6.9 Hz, H-5′), 3.62 (1H, dd, J3′,4′ 3.3 Hz, H-3′). 13C NMR (90 MHz, CD3OD) δ (ppm) 171.2 (C-5), 165.1 (C-3), 131.5–126.7 (Ar), 99.7 (C-4), 81.1 (C-5′), 76.5 (C-1′), 76.1 (C-3′), 71.5 (C-2′), 70.8 (C-4′), 62.9 (C-6′). HR-ESI-MS positive mode (m/z): calcd. for C15H17NO6 (307.11) [M + H]+ = 308.1129, found: [M + H]+ = 308.1129.
![Ijms 26 08167 i063 Ijms 26 08167 i063]()
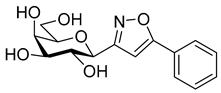
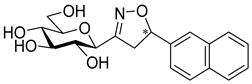
3-(β-d-Galactopyranosyl)-5-(naphth-2-yl)isoxazole (10b)
Prepared from isoxazole 6b (0.06 g, 0.12 mmol) according to the General Procedure II. Purified by column chromatography (6:1 CHCl3–MeOH) to yield 19 mg (43%) of 10b as a white amorphous product. Rf: 0.43 (1:1 CHCl3–MeOH); [α]D+32 (c 0.10, MeOH). 1H NMR (700 MHz, CD3OD) δ (ppm) 8.38 (1H, bs, Ar), 8.01–7.96 (1H, m, Ar), 7.99 (1H, d, J 8.4 Hz, Ar), 7.93–7.88 (1H, m, Ar), 7.90 (1H, dd, J 1.7, 8.5 Hz, Ar), 7.60–7.54 (2H, m, Ar), 7.08 (1H, s, H-4), 4.38 (1H, d, J1′,2′ 9.7 Hz, H-1′), 4.00 (1H, dd, J4′,5′ 0.4 Hz, H-4′), 3.90 (1H, pseudo t, J2′,3′ 9.4 Hz, H-2′), 3.81 (1H, dd, J6a′,6b′ 11.3 Hz, H-6a′), 3.74 (1H, dd, H-6b′), 3.72 (1H, ddd, J5′,6a′ 4.9, J5′,6b′ 6.8 Hz, H-5′), 3.63 (1H, dd, J3′,4′ 3.3 Hz, H-3′). 13C NMR (175 MHz, CD3OD) δ (ppm) 171.3 (C-5), 165.2 (C-3), 135.5–123.8 (Ar), 100.2 (C-4), 81.2 (C-5′), 76.6 (C-1′), 76.1 (C-3′), 71.6 (C-2′), 70.9 (C-4′), 62.9 (C-6′). HR-ESI-MS positive mode (m/z): calcd. for C19H19NO6 (357.12) [M + Na]+ = 380.1105, found: [M + Na]+ = 380.1106.
![Ijms 26 08167 i064 Ijms 26 08167 i064]()
3-(β-d-Galactopyranosyl)-5-(naphth-1-yl)isoxazole (10c)
Prepared from isoxazole 6c (0.05 g, 0.10 mmol) according to the General Procedure II. Purified by column chromatography (3.5:1 CHCl3–MeOH) to yield 26 mg (75%) of 10c as a white amorphous product. Rf: 0.46 (7:3 CHCl3–MeOH); [α]D+21 (c 0.10, MeOH). 1H NMR (500 MHz, CD3OD) δ (ppm) 8.31 (1H, d, J 8.1 Hz, Ar), 8.02 (1H, d, J 8.2 Hz, Ar), 7.97 (1H, dd, J 1.9, 7.5 Hz, Ar), 7.83 (1H, dd, J 1.1, 7.2 Hz, Ar), 7.63–7.54 (3H, m, Ar), 7.02 (1H, s, H-4), 4.44 (1H, d, J1′,2′ 9.7 Hz, H-1′), 4.01 (1H, dd, J4′,5′ 0.5 Hz, H-4′), 3.95 (1H, pseudo t, J2′,3′ 9.4 Hz, H-2′), 3.83 (1H, dd, J5′,6a′ 8.3, J6a′,6b′ 12.6 Hz, H-6a′), 3.79–3.71 (2H, m, H-5′, H-6b′), 3.66 (1H, dd, J3′,4′ 3.3 Hz, H-3′). 13C NMR (125 MHz, CD3OD) δ (ppm) 171.2 (C-5), 164.8 (C-3), 135.5–125.7 (Ar), 103.8 (C-4), 81.2 (C-5′), 76.6 (C-1′), 76.1 (C-3′), 71.6 (C-2′), 70.9 (C-4′), 62.9 (C-6′). HR-ESI-MS positive mode (m/z): calcd. for C19H19NO6 (357.12) [M + Na]+ = 380.1105, found: [M + Na]+ = 380.1104.
![Ijms 26 08167 i065 Ijms 26 08167 i065]()
3-(β-d-Galactopyranosyl)-5-(pyridin-2-yl)isoxazole (10d)
Prepared from isoxazole 6d (0.11 g, 0.23 mmol) according to the General Procedure II. Purified by column chromatography (7:3 CHCl3–MeOH) to yield 45 mg (63%) of 10d as a white amorphous product. Rf: 0.36 (4:1 CHCl3–MeOH); [α]D+28 (c 0.20, MeOH). 1H NMR (700 MHz, CD3OD) δ (ppm) 8.68–8.65 (1H, m, Ar), 8.00–7.95 (2H, m, Ar), 7.49 (1H, ddd, J 1.6, 4.9, 6.7 Hz, Ar), 7.19 (1H, s, H-4), 4.39 (1H, d, J1′,2′ 9.7 Hz, H-1′), 3.99 (1H, dd, J4′,5′ 0.6 Hz, H-4′), 3.89 (1H, pseudo t, J2′,3′ 9.4 Hz, H-2′), 3.80 (1H, dd, J6a′,6b′ 11.1 Hz, H-6a′), 3.72 (1H, dd, H-6b′), 3.70 (1H, ddd, J5′,6b′ 4.9, J5′,6a′ 6.6 Hz, H-5′), 3.62 (1H, dd, J3′,4′ 3.3 Hz, H-3′). 13C NMR (100 MHz, CD3OD) δ (ppm) 170.0 (C-5), 165.2 (C-3), 151.6–121.5 (Ar), 102.6 (d, J 15.7 Hz, C-4), 81.2 (C-5′), 76.5 (d, J 10.1 Hz, C-1′), 76.1 (C-3′), 71.5 (C-2′), 70.8 (d, J 6.2 Hz, C-4′), 62.9 (C-6′). HR-ESI-MS positive mode (m/z): calcd. for C14H16N2O6 (308.10) [M + H]+ = 309.1081, found: [M + H]+ = 309.1081.
![Ijms 26 08167 i066 Ijms 26 08167 i066]()
3-(β-d-Glucopyranosyl)-5-phenylisoxazole (11a)
Prepared from isoxazole
7a (0.06 g, 0.08 mmol) according to the General Procedure II. Purified by column chromatography (4:1 CHCl
3–MeOH) to yield 19 mg (80%) of
11a as a pale orange amorphous product. R
f: 0.44 (4:1 CHCl
3–MeOH).
1H NMR (500 MHz, CD
3OD) δ (ppm) 7.83 (2H, d,
J 7.7 Hz, Ar), 7.50 (2H, d,
J 7.9 Hz, Ar), 7.54–7.42 (1H, m, Ar), 6.91 (1H, s, H-4), 4.42 (1H, d,
J1′,2′ 8.9 Hz, H-1′), 3.90 (1H, dd,
J6a′,6b′ 12.1 Hz, H-6
a′), 3.72 (1H, dd, H-6
b′), 3.54 (1H, pseudo t,
J2′,3′ 9.0 Hz, H-2′), 3.51 (1H, pseudo t,
J3′,4′ 8.7 Hz, H-3′), 3.45 (1H, pseudo t,
J4′,5′ 8.7 Hz, H-4′), 3.45 (1H, ddd,
J5′,6a′ < 1.0,
J5′,6b′ 2.9 Hz, H-5′).
13C NMR (125 MHz, CD
3OD) δ (ppm) 171.2 (C-5), 165.0 (C-3), 131.6–126.5 (Ar), 99.8 (C-4), 82.4 (C-5′), 79.5 (C-3′), 76.1 (C-1′), 74.7 (C-2′), 71.4 (C-4′), 62.9 (C-6′). C
15H
17NO
6 (307.11). NMR spectra are identical with those reported [
16].
![Ijms 26 08167 i067 Ijms 26 08167 i067]()
3-(β-d-Glucopyranosyl)-5-(pyridin-2-yl)isoxazole (11d)
Prepared from isoxazole 7d (0.03 g, 0.04 mmol) according to the General Procedure II. Purified by column chromatography (4:1 CHCl3–MeOH) to yield 9 mg (67%) of 11d as a colourless amorphous product. Rf: 0.32 (4:1 CHCl3–MeOH); [α]D+21 (c 0.15, MeOH). 1H NMR (500 MHz, CD3OD) δ (ppm) 8.66 (1H, dt, J 4.9, 1.2 Hz, Ar), 8.02–7.95 (2H, m, Ar), 7.49 (1H, ddd, J 6.9, 4.9, 2.3 Hz, Ar), 7.13 (1H, s, H-4), 4.46 (1H, d, J1′,2′ 9.3 Hz, H-1′), 3.90 (1H, dd, J6a′,6b′ 12.2 Hz, H-6a′), 3.72 (1H, strongly coupled, H-6b′), 3.54 (1H, pseudo t, J2′,3′ 8.9 Hz, H-2′), 3.51 (1H, pseudo t, J3′,4′ 8.9 Hz, H-3′), 3.45 (1H, pseudo t, J4′,5′ 9.5 Hz, H-4′), 3.45 (1H, ddd, J5′,6a′ 1.4, J5′,6b′ 5.1 Hz, H-5′). 13C NMR (125 MHz, CD3OD) δ (ppm) 170.0 (C-5), 165.2 (C-3), 151.3–122.2 (Ar), 102.5 (C-4), 82.5 (C-5′), 79.5 (C-3′), 76.0 (C-1′), 74.7 (C-2′), 71.5 (C-4′), 62.9 (C-6′). HR-ESI-MS positive mode (m/z): calcd. for C14H16N2O6 (308.10) [M + Na]+ = 331.0901, found: [M + Na]+ = 331.0900.
![Ijms 26 08167 i068 Ijms 26 08167 i068]()
3-(β-d-Glucopyranosylisoxazol-5-yl)methanol (11f)
Prepared from isoxazole 7f (0.05 g, 0.08 mmol) according to the General Procedure II. Purified by column chromatography (4:1 CHCl3–MeOH) to yield 21 mg (99%) of 11f as a colourless amorphous product. Rf: 0.07 (4:1 CHCl3–MeOH); [α]D+17 (c 0.26, MeOH). 1H NMR (500 MHz, CD3OD) δ (ppm) 6.45 (1H, s, H-4), 4.66 (2H, s, CH2OH), 4.36 (1H, d, J1′,2′ 9.3 Hz, H-1′), 3.87 (1H, dd, J6a′,6b′ 12.2 Hz, H-6a′), 3.69 (1H, strongly coupled, H-6b′), 3.50–3.43 (2H, m, H-2′, H-3′), 3.41 (1H, pseudo t, J4′,5′ 9.4 Hz, H-4′), 3.40 (1H, ddd, J5′,6a′ 1.2, J5′,6b′ 5.2 Hz, H-5′). 13C NMR (125 MHz, CD3OD) δ (ppm) 173.8 (C-5), 164.2 (C-3), 101.7 (C-4), 82.4 (C-5′), 79.5 (C-3′), 76.0 (C-1′), 74.7 (C-2′), 71.4 (C-4′), 62.8 (C-6′), 56.4 (CH2OH). HR-ESI-MS positive mode (m/z): calcd. for C10H15NO7 (261.08) [M + Na]+ = 284.0741, found: [M + Na]+ = 284.0738.
![Ijms 26 08167 i069 Ijms 26 08167 i069]()
3-(β-d-Galactopyranosyl)-5-phenylisoxazolines (12a)
Prepared from isoxazolines 8a (0.09 g, 0.18 mmol) according to the General Procedure II. Purified by column chromatography (4:1 CHCl3–MeOH) to yield 18 mg (76%) of 12a (diastereomeric ratio: 1.1:1) as a white amorphous product. Rf: 0.52 (7:3 CHCl3–MeOH). 12a-I 1H NMR (400 MHz, CD3OD) δ (ppm) 7.41–7.27 (5H, m, Ar), 5.61 (1H, dd, J4a,5 11.0, J4b,5 8.4 Hz, H-5), 4.08 (1H, dd, J1′,2′ 9.7 Hz, H-1′), 3.92 (1H, dd, J3′,4′ 3.3, J4′,5′ 0.6 Hz, H-4′), 3.81–3.51 (6H, m, H-2′, H-3′, H-5′, H-6a′, H-6b′, H-4a), 3.15 (1H, dd, J4a,4b 17.6 Hz, H-4b). 13C NMR (90 MHz, CD3OD) δ (ppm) 158.8 (C-3), 142.6–126.7 (Ar), 83.4 (C-5), 80.9 (C-5′), 77.1 (C-1′), 75.9 (C-3′), 70.8 (C-4′), 69.8 (C-2′), 62.8 (C-6′), 42.5 (C-4). 12a-II 1H NMR (400 MHz, CD3OD) δ (ppm) 7.41–7.27 (5H, m, Ar), 5.57 (1H, dd, J4a,5 10.9, J4b,5 9.3 Hz, H-5), 4.09 (1H, dd, J1′,2′ 9.7 Hz, H-1′), 3.92 (1H, dd, J3′,4′ 3.3, J4′,5′ 0.6 Hz, H-4′), 3.81–3.51 (6H, m, H-2′, H-3′, H-5′, H-6a′, H-6b′, H-4a), 3.09 (1H, dd, J4a,4b 17.5 Hz, H-4b). 13C NMR (90 MHz, CD3OD) δ (ppm) 159.1 (C-3), 142.6–126.7 (Ar), 83.7 (C-5), 80.9 (C-5′), 77.1 (C-1′), 76.1 (C-3′), 70.8 (C-4′), 69.8 (C-2′), 62.8 (C-6′), 42.6 (C-4). HR-ESI-MS positive mode (m/z): calcd. for C15H19NO6 (309.12) [M + H]+ = 310.1285, found: [M + H]+ = 310.1286.
![Ijms 26 08167 i070 Ijms 26 08167 i070]()
3-(β-d-Galactopyranosyl)-5-(naphth-2-yl)isoxazolines (12b)
Prepared from isoxazolines 8b (0.06 g, 0.10 mmol) according to the General Procedure II. Purified by column chromatography (4:1 CHCl3–MeOH) to yield 26 mg (70%) of 12b (diastereomeric ratio: 1.1:1) as a brownish amorphous product. Rf: 0.59 (7:3 CHCl3–MeOH). 12b-I 1H NMR (400 MHz, CD3OD) δ (ppm) 7.91–7.80 (4H, m, Ar), 7.56–7.42 (3H, m, Ar), 5.78 (1H, dd, J4a,5 10.9, J4b,5 8.3 Hz, H-5), 4.12 (1H, dd, J1′,2′ 9.7 Hz, H-1′), 3.92 (1H, dd, J3′,4′ 3.2, J4′,5′ 0.9 Hz, H-4′), 3.81 (1H, dd, J2′,3′ 9.5 Hz, H-2′), 3.80–3.59 (4H, m, H-5′, H-6a′, H-6b′, H-4a), 3.56 (1H, dd, H-3′), 3.26 (1H, dd, J4a,4b 17.6 Hz, H-4b). 13C NMR (90 MHz, CD3OD) δ (ppm) 158.9 (C-3), 139.9–124.7 (Ar), 83.5 (C-5), 80.9 (C-5′), 77.2 (C-1′), 76.1 (C-3′), 70.8 (C-4′), 69.8 (C-2′), 62.8 (C-6′), 42.5 (C-4). 12b-II 1H NMR (400 MHz, CD3OD) δ (ppm) 7.91–7.80 (4H, m, Ar), 7.56–7.42 (3H, m, Ar), 5.73 (1H, dd, J4a,5 10.8, J4b,5 9.1 Hz, H-5), 4.13 (1H, dd, J1′,2′ 9.7 Hz, H-1′), 3.92 (1H, dd, J3′,4′ 3.2, J4′,5′ 0.9 Hz, H-4′), 3.80–3.59 (5H, m, H-2′, H-5′, H-6a′, H-6b′, H-4a), 3.55 (1H, dd, H-3′), 3.19 (1H, dd, J4a,4b 17.4 Hz, H-4b). 13C NMR (90 MHz, CD3OD) δ (ppm) 159.1 (C-3), 139.9–124.7 (Ar), 83.8 (C-5), 80.9 (C-5′), 77.1 (C-1′), 75.9 (C-3′), 70.8 (C-4′), 69.8 (C-2′), 62.8 (C-6′), 42.6 (C-4). HR-ESI-MS positive mode (m/z): calcd. for C19H21NO6 (359.14) [M + H]+ = 360.1442, found: [M + H]+ = 360.1444.
![Ijms 26 08167 i071 Ijms 26 08167 i071]()
3-(β-d-Glucopyranosyl)-5-phenylisoxazolines (13a)
Prepared from isoxazolines 3a (0.07 g, 0.10 mmol) according to the General Procedure II. Purified by column chromatography (4:1 CHCl3–MeOH) to yield 24 mg (80%) of 13a (diastereomeric ratio: 1.3:1) as a pale orange amorphous product. Rf: 0.44 (4:1 CHCl3–MeOH). 13a-I 1H NMR (500 MHz, CD3OD) δ (ppm) 7.42–7.26 (5H, m, Ar), 5.61 (1H, dd, J4a,5 11.1, J4b,5 8.6 Hz, H-5), 4.14 (1H, dd, J1′,2′ 9.2 Hz, H-1′), 3.85 (1H, dd, J5′,6a′ 1.3, J6a′,6b′ 11.7 Hz, H-6a′), 3.65 (1H, dd, J5′,6b′ 4.9 Hz, H-6b′), 3.54 (1H, dd, J4a,4b 17.5, J4a,5 11.4 Hz, H-4a), 3.47–3.27 (3H, m, H-3′, H-4′, H-5′), 3.41 (1H, pseudo t, J2′,3′ 8.7 Hz, H-2′), 3.15 (1H, dd, J4b,5 8.5 Hz, H-4b). 13C NMR (125 MHz, CD3OD) δ (ppm) 158.8 (C-3), 142.5–126.9 (Ar), 83.4 (C-5), 82.3 (C-5′), 79.3 (C-3′), 76.7 (C-1′), 73.0 (C-2′), 71.4 (C-4′), 62.7 (C-6′), 42.6 (C-4). 13a-II 1H NMR (500 MHz, CD3OD) δ (ppm) 7.42–7.26 (5H, m, Ar), 5.57 (1H, dd, J4a,5 10.8, J4b,5 10.0 Hz, H-5), 4.13 (1H, dd, J1′,2′ 9.3 Hz, H-1′), 3.85 (1H, dd, J5′,6a′ 1.3, J6a′,6b′ 11.7 Hz, H-6a′), 3.67 (1H, dd, J5′,6b′ 5.4 Hz, H-6b′), 3.60 (1H, dd, J4a,4b 17.4, J4a,5 11.1 Hz, H-4a), 3.47–3.27 (3H, m, H-3′, H-4′, H-5′), 3.41 (1H, pseudo t, J2′,3′ 8.7 Hz, H-2′), 3.06 (1H, dd, J4b,5 9.2 Hz, H-4b). 13C NMR (125 MHz, CD3OD) δ (ppm) 159.0 (C-3), 142.5–126.9 (Ar), 83.7 (C-5), 82.3 (C-5′), 79.5 (C-3′), 76.6 (C-1′), 73.1 (C-2′), 71.4 (C-4′), 62.8 (C-6′), 42.7 (C-4). HR-ESI-MS positive mode (m/z): calcd. for C15H19NO6 (309.12) [M + Na]+ = 332.1105, found: [M + Na]+ = 332.1104.
![Ijms 26 08167 i072 Ijms 26 08167 i072]()
3-(β-d-Glucopyranosyl)-5-(naphth-2-yl)isoxazolines (13b)
Prepared from isoxazolines 3b (0.08 g, 0.10 mmol) according to the General Procedure II. Purified by column chromatography (4:1 CHCl3–MeOH) to yield 16 mg (46%) of 13b (diastereomeric ratio: 1:1) as a white amorphous product. Rf: 0.40 (4:1 CHCl3–MeOH). 13b-I 1H NMR (500 MHz, CD3OD) δ (ppm) 8.11–7.77 (4H, m, Ar), 7.65–7.39 (3H, m, Ar), 5.79 (1H, dd, J4a,5 11.2, J4b,5 8.7 Hz, H-5), 4.16 (1H, dd, J1′,2′ 9.1 Hz, H-1′), 3.84 (1H, dd, J5′,6a′ 1.6, J6a′,6b′ 12.0 Hz, H-6a′), 3.71–3.54 (2H, m, H-4a, H-6b′), 3.50–3.41 (2H, m, H-2′, H-3′), 3.41–3.29 (2H, m, H-4′, H-5′), 3.25 (1H, dd, J4a,4b 17.5, J4b,5 8.4 Hz, H-4b). 13C NMR (125 MHz, CD3OD) δ 158.9 (C-3), 139.8–124.7 (Ar), 83.6 (C-5), 82.3 (C-5′), 79.4 (C-3′), 76.7 (C-1′), 73.1 (C-2′), 71.4 (C-4′), 62.7 (C-6′), 42.6 (C-4). 13b-II 1H NMR (500 MHz, CD3OD) δ (ppm) 8.11–7.77 (4H, m, Ar), 7.65–7.39 (3H, m, Ar), 5.75 (1H, dd, J4a,5 11.3, J4b,5 9.7 Hz, H-5), 4.17 (1H, dd, J1′,2′ 9.3 Hz, H-1′), 3.88 (1H, dd, J5′,6a′ 2.0, J6a′,6b′ 12.1 Hz, H-6a′), 3.71–3.54 (2H, m, H-4a, H-6b′), 3.50–3.41 (2H, m, H-2′, H-3′), 3.41–3.29 (2H, m, H-4′, H-5′), 3.17 (1H, dd, J4a,4b 17.4, J4b,5 9.2 Hz, H-4b). 13C NMR (125 MHz, CD3OD) δ (ppm) 159.1 (C-3), 139.8–124.7 (Ar), 83.9 (C-5), 82.3 (C-5′), 79.5 (C-3′), 76.7 (C-1′), 73.1 (C-2′), 71.4 (C-4′), 62.8 (C-6′), 42.6 (C-4). HR-ESI-MS positive mode (m/z): calcd. for C19H21NO6 (359.14) [M + Na]+ = 382.1261, found: [M + Na]+ = 382.1258.
![Ijms 26 08167 i073 Ijms 26 08167 i073]()
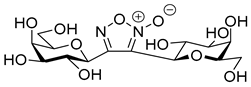
3,4-Di(β-d-Galactopyranosyl)-1,2,5-oxadiazole-2-oxide (14)
Prepared from isoxazole 4b (0.10 g, 0.13 mmol) according to the General Procedure II. Purified by column chromatography (4:1 CHCl3–MeOH) to yield 19 mg (34%) of 14 as a white amorphous product. Rf: 0.16 (1:1 CHCl3–MeOH); [α]D+82 (c 0.08, DMSO). 1H NMR (500 MHz, CD3OD) δ (ppm) 4.44 (1H, d, J1′,2′ 9.9 Hz, H-1′), 4.42 (1H, d, J1″,2″ 9.8 Hz, H-1″), 4.31 (1H, pseudo t, J2′,3′ 9.6 Hz, H-2′), 4.29 (1H, pseudo t, J2″,3″ 9.6 Hz, H-2″), 3.92 (1H, dd, J4″,5″ 0.5 Hz, H-4″), 3.89 (1H, dd, J4′,5′ 0.5 Hz, H-4′), 3.91–3.83 (2H, m, H-6a′, H-6a″), 3.75–3.66 (4H, m, H-5′, H-5″, H-6b′, H-6b″), 3.56 (1H, dd, J3″,4″ 3.3 Hz, H-3″), 3.55 (1H, dd, J3′,4′ 3.4 Hz, H-3′). 13C NMR (125 MHz, CD3OD) δ (ppm) 157.2 (C-4), 115.6 (C-3), 82.2 (C-5′), 81.8 (C-5″), 77.9 (C-1″), 76.2 (C-3″), 76.1 (C-3′), 75.3 (C-1′), 71.0 (C-4′, C-4″), 70.2 (C-2′), 69.9 (C-2″), 62.7 (C-6″), 62.6 (C-6′). HR-ESI-MS positive mode (m/z): calcd. for C14H22N2O11 (410.33) [M + H]+ = 411.1246, found: [M + H]+ = 411.1246.