Unlocking Casein Bioactivity: Lactic Acid Bacteria and Molecular Strategies for Peptide Release
Abstract
1. Introduction
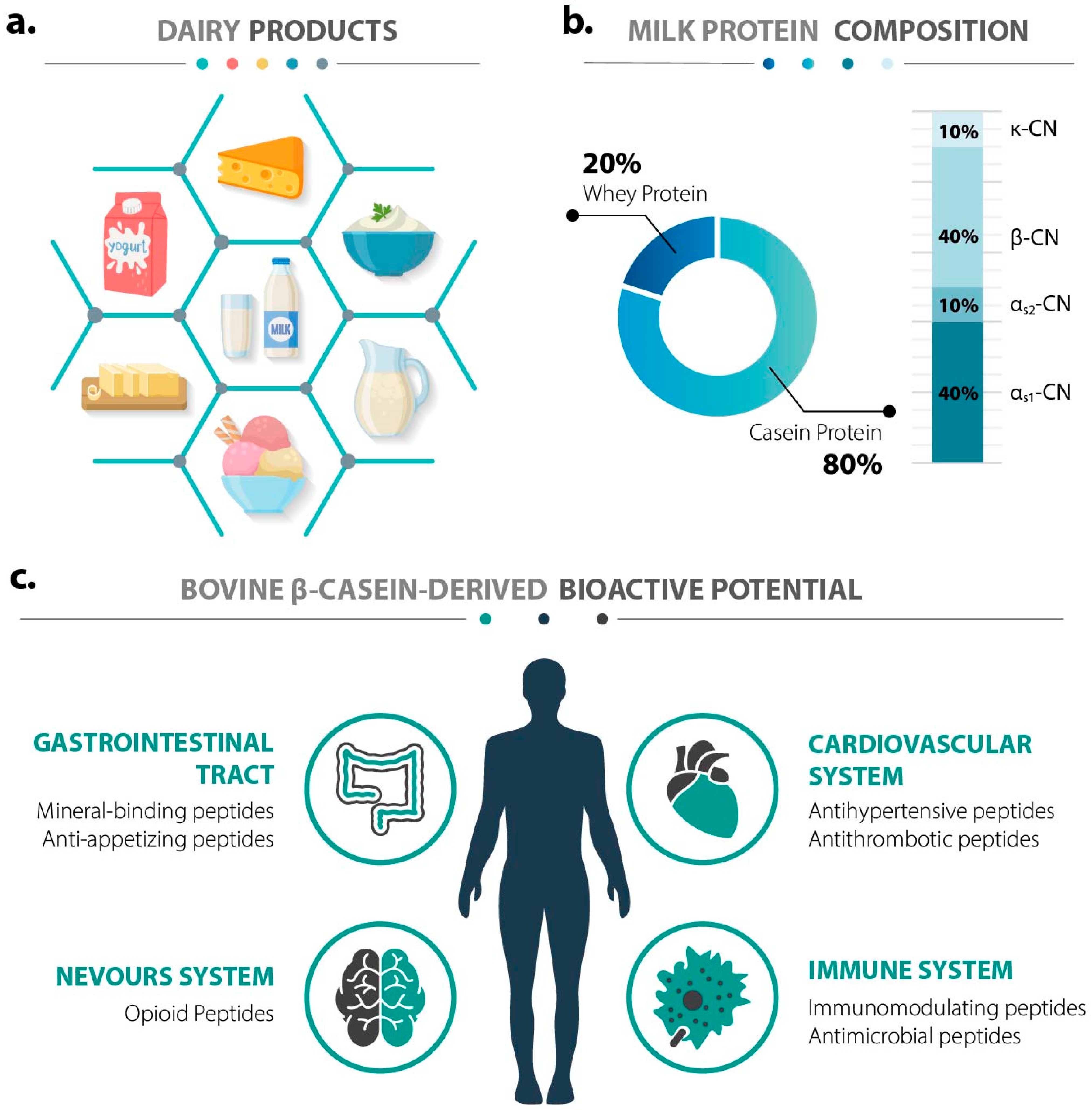
2. Functional Diversity of Bioactive Peptides from Bovine β-Casein
2.1. Caseinophosphopeptides (CPPs): Mineral-Binding and Immunomodulatory Peptides
2.2. β-Casomorphins (β-CMs): Opioid-like Peptides
2.3. DPP-IV Inhibitory Peptides: Metabolic Regulation
2.4. Antimicrobial Peptides (AMPs)
2.5. Immunomodulatory Peptides
2.6. ACE-Inhibitory Peptides: Antihypertensive Potential
2.7. Other Bioactivities
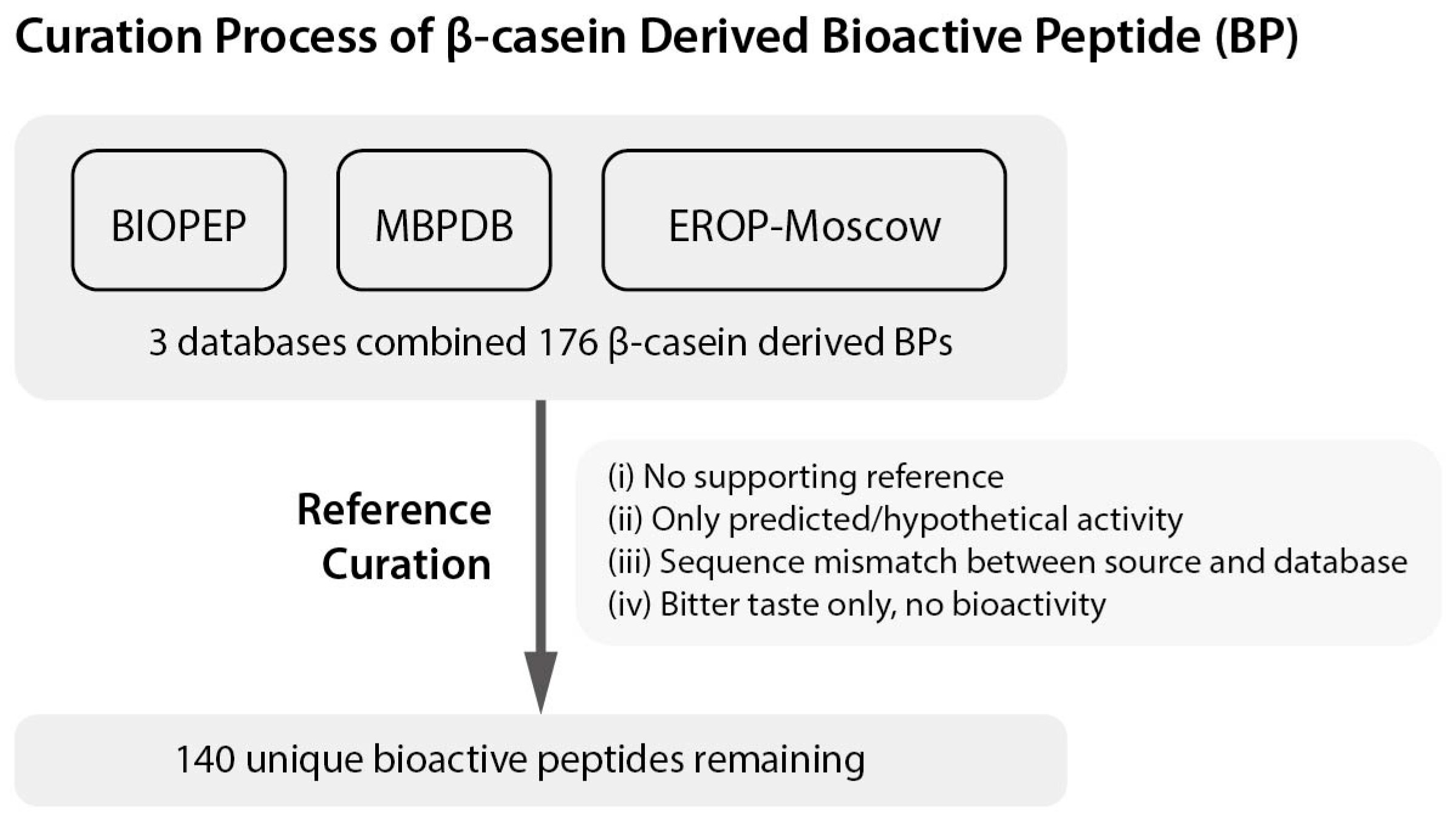
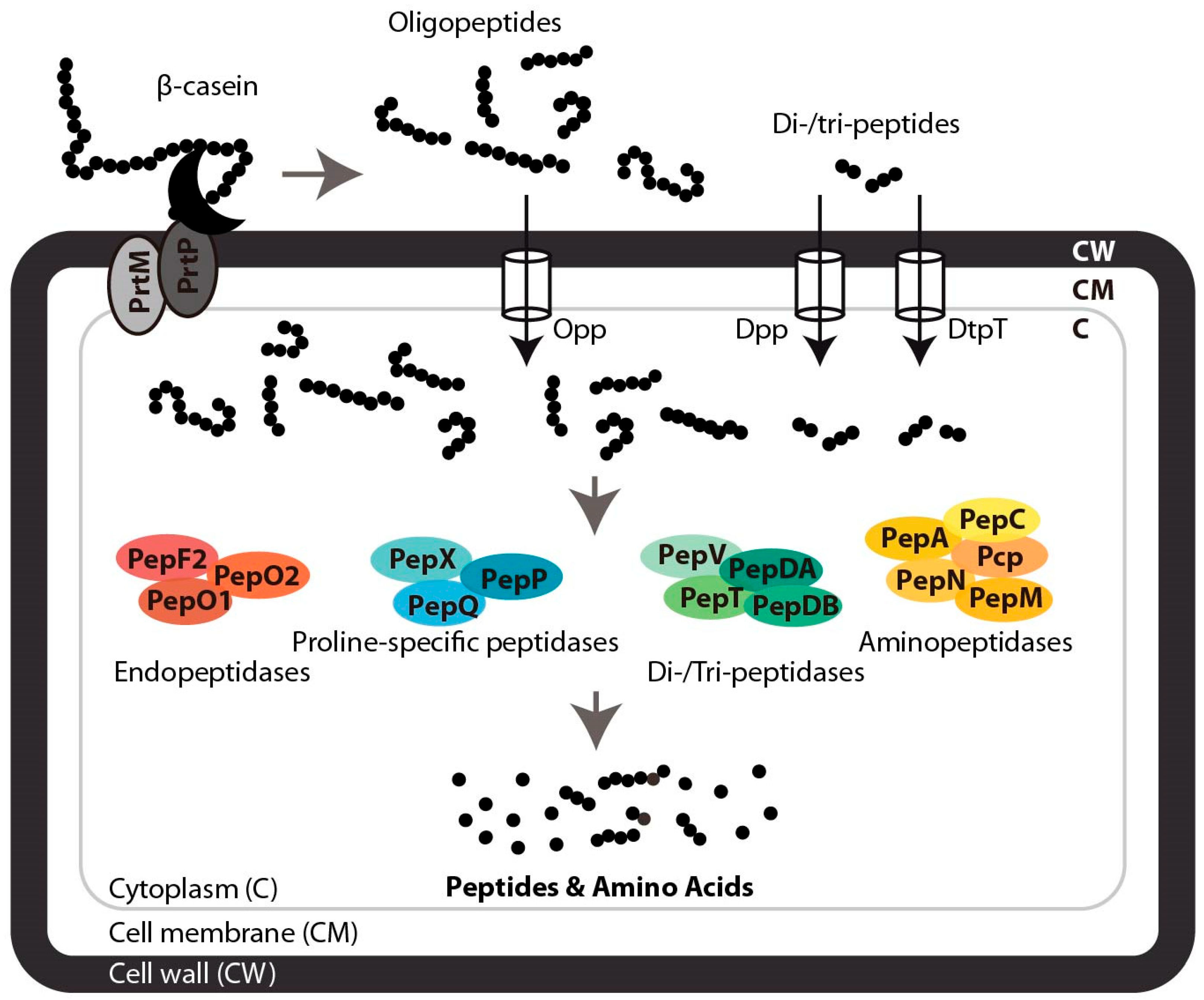
3. Proteolytic Systems of Lactic Acid Bacteria and Natural Peptide Liberation from β-Casein
3.1. Extracellular Proteolysis of β-Casein
3.2. Peptide Transport and Intracellular Degradation
3.3. Release of Functional Peptides
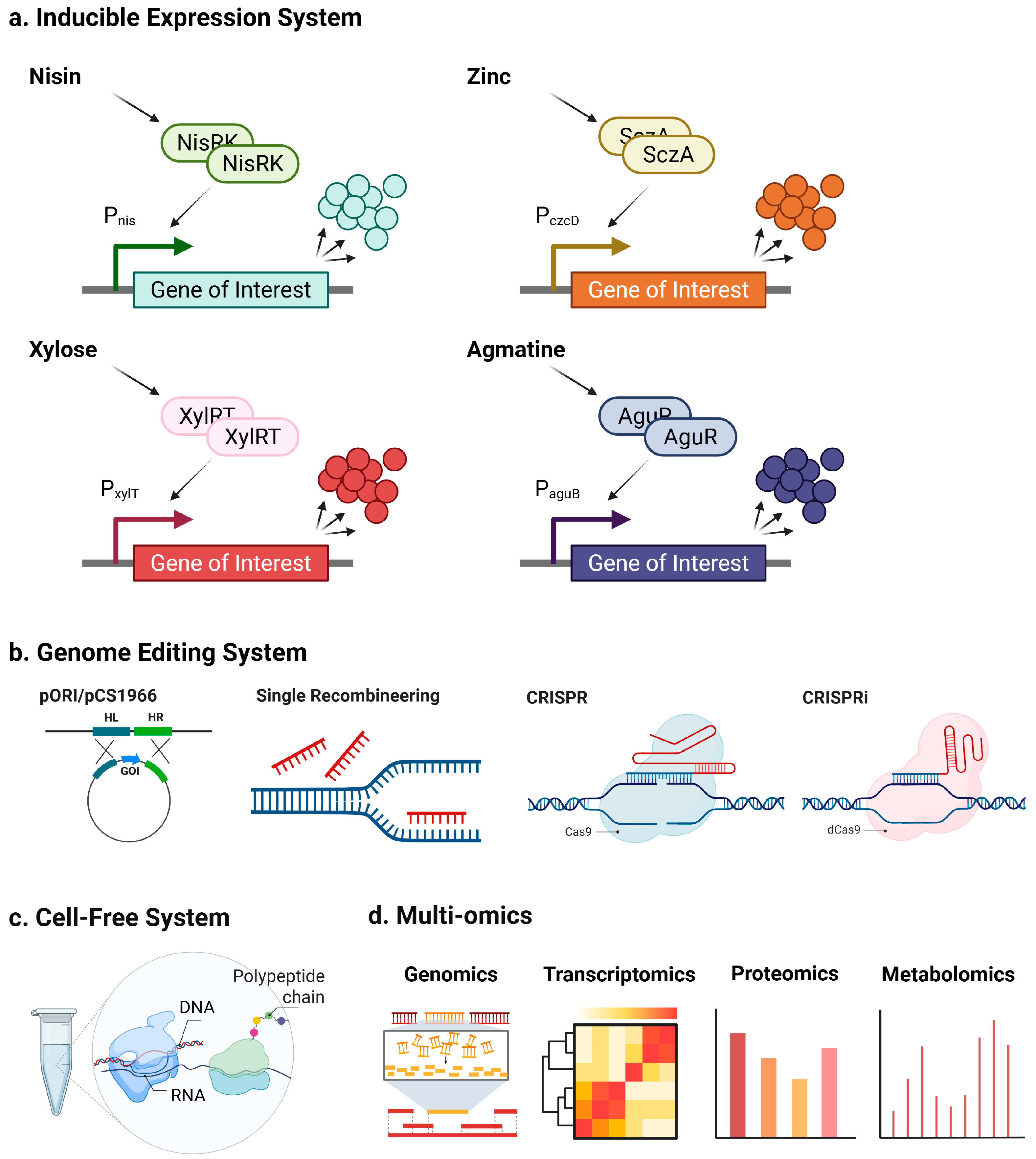
4. Molecular Strategies for Enhancing LAB-Mediated Liberation of Bioactive Peptides
4.1. Inducible Systems for Targeted Regulation of Proteolysis
4.2. Genome Editing Tools for Stable Manipulation of Proteolytic Pathways
4.3. Cell-Free Protein Synthesis for Rapid Discovery and Screening
4.4. Multi-Omics Strategies for System-Level Optimization
5. Challenges, Regulatory Considerations, and Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LAB | lactic acid bacteria |
| AA | amino acid |
| CPPs | caseinophosphopeptides |
| β-CMs | β-casomorphins |
| ACE | angiotensin-converting enzyme |
| DPP-IV | dipeptidyl peptidase-4 |
| GIP | insulinotropic polypeptide |
| GLP-1 | glucagon-like peptide-1 |
| MS | mass spectrometry |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| AMPs | antimicrobial peptides |
| GRAS | generally recognized as safe |
| FDA | U.S. Food and Drug Administration |
| NICE | NIsin-Controlled Expression |
| XIES | xylose-inducible expression system |
| CRISP/Cas | clustered regularly interspaced short palindromic repeats associated system |
| NHEJ | non-homologous end joining |
| SpCas9D10A | S. pyogenes Cas9D10A nickase |
| SSBs | single-strand breaks |
| DSBs | double-strand breaks |
| CFPS | cell-free protein synthesis |
| CFCS | cell-free culture supernatants |
| GM-LAB | genetically modified lactic acid bacteria |
| QPS | Qualified Presumption of Safety |
| GMOs | genetically modified organisms |
| AI | artificial intelligence |
References
- Turner-Ravana, N. Milk and Dairy Foods: Their Functionality in Human Health and Disease. J. Nutr. Educ. Behav. 2021, 53, 546. [Google Scholar] [CrossRef]
- OECD Publishing. OECD-FAO Agricultural Outlook 2024–2033; Organisation for Economic Co-operation and Development OECD: Paris, France, 2024; ISBN 978-92-64-59211-7. [Google Scholar]
- Saxelin, M.; Korpela, R.; Mäyrä-Mäkinen, A. Introduction: Classifying Functional Dairy Products; Woodhead Publishing Ltd.: Cambridge, UK, 2003. [Google Scholar]
- Giromini, C.; Fekete, Á.A.; Pinotti, L.; Baldi, A. Milk Proteins: Their Role in Cardiovascular Health. Milk Dairy Foods 2020, 145–172. [Google Scholar] [CrossRef]
- Eigel, W.; Butler, J.; Ernstrom, C.; Farrell, H., Jr.; Harwalkar, V.; Jenness, R.; Whitney, R.M. Nomenclature of Proteins of Cow’s Milk: Fifth Revision. J. Dairy Sci. 1984, 67, 1599–1631. [Google Scholar] [CrossRef]
- Atamer, Z.; Post, A.E.; Schubert, T.; Holder, A.; Boom, R.M.; Hinrichs, J. Bovine β-Casein: Isolation, Properties and Functionality. A Review. Int. Dairy J. 2017, 66, 115–125. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive Peptides: Production and Functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Gobbetti, M.; Minervini, F.; Rizzello, C.G. Bioactive Peptides in Dairy Products. Handb. Food Prod. Manuf. 2007, 489–517. [Google Scholar] [CrossRef]
- Kilara, A.; Panyam, D. Peptides from Milk Proteins and Their Properties. Crit. Rev. Food Sci. Nutr. 2003, 43, 607–633. [Google Scholar] [CrossRef]
- Korhonen, H. Milk-Derived Bioactive Peptides: From Science to Applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Chen, X.; Fan, R.; Wang, Y.; Munir, M.; Li, C.; Wang, C.; Hou, Z.; Zhang, G.; Liu, L.; He, J. Bovine Milk β-Casein: Structure, Properties, Isolation, and Targeted Application of Isolated Products. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13311. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, H.; Hati, S. Production and Biofunctionality of Milk-Derived Bioactive Peptides. In Microbiome-Gut-Brain Axis: Implications on Health; Sayyed, R.Z., Khan, M., Eds.; Springer Nature: Singapore, 2022; pp. 297–316. ISBN 978-981-16-1626-6. [Google Scholar]
- Auestad, N.; Layman, D.K. Dairy Bioactive Proteins and Peptides: A Narrative Review. Nutr. Rev. 2021, 79, 36–47. [Google Scholar] [CrossRef]
- Kok, J. Genetics of the Proteolytic System of Lactic Acid Bacteria. FEMS Microbiol. Rev. 1990, 7, 15–41. [Google Scholar] [CrossRef]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic Systems of Lactic Acid Bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef]
- Huang, C.; Kok, J. Editing of the Proteolytic System of Lactococcus lactis Increases Its Bioactive Potential. Appl. Environ. Microbiol. 2020, 86, e01319-20. [Google Scholar] [CrossRef]
- Caballero, B.; Finglas, P.; Toldrá, F. Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2015; ISBN 0-12-384953-5. [Google Scholar]
- FitzGerald, R. Potential Uses of Caseinophosphopeptides. Int. Dairy J. 1998, 8, 451–457. [Google Scholar] [CrossRef]
- Kawahara, T.; Katayama, D.; Otani, H. Effect of β-Casein (1-28) on Proliferative Responses and Secretory Functions of Human Immunocompetent Cell Lines. Biosci. Biotechnol. Biochem. 2004, 68, 2091–2095. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Shindo, M.; Gunshin, H.; Noguchi, T.; Naito, H. Characterization of Phosphopeptide Derived from Bovine β-Casein: An Inhibitor to Intra-Intestinal Precipitation of Calcium Phosphate. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1991, 1077, 413–415. [Google Scholar] [CrossRef]
- Tobita, K.; Kawahara, T.; Otani, H. Bovine β-Casein (1−28), a Casein Phosphopeptide, Enhances Proliferation and IL-6 Expression of Mouse CD19+ Cells via Toll-like Receptor 4. J. Agric. Food Chem. 2006, 54, 8013–8017. [Google Scholar] [CrossRef] [PubMed]
- Hata, I.; Higashiyama, S.; Otani, H. Identification of a Phosphopeptide in Bovine As1-Casein Digest as a Factor Influencing Proliferation and Immunoglobulin Production in Lymphocyte Cultures. J. Dairy Res. 1998, 65, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Contet, C.; Kieffer, B.L.; Befort, K. Mu Opioid Receptor: A Gateway to Drug Addiction. Curr. Opin. Neurobiol. 2004, 14, 370–378. [Google Scholar] [CrossRef]
- Mansour, A.; Watson, S. Anatomical Distribution of Opioid Receptors in Mammalians: An Overview. Opioids 1993, 79–105. [Google Scholar] [CrossRef]
- Brantl, V.; Teschemacher, H.; Henschen, A.; Lottspeich, F. Novel Opioid Peptides Derived from Casein (β-Casomorphins). I. Isolation from Bovine Casein Peptone. Hoppe Seyler’s Z. Physiol. Chem. 1979, 360, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, S.; Trompette, A.; Claustre, J.; Homsi, M.E.; Garzón, J.; Jourdan, G.; Scoazec, J.-Y.; Plaisancié, P. β-Casomorphin-7 Regulates the Secretion and Expression of Gastrointestinal Mucins through a μ-Opioid Pathway. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 290, G1105–G1113. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Amigo, L.; Ramos, M.; Recio, I. Release of Angiotensin Converting Enzyme-Inhibitory Peptides by Simulated Gastrointestinal Digestion of Infant Formulas. Int. Dairy J. 2004, 14, 889–898. [Google Scholar] [CrossRef]
- Yin, H.; Miao, J.; Ma, C.; Sun, G.; Zhang, Y. β-Casomorphin-7 Cause Decreasing in Oxidative Stress and Inhibiting NF-κB-iNOS-NO Signal Pathway in Pancreas of Diabetes Rats. J. Food Sci. 2012, 77, C278–C282. [Google Scholar] [CrossRef]
- Escobar-Charry, M.A.; Quintanilla-Carvajal, M.X. An Overview of Bovine Beta-Casomorphin-7 (b-BCM7) and Its Potential Impact on Microbiota Regulation and Digestive Health. J. Funct. Foods 2025, 128, 106812. [Google Scholar] [CrossRef]
- Robinson, S.R.; Greenway, F.L.; Deth, R.C.; Fayet-Moore, F. Effects of Different Cow-Milk Beta-Caseins on the Gut–Brain Axis: A Narrative Review of Preclinical, Animal, and Human Studies. Nutr. Rev. 2025, 83, e1259–e1269. [Google Scholar] [CrossRef]
- Dalziel, J.; Spencer, N.; Dunstan, K.; Lynch, A.; Haggarty, N.; Gopal, P.; Roy, N. An In Vitro Rat Model of Colonic Motility to Determine the Effect of β-Casomorphin-5 on Propagating Contractions. Food Funct. 2014, 5, 2768–2774. [Google Scholar] [CrossRef]
- Daniel, H.; Vohwinkel, M.; Rehner, G. Effect of Casein and β-Casomorphins on Gastrointestinal Motility in Rats. J. Nutr. 1990, 120, 252–257. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Murayama, K.; Jinsmaa, Y.; Yoshikawa, M.; Matsumura, E. Neurite Outgrowth-Stimulating Activities of β-Casomorphins in Neuro-2a Mouse Neuroblastoma Cells. Biosci. Biotechnol. Biochem. 2003, 67, 2541–2547. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Koseki, M.; Wakamatsu, M.; Matsumura, E. Effects of Systemic Administration of β-Casomorphin-5 on Learning and Memory in Mice. Eur. J. Pharmacol. 2006, 530, 81–87. [Google Scholar] [CrossRef]
- Tulipano, G. Role of Bioactive Peptide Sequences in the Potential Impact of Dairy Protein Intake on Metabolic Health. Int. J. Mol. Sci. 2020, 21, 8881. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Prospects for the Management of Type 2 Diabetes Using Food Protein-Derived Peptides with Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Activity. Curr. Opin. Food Sci. 2016, 8, 19–24. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the Antibiotic Resistance Crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Birkemo, G.; O’sullivan, O.; Ross, R.; Hill, C. Antimicrobial Activity of Two Peptides Casecidin 15 and 17, Found Naturally in Bovine Colostrum. J. Appl. Microbiol. 2009, 106, 233–240. [Google Scholar] [CrossRef]
- Sedaghati, M.; Ezzatpanah, H.; Boojar, M.M.A.; Ebrahimi, M.T.; Kobarfard, F. Isolation and Identification of Some Antibacterial Peptides in the Plasmin-Digest of β-Casein. LWT-Food Sci. Technol. 2016, 68, 217–225. [Google Scholar] [CrossRef]
- Gertsch, J.; Viveros-Paredes, J.M.; Taylor, P. Plant Immunostimulants—Scientific Paradigm or Myth? J. Ethnopharmacol. 2011, 136, 385–391. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and Anticancer Protein Hydrolysates (Peptides) from Food Proteins: A Review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Díaz, A.; González-Córdova, A.F.; Hernández-Mendoza, A.; Reyes-Díaz, R.; Vallejo-Cordoba, B. Immunomodulation by Hydrolysates and Peptides Derived from Milk Proteins. Int. J. Dairy Technol. 2018, 71, 1–9. [Google Scholar] [CrossRef]
- Liu, T.; Feng, J.; Han, H.; Huang, J.; Zhang, L.; Hettinga, K.; Zhou, P. Anti-Inflammatory Effects of Dietary β-Casein Peptides and Its Peptide QEPVL in a DSS-Induced Inflammatory Bowel Disease Mouse Model. Food Biosci. 2023, 56, 103375. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Khan, Z.; Giani, J.F.; Cao, D.-Y.; Bernstein, E.A.; Shen, X.Z. Angiotensin-Converting Enzyme in Innate and Adaptive Immunity. Nat. Rev. Nephrol. 2018, 14, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Sheih, I.-C.; Fang, T.J.; Wu, T.-K. Isolation and Characterisation of a Novel Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptide from the Algae Protein Waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Messerli, F.H.; Williams, B.; Ritz, E. Essential Hypertension. Lancet 2007, 370, 591–603. [Google Scholar] [CrossRef]
- Irvin, J.D.; Viau, J.M. Safety Profiles of the Angiotensin Converting Enzyme Inhibitors Captopril and Enalapril. Am. J. Med. 1986, 81, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Okubo, A.; Yamazaki, S.; Takano, T. Purification and Characterization of Angiotensin I-Converting Enzyme Inhibitors from Sour Milk. J. Dairy Sci. 1995, 78, 777–783. [Google Scholar] [CrossRef]
- Rai, A.K.; Sanjukta, S.; Jeyaram, K. Production of Angiotensin I Converting Enzyme Inhibitory (ACE-I) Peptides during Milk Fermentation and Their Role in Reducing Hypertension. Crit. Rev. Food Sci. Nutr. 2017, 57, 2789–2800. [Google Scholar] [CrossRef]
- Tonouchi, H.; Suzuki, M.; Uchida, M.; Oda, M. Antihypertensive Effect of an Angiotensin Converting Enzyme Inhibitory Peptide from Enzyme Modified Cheese. J. Dairy Res. 2008, 75, 284–290. [Google Scholar] [CrossRef]
- Farvin, K.S.; Baron, C.P.; Nielsen, N.S.; Otte, J.; Jacobsen, C. Antioxidant Activity of Yoghurt Peptides: Part 2–Characterisation of Peptide Fractions. Food Chem. 2010, 123, 1090–1097. [Google Scholar] [CrossRef]
- Smacchi, E.; Gobbetti, M. Peptides from Several Italian Cheeses Inhibitory to Proteolytic Enzymes of Lactic Acid Bacteria, Pseudomonas fluorescens ATCC 948 and to the Angiotensin I-Converting Enzyme. Enzym. Microb. Technol. 1998, 22, 687–694. [Google Scholar] [CrossRef]
- Liu, H.; Tu, M.; Cheng, S.; Chen, H.; Wang, Z.; Du, M. An Anticoagulant Peptide from Beta-Casein: Identification, Structure and Molecular Mechanism. Food Funct. 2019, 10, 886–892. [Google Scholar] [CrossRef]
- Jiang, X.; Pan, D.; Zhang, T.; Liu, C.; Zhang, J.; Su, M.; Wu, Z.; Zeng, X.; Sun, Y.; Guo, Y. Novel Milk Casein–Derived Peptides Decrease Cholesterol Micellar Solubility and Cholesterol Intestinal Absorption in Caco-2 Cells. J. Dairy Sci. 2020, 103, 3924–3936. [Google Scholar] [CrossRef]
- Gong, H.; Gao, J.; Wang, Y.; Luo, Q.W.; Guo, K.R.; Ren, F.Z.; Mao, X.Y. Identification of Novel Peptides from Goat Milk Casein That Ameliorate High-Glucose-Induced Insulin Resistance in HepG2 Cells. J. Dairy Sci. 2020, 103, 4907–4918. [Google Scholar] [CrossRef]
- Lee, S.; Youn, B. Hypolipidemic Roles of Casein-Derived Peptides by Regulation of Trans-Intestinal Cholesterol Excretion and Bile Acid Synthesis. Nutrients 2020, 12, 3058. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP Database and Other Programs for Processing Bioactive Peptide Sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk Bioactive Peptide Database: A Comprehensive Database of Milk Protein-Derived Bioactive Peptides and Novel Visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Zamyatnin, A.A.; Borchikov, A.S.; Vladimirov, M.G.; Voronina, O.L. The EROP-Moscow Oligopeptide Database. Nucleic Acids Res. 2006, 34, D261–D266. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Wang, W.; Feng, F.; Shan, W. A Novel Angiotensin I Converting Enzyme Inhibitory Peptide from the Milk Casein: Virtual Screening and Docking Studies. Agric. Sci. China 2011, 10, 463–467. [Google Scholar] [CrossRef]
- Otani, H.; Nakano, K.; Kawahara, T. Stimulatory Effect of a Dietary Casein Phosphopeptide Preparation on the Mucosal IgA Response of Mice to Orally Ingested Lipopolysaccharide from Salmonella typhimurium. Biosci. Biotechnol. Biochem. 2003, 67, 729–735. [Google Scholar] [CrossRef]
- Kawahara, T.; Otani, H. Stimulatory Effects of Casein Phosphopeptide (CPP-III) on mRNA Expression of Cytokines in Caco-2 Cells. Biosci. Biotechnol. Biochem. 2004, 68, 1779–1781. [Google Scholar] [CrossRef] [PubMed]
- Otani, H.; Watanabe, T.; Tashiro, Y. Effects of Bovine β-Casein (1-28) and Its Chemically Synthesized Partial Fragments on Proliferative Responses and Immunoglobulin Production in Mouse Spleen Cell Cultures. Biosci. Biotechnol. Biochem. 2001, 65, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N.; Shamay, A.; Shinder, D.; Moran, A. Stress down Regulates Milk Yield in Cows by Plasmin Induced β-Casein Product That Blocks K+ Channels on the Apical Membranes. Life Sci. 2000, 67, 2201–2212. [Google Scholar] [CrossRef]
- Pizzano, R.; Nicolai, M.A.; Padovano, P.; Ferranti, P.; Barone, F.; Addeo, F. Immunochemical Evaluation of Bovine β-Casein and Its 1−28 Phosphopeptide in Cheese during Ripening. J. Agric. Food Chem. 2000, 48, 4555–4560. [Google Scholar] [CrossRef]
- Gagnaire, V.; Carpino, S.; Pediliggieri, C.; Jardin, J.; Lortal, S.; Licitra, G. Uncommonly Thorough Hydrolysis of Peptides during Ripening of Ragusano Cheese Revealed by Tandem Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 12443–12452. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Caira, S.; Cuollo, M.; Lilla, S.; Chianese, L.; Addeo, F. Bioactive Casein Phosphopeptides in Dairy Products as Nutraceuticals for Functional Foods. In Milk Protein; IntechOpen: London, UK, 2012; ISBN 953-51-0743-7. [Google Scholar][Green Version]
- Gobbetti, M.; Ferranti, P.; Smacchi, E.; Goffredi, F.; Addeo, F. Production of Angiotensin-I-Converting-Enzyme-Inhibitory Peptides in Fermented Milks Started by Lactobacillus delbrueckii subsp. Bulgaricus SS1 and Lactococcus lactis subsp. Cremoris FT4. Appl. Environ. Microbiol. 2000, 66, 3898–3904. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Structure Activity Relationship Modelling of Milk Protein-Derived Peptides with Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Activity. Peptides 2016, 79, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Exposito, I.; Minervini, F.; Amigo, L.; Recio, I. Identification of Antibacterial Peptides from Bovine κ-Casein. J. Food Prot. 2006, 69, 2992–2997. [Google Scholar] [CrossRef]
- Donkor, O.; Henriksson, A.; Singh, T.; Vasiljevic, T.; Shah, N.P. ACE-Inhibitory Activity of Probiotic Yoghurt. Int. Dairy J. 2007, 17, 1321–1331. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Ferreira, A.K.; Auada, A.V.V.; Pasqualoto, K.F.M.; Marques-Porto, R.; Maria, D.A.; Lebrun, I. Antitumor Effect of Cationic INKKI Peptide from Bovine β-Casein on Melanoma B16F10. J. Cancer Ther. 2012, 3. [Google Scholar] [CrossRef]
- Lebrun, I.; Cavallaro, V.; Juliano, L.; Juliano, M.A.; de Sousa e Silva, M.C.C. Effects Of′ Casoparan′, a Peptide Isolated from Casein Hydrolysates with Mastoparan-like Properties. Mediat. Inflamm. 2004, 13, 263–268. [Google Scholar] [CrossRef]
- Yamamoto, N.; Akino, A.; Takano, T. Antihypertensive Effect of the Peptides Derived from Casein by an Extracellular Proteinase from Lactobacillus helveticus CP790. J. Dairy Sci. 1994, 77, 917–922. [Google Scholar] [CrossRef]
- Kohmura, M.; Nio, N.; Ariyoshi, Y. Inhibition of Angiotensin-Converting Enzyme by Synthetic Peptide Fragments of Various β-Caseins. Agric. Biol. Chem. 1990, 54, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Nio, N.; Ariyoshi, Y. Inhibition of Prolyl Endopeptidase by Synthetic β-Casein Peptides and Their Derivatives with a C-Terminal Prolinol or Prolinal. Biosci. Biotechnol. Biochem. 1992, 56, 976–977. [Google Scholar] [CrossRef]
- Minervini, F.; Algaron, F.; Rizzello, C.G.; Fox, P.; Monnet, V.; Gobbetti, M. Angiotensin I-Converting-Enzyme-Inhibitory and Antibacterial Peptides from Lactobacillus helveticus PR4 Proteinase-Hydrolyzed Caseins of Milk from Six Species. Appl. Environ. Microbiol. 2003, 69, 5297–5305. [Google Scholar] [CrossRef]
- Otte, J.; Shalaby, S.M.; Zakora, M.; Nielsen, M.S. Fractionation and Identification of ACE-Inhibitory Peptides from α-Lactalbumin and β-Casein Produced by Thermolysin-Catalysed Hydrolysis. Int. Dairy J. 2007, 17, 1460–1472. [Google Scholar] [CrossRef]
- Quirós, A.; Ramos, M.; Muguerza, B.; Delgado, M.A.; Miguel, M.; Aleixandre, A.; Recio, I. Identification of Novel Antihypertensive Peptides in Milk Fermented with Enterococcus faecalis. Int. Dairy J. 2007, 17, 33–41. [Google Scholar] [CrossRef]
- Pihlanto, A.; Virtanen, T.; Korhonen, H. Angiotensin I Converting Enzyme (ACE) Inhibitory Activity and Antihypertensive Effect of Fermented Milk. Int. Dairy J. 2010, 20, 3–10. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, C.; Gibson, T.; Jauregi, P. Novel Probiotic-Fermented Milk with Angiotensin I-Converting Enzyme Inhibitory Peptides Produced by Bifidobacterium bifidum MF 20/5. Int. J. Food Microbiol. 2013, 167, 131–137. [Google Scholar] [CrossRef]
- Nakano, D.; Ogura, K.; Miyakoshi, M.; Ishii, F.; Kawanishi, H.; Kurumazuka, D.; Kwak, C.; Ikemura, K.; Takaoka, M.; Moriguchi, S. Antihypertensive Effect of Angiotensin I-Converting Enzyme Inhibitory Peptides from a Sesame Protein Hydrolysate in Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 2006, 70, 1118–1126. [Google Scholar] [CrossRef]
- Eisele, T.; Stressler, T.; Kranz, B.; Fischer, L. Bioactive Peptides Generated in an Enzyme Membrane Reactor Using Bacillus lentus Alkaline Peptidase. Eur. Food Res. Technol. 2013, 236, 483–490. [Google Scholar] [CrossRef]
- Abubakar, A.; Saito, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Structural Analysis of New Antihypertensive Peptides Derived from Cheese Whey Protein by Proteinase K Digestion. J. Dairy Sci. 1998, 81, 3131–3138. [Google Scholar] [CrossRef]
- Chang, K.-J.; Su, Y.F.; Brent, D.A.; Chang, J. Isolation of a Specific Mu-Opiate Receptor Peptide, Morphiceptin, from an Enzymatic Digest of Milk Proteins. J. Biol. Chem. 1985, 260, 9706–9712. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, R.; Meisel, H. Milk Protein Hydrolysates and Bioactive Peptides. In Advanced Dairy Chemistry—1 Proteins: Part A/Part B; Springer: Berlin/Heidelberg, Germany, 2003; pp. 675–698. [Google Scholar]
- Meisel, H. Chemical Characterization and Opioid Activity of an Exorphin Isolated from In Vivo Digests of Casein. FEBS Lett. 1986, 196, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Apostolopoulos, V.; Nurgali, K.; Mishra, V.K. Evaluation of in Silico Approach for Prediction of Presence of Opioid Peptides in Wheat. J. Funct. Foods 2018, 41, 34–40. [Google Scholar] [CrossRef]
- Meisel, H.; Frister, H.; Schlimme, E. Biologically Active Peptides in Milk Proteins. Z. Für Ernährungswiss. 1989, 28, 267–278. [Google Scholar] [CrossRef]
- Sienkiewicz-Szłapka, E.; Jarmołowska, B.; Krawczuk, S.; Kostyra, E.; Kostyra, H.; Iwan, M. Contents of Agonistic and Antagonistic Opioid Peptides in Different Cheese Varieties. Int. Dairy J. 2009, 19, 258–263. [Google Scholar] [CrossRef]
- Haq, M.R.U.; Kapila, R.; Saliganti, V. Consumption of β-Casomorphins-7/5 Induce Inflammatory Immune Response in Mice Gut Through Th2 Pathway. J. Funct. Foods 2014, 8, 150–160. [Google Scholar] [CrossRef]
- Claustre, J.; Toumi, F.; Trompette, A.; Jourdan, G.; Guignard, H.; Chayvialle, J.A.; Plaisancié, P. Effects of Peptides Derived from Dietary Proteins on Mucus Secretion in Rat Jejunum. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 283, G521–G528. [Google Scholar] [CrossRef] [PubMed]
- Osborne, S.; Chen, W.; Addepalli, R.; Colgrave, M.; Singh, T.; Tran, C.; Day, L. In Vitro Transport and Satiety of a Beta-Lactoglobulin Dipeptide and Beta-Casomorphin-7 and Its Metabolites. Food Funct. 2014, 5, 2706–2718. [Google Scholar] [CrossRef]
- Dubynin, V.; Asmakova, L.; Sokhanenkova, N.Y.; Bespalova, Z.D.; Nezavibat’ko, V.; Kamenskii, A. Comparative Analysis of Neurotropic Activity of Exorphines, Derivatives of Dietary Proteins. Bull. Exp. Biol. Med. 1998, 125, 131–134. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; De Pascual-Teresa, S.; Reverón, I.; Muñoz, R.; Recio, I. Food-Derived Peptides Stimulate Mucin Secretion and Gene Expression in Intestinal Cells. J. Agric. Food Chem. 2012, 60, 8600–8605. [Google Scholar] [CrossRef]
- Trompette, A.; Claustre, J.; Caillon, F.; Jourdan, G.; Chayvialle, J.A.; Plaisancie, P. Milk Bioactive Peptides and β-Casomorphins Induce Mucus Release in Rat Jejunum. J. Nutr. 2003, 133, 3499–3503. [Google Scholar] [CrossRef]
- Kayser, H.; Meisel, H. Stimulation of Human Peripheral Blood Lymphocytes by Bioactive Peptides Derived from Bovine Milk Proteins. FEBS Lett. 1996, 383, 18–20. [Google Scholar] [CrossRef]
- Hatzoglou, A.; Bakogeorgou, E.; Hatzoglou, C.; Martin, P.-M.; Castanas, E. Antiproliferative and Receptor Binding Properties of α-and β-Casomorphins in the T47D Human Breast Cancer Cell Line. Eur. J. Pharmacol. 1996, 310, 217–223. [Google Scholar] [CrossRef]
- Brantl, V.; Teschemacher, H.; Bläsig, J.; Henschen, A.; Lottspeich, F. Opioid Activities of β-Casomorphins. Life Sci. 1981, 28, 1903–1909. [Google Scholar] [CrossRef]
- Gach, K.; Do-Rego, J.C.; Fichna, J.; Storr, M.; Delbro, D.; Toth, G.; Janecka, A. Synthesis and Biological Evaluation of Novel Peripherally Active Morphiceptin Analogs. Peptides 2010, 31, 1617–1624. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Yoshikawa, M. Enzymatic Release of Neocasomorphin and β-Casomorphin from Bovine β-Casein. Peptides 1999, 20, 957–962. [Google Scholar] [CrossRef]
- Saito, T.; Nakamura, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Isolation and Structural Analysis of Antihypertensive Peptides That Exist Naturally in Gouda Cheese. J. Dairy Sci. 2000, 83, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Uenishi, H.; Kabuki, T.; Seto, Y.; Serizawa, A.; Nakajima, H. Isolation and Identification of Casein-Derived Dipeptidyl-Peptidase 4 (DPP-4)-Inhibitory Peptide LPQNIPPL from Gouda-Type Cheese and Its Effect on Plasma Glucose in Rats. Int. Dairy J. 2012, 22, 24–30. [Google Scholar] [CrossRef]
- Wang, W.; Gu, F.; Wei, C.; Tang, Y.; Zheng, X.; Ren, M.; Qin, Y. PGPIPN, a Therapeutic Hexapeptide, Suppressed Human Ovarian Cancer Growth by Targeting BCL2. PLoS ONE 2013, 8, e60701. [Google Scholar] [CrossRef]
- Migliore-Samour, D.; Floc’h, F.; Jolles, P. Biologically Active Casein Peptides Implicated in Immunomodulation. J. Dairy Res. 1989, 56, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Migliore-Samour, D.; Jolles, P. Casein, a Prohormone with an Immunomodulating Role for the Newborn? Experientia 1988, 44, 188–193. [Google Scholar] [CrossRef]
- Zhao, M.; Wei, C.; Yang, X.; Zhou, J.; Wang, J.; Gu, F.; Lei, T.; Qin, Y. The Milk-Derived Hexapeptide PGPIPN Inhibits the Invasion and Migration of Human Ovarian Cancer Cells by Regulating the Expression of MTA1 and NM23H1 Genes. Int. J. Oncol. 2016, 48, 1721–1729. [Google Scholar] [CrossRef]
- Qi, N.; Liu, C.; Yang, H.; Shi, W.; Wang, S.; Zhou, Y.; Wei, C.; Gu, F.; Qin, Y. Therapeutic Hexapeptide (PGPIPN) Prevents and Cures Alcoholic Fatty Liver Disease by Affecting the Expressions of Genes Related with Lipid Metabolism and Oxidative Stress. Oncotarget 2017, 8, 88079. [Google Scholar] [CrossRef]
- Gu, F.; Xi, H.; Ruan, X.; Xu, Q.; Wang, S.; Qin, Y.-D. Protective Effect of Immunomodulating Peptide (PGPIPN) Derived from Beta-Casomorphin in Bovine Milk on Acute Alcohol-Induced Liver Injury. Chin. Pharmacol. Bull. 2018, 712–716. [Google Scholar] [CrossRef]
- Norris, R.; Poyarkov, A.; O’Keeffe, M.B.; FitzGerald, R.J. Characterisation of the Hydrolytic Specificity of Aspergillus niger Derived Prolyl Endoproteinase on Bovine β-Casein and Determination of ACE Inhibitory Activity. Food Chem. 2014, 156, 29–36. [Google Scholar] [CrossRef]
- Quirós, A.; del Mar Contreras, M.; Ramos, M.; Amigo, L.; Recio, I. Stability to Gastrointestinal Enzymes and Structure–Activity Relationship of β-Casein-Peptides with Antihypertensive Properties. Peptides 2009, 30, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Narva, M.; Rissanen, J.; Halleen, J.; Vapaatalo, H.; Väänänen, K.; Korpela, R. Effects of Bioactive Peptide, Valyl-Prolyl-Proline (VPP), and Lactobacillus helveticus Fermented Milk Containing VPP on Bone Loss in Ovariectomized Rats. Ann. Nutr. Metab. 2007, 51, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Rosticci, M.; Veronesi, M.; Bacchelli, S.; Strocchi, E.; Melegari, C.; Grandi, E.; Borghi, C. Hemodynamic Effects of Lactotripeptides from Casein Hydrolysate in Mediterranean Normotensive Subjects and Patients with High-Normal Blood Pressure: A Randomized, Double-Blind, Crossover Clinical Trial. J. Med. Food 2010, 13, 1363–1368. [Google Scholar] [CrossRef]
- Jäkälä, P.; Turpeinen, A.M.; Rajakari, K.; Korpela, R.; Vapaatalo, H. Biological Effects of Casein-Derived Tripeptide Powders Are Not Affected by Fermentation Process. Int. Dairy J. 2010, 20, 366–370. [Google Scholar] [CrossRef]
- Yamada, A.; Sakurai, T.; Ochi, D.; Mitsuyama, E.; Yamauchi, K.; Abe, F. Antihypertensive Effect of the Bovine Casein-Derived Peptide Met-Lys-Pro. Food Chem. 2015, 172, 441–446. [Google Scholar] [CrossRef]
- Jing, P.; Qian, B.; He, Y.; Zhao, X.; Zhang, J.; Zhao, D.; Lv, Y.; Deng, Y. Screening Milk-Derived Antihypertensive Peptides Using Quantitative Structure Activity Relationship (QSAR) Modelling and In Vitro/In Vivo Studies on Their Bioactivity. Int. Dairy J. 2014, 35, 95–101. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jahandideh, F.; Davidge, S.T.; Wu, J. Milk-Derived Tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) Enhance Insulin Sensitivity and Prevent Insulin Resistance in 3T3-F442A Preadipocytes. J. Agric. Food Chem. 2018, 66, 10179–10187. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Liao, W.; Davidge, S.T.; Wu, J. Milk-Derived Tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) Differentially Modulate Angiotensin II Effects on Vascular Smooth Muscle Cells. J. Funct. Foods 2017, 30, 151–158. [Google Scholar] [CrossRef]
- Aihara, K.; Osaka, M.; Yoshida, M. Oral Administration of the Milk Casein-Derived Tripeptide Val-Pro-Pro Attenuates High-Fat Diet-Induced Adipose Tissue Inflammation in Mice. Br. J. Nutr. 2014, 112, 513–519. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Wu, J. Milk-Derived Tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) Promote Adipocyte Differentiation and Inhibit Inflammation in 3T3-F442A Cells. PLoS ONE 2015, 10, e0117492. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Inhibition of Dipeptidyl Peptidase IV (DPP-IV) by Proline Containing Casein-Derived Peptides. J. Funct. Foods 2013, 5, 1909–1917. [Google Scholar] [CrossRef]
- Bruno, J.; Nicolas, A.; Pesenti, S.; Schwarz, J.; Simon, J.-L.; Léonil, J.; Plaisancié, P. Variants of β-Casofensin, a Bioactive Milk Peptide, Differently Modulate the Intestinal Barrier: In Vivo and Ex Vivo Studies in Rats. J. Dairy Sci. 2017, 100, 3360–3372, Erratum in J. Dairy Sci. 2018, 101, 5667. [Google Scholar] [CrossRef]
- Plaisancié, P.; Boutrou, R.; Estienne, M.; Henry, G.; Jardin, J.; Paquet, A.; Léonil, J. β-Casein (94-123)-Derived Peptides Differently Modulate Production of Mucins in Intestinal Goblet Cells. J. Dairy Res. 2015, 82, 36–46. [Google Scholar] [CrossRef]
- Plaisancié, P.; Claustre, J.; Estienne, M.; Henry, G.; Boutrou, R.; Paquet, A.; Léonil, J. A Novel Bioactive Peptide from Yoghurts Modulates Expression of the Gel-Forming MUC2 Mucin as Well as Population of Goblet Cells and Paneth Cells along the Small Intestine. J. Nutr. Biochem. 2013, 24, 213–221. [Google Scholar] [CrossRef]
- Bessette, C.; Benoit, B.; Sekkal, S.; Bruno, J.; Estienne, M.; Léonil, J.; Ferrier, L.; Théodorou, V.; Plaisancié, P. Protective Effects of Β-casofensin, a Bioactive Peptide from Bovine Β-casein, against Indomethacin-induced Intestinal Lesions in Rats. Mol. Nutr. Food Res. 2016, 60, 823–833. [Google Scholar] [CrossRef]
- Rival, S.G.; Boeriu, C.G.; Wichers, H.J. Caseins and Casein Hydrolysates. 2. Antioxidative Properties and Relevance to Lipoxygenase Inhibition. J. Agric. Food Chem. 2001, 49, 295–302. [Google Scholar] [CrossRef]
- Pan, M.; Huo, Y.; Wang, C.; Zhang, Y.; Dai, Z.; Li, B. Positively Charged Peptides from Casein Hydrolysate Show Strong Inhibitory Effects on LDL Oxidation and Cellular Lipid Accumulation in Raw264.7 Cells. Int. Dairy J. 2019, 91, 119–128. [Google Scholar] [CrossRef]
- Miao, J.; Liao, W.; Pan, Z.; Wang, Q.; Duan, S.; Xiao, S.; Yang, Z.; Cao, Y. Isolation and Identification of Iron-Chelating Peptides from Casein Hydrolysates. Food Funct. 2019, 10, 2372–2381. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A.; Rokka, T.; Korhonen, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Bovine Milk Proteins. Int. Dairy J. 1998, 8, 325–331. [Google Scholar] [CrossRef]
- Perpetuo, E.A.; Juliano, L.; Lebrun, I. Biochemical and Pharmacological Aspects of Two Bradykinin-Potentiating Peptides Obtained from Tryptic Hydrolysis of Casein. J. Protein Chem. 2003, 22, 601–606. [Google Scholar] [CrossRef]
- Hayes, M.; Stanton, C.; Slattery, H.; O’sullivan, O.; Hill, C.; Fitzgerald, G.; Ross, R. Casein Fermentate of Lactobacillus Animalis DPC6134 Contains a Range of Novel Propeptide Angiotensin-Converting Enzyme Inhibitors. Appl. Environ. Microbiol. 2007, 73, 4658–4667. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Identification of Novel Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides in Camel Milk Protein Hydrolysates. Food Chem. 2018, 244, 340–348. [Google Scholar] [CrossRef]
- Sanchez-Rivera, L.; Ares, I.; Miralles, B.; Gómez-Ruiz, J.Á.; Recio, I.; Martínez-Larrañaga, M.R.; Anadon, A.; Martínez, M.A. Bioavailability and Kinetics of the Antihypertensive Casein-Derived Peptide HLPLP in Rats. J. Agric. Food Chem. 2014, 62, 11869–11875. [Google Scholar] [CrossRef]
- Sánchez-Rivera, L.; Santos, P.F.; Miralles, B.; Carrón, R.; Montero, M.J.; Recio, I. Peptide Fragments from β-Casein f (134–138), HLPLP, Generated by the Action of Rat Blood Plasma Peptidases Show Potent Antihypertensive Activity. Food Res. Int. 2016, 88, 348–353. [Google Scholar] [CrossRef]
- Robert, M.-C.; Razaname, A.; Mutter, M.; Juillerat, M.A. Identification of Angiotensin-I-Converting Enzyme Inhibitory Peptides Derived from Sodium Caseinate Hydrolysates Produced by Lactobacillus helveticus NCC 2765. J. Agric. Food Chem. 2004, 52, 6923–6931. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Susceptibility of Milk Protein-Derived Peptides to Dipeptidyl Peptidase IV (DPP-IV) Hydrolysis. Food Chem. 2014, 145, 845–852. [Google Scholar] [CrossRef]
- Maeno, M.; Yamamoto, N.; Takano, T. Identification of an Antihypertensive Peptide from Casein Hydrolysate Produced by a Proteinase from Lactobacillus helveticus CP790. J. Dairy Sci. 1996, 79, 1316–1321. [Google Scholar] [CrossRef]
- Phelan, M.; Khaldi, N.; Shields, D.C.; Kerins, D.M. Angiotensin Converting Enzyme and Nitric Oxide Inhibitory Activities of Novel Milk Derived Peptides. Int. Dairy J. 2014, 35, 38–42. [Google Scholar] [CrossRef]
- Liu, D.; Sun, H.; Zhang, L.; Li, S.; Qin, Z. High-Level Expression of Milk-Derived Antihypertensive Peptide in Escherichia coli and Its Bioactivity. J. Agric. Food Chem. 2007, 55, 5109–5112, Erratum in J. Agric. Food Chem. 2008, 56, 1524. [Google Scholar] [CrossRef]
- Kumar, N.; Devi, S.; Mada, S.B.; Reddi, S.; Kapila, R.; Kapila, S. Anti-Apoptotic Effect of Buffalo Milk Casein Derived Bioactive Peptide by Directing Nrf2 Regulation in Starving Fibroblasts. Food Biosci. 2020, 35, 100566. [Google Scholar] [CrossRef]
- Maruyama, S.; Nakagomi, K.; Tomizuka, N.; Suzuki, H. Angiotensin I-Converting Enzyme Inhibitor Derived from an Enzymatic Hydrolysate of Casein. II. Isolation and Bradykinin-Potentiating Activity on the Uterus and the Ileum of Rats. Agric. Biol. Chem. 1985, 49, 1405–1409. [Google Scholar]
- Maruyama, S.; Mitachi, H.; Tanaka, H.; Tomizuka, N.; Suzuki, H. Studies on the Active Site and Antihypertensive Activity of Angiotensin I-Converting Enzyme Inhibitors Derived from Casein. Agric. Biol. Chem. 1987, 51, 1581–1586. [Google Scholar] [CrossRef]
- Tsopmo, A.; Romanowski, A.; Banda, L.; Lavoie, J.C.; Jenssen, H.; Friel, J.K. Novel Anti-Oxidative Peptides from Enzymatic Digestion of Human Milk. Food Chem. 2011, 126, 1138–1143. [Google Scholar] [CrossRef]
- Hernandez-Ledesma, B.; Quiros, A.; Amigo, L.; Recio, I. Identification of Bioactive Peptides after Digestion of Human Milk and Infant Formula with Pepsin and Pancreatin. Int. Dairy J. 2007, 17, 42–49. [Google Scholar] [CrossRef]
- Vukic, V.R.; Vukic, D.V.; Milanovic, S.D.; Ilicic, M.D.; Kanuric, K.G.; Johnson, M.S. In Silico Identification of Milk Antihypertensive Di-and Tripeptides Involved in Angiotensin I–Converting Enzyme Inhibitory Activity. Nutr. Res. 2017, 46, 22–30. [Google Scholar] [CrossRef]
- Malinowski, J.; Klempt, M.; Clawin-Rädecker, I.; Lorenzen, P.C.; Meisel, H. Identification of a NFκB Inhibitory Peptide from Tryptic β-Casein Hydrolysate. Food Chem. 2014, 165, 129–133. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Isolation and Characterization of Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Ulva rigida C. Agardh Protein Hydrolysate. J. Funct. Foods 2016, 26, 65–76. [Google Scholar] [CrossRef]
- Berthou, J.; Migliore-Samour, D.; Lifchitz, A.; Delettré, J.; Floc’h, F.; Jollès, P. Immunostimulating Properties and Three-Dimensional Structure of Two Tripeptides from Human and Cow Caseins. FEBS Lett. 1987, 218, 55–58. [Google Scholar] [CrossRef]
- Coste, M.; Rochet, V.; Léonil, J.; Mollé, D.; Bouhallab, S.; Tomé, D. Identification of C-Terminal Peptides of Bovine β-Casein That Enhance Proliferation of Rat Lymphocytes. Immunol. Lett. 1992, 33, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Sandré, C.; Gleizes, A.; Forestier, F.; Gorges-Kergot, R.; Chilmonczyk, S.; Léonil, J.; Moreau, M.-C.; Labarre, C. A Peptide Derived from Bovine β-Casein Modulates Functional Properties of Bone Marrow-Derived Macrophages from Germfree and Human Flora-Associated Mice. J. Nutr. 2001, 131, 2936–2942. [Google Scholar] [CrossRef]
- Rojas-Ronquillo, R.; Cruz-Guerrero, A.; Flores-Nájera, A.; Rodríguez-Serrano, G.; Gómez-Ruiz, L.; Reyes-Grajeda, J.P.; Jiménez-Guzmán, J.; García-Garibay, M. Antithrombotic and Angiotensin-Converting Enzyme Inhibitory Properties of Peptides Released from Bovine Casein by Lactobacillus casei Shirota. Int. Dairy J. 2012, 26, 147–154. [Google Scholar] [CrossRef]
- Lu, Y.; Govindasamy-Lucey, S.; Lucey, J. Angiotensin-I-Converting Enzyme-Inhibitory Peptides in Commercial Wisconsin Cheddar Cheeses of Different Ages. J. Dairy Sci. 2016, 99, 41–52. [Google Scholar] [CrossRef]
- Sowmya, K.; Bhat, M.I.; Bajaj, R.K.; Kapila, S.; Kapila, R. Buffalo Milk Casein Derived Decapeptide (YQEPVLGPVR) Having Bifunctional Anti-Inflammatory and Antioxidative Features under Cellular Milieu. Int. J. Pept. Res. Ther. 2019, 25, 623–633. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, L.; Xu, H.; Gao, Y.; Jin, Y.; Zhao, L.; David, X.A.L.; Zhan, D.; Zhang, S. Immunomodulating Effects of Casein-Derived Peptides QEPVL and QEPV on Lymphocytes In Vitro and In Vivo. Food Funct. 2014, 5, 2061–2069. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, F.; Wang, L.; Bai, F.; Dziugan, P.; Walczak, P.; Zhang, B. Characterization of a Bioactive Peptide with Cytomodulatory Effect Released from Casein. Eur. Food Res. Technol. 2014, 238, 315–322. [Google Scholar] [CrossRef]
- Vermeirssen, V.; van der Bent, A.; Van Camp, J.; van Amerongen, A.; Verstraete, W. A Quantitative in Silico Analysis Calculates the Angiotensin I Converting Enzyme (ACE) Inhibitory Activity in Pea and Whey Protein Digests. Biochimie 2004, 86, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Aihara, K.; Ishii, H.; Yoshida, M. Casein-Derived Tripeptide, Val-Pro-Pro (VPP), Modulates Monocyte Adhesion to Vascular Endothelium. J. Atheroscler. Thromb. 2009, 16, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, J.Á.; Ramos, M.; Recio, I. Angiotensin-Converting Enzyme-Inhibitory Peptides in Manchego Cheeses Manufactured with Different Starter Cultures. Int. Dairy J. 2002, 12, 697–706. [Google Scholar] [CrossRef]
- Hashimoto, A.; Aoyagi, H.; Izumiya, N. Synthetic Identification of Bitter Heptapeptide in Tryptic Hydrolysate of Casein. Bull. Chem. Soc. Jpn. 1980, 53, 2926–2928. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Amigo, L.; Ramos, M.; Recio, I. Angiotensin Converting Enzyme Inhibitory Activity in Commercial Fermented Products. Formation of Peptides under Simulated Gastrointestinal Digestion. J. Agric. Food Chem. 2004, 52, 1504–1510. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, K.J. Cathepsin B Inhibitory Peptides Derived from β-Casein. Peptides 2000, 21, 807–809. [Google Scholar] [CrossRef]
- Gómez-Ruiz, J.A.; Taborda, G.; Amigo, L.; Recio, I.; Ramos, M. Identification of ACE-Inhibitory Peptides in Different Spanish Cheeses by Tandem Mass Spectrometry. Eur. Food Res. Technol. 2006, 223, 595–601. [Google Scholar] [CrossRef]
- Wessels, S.; Axelsson, L.; Hansen, E.B.; De Vuyst, L.; Laulund, S.; Lähteenmäki, L.; Lindgren, S.; Mollet, B.; Salminen, S.; von Wright, A. The Lactic Acid Bacteria, the Food Chain, and Their Regulation. Trends Food Sci. Technol. 2004, 15, 498–505. [Google Scholar] [CrossRef]
- Fuquay, J.W.; McSweeney, P.L.; Fox, P.F. Encyclopedia of Dairy Sciences; Academic Press: Cambridge, MA, USA, 2011; ISBN 0-12-374407-5. [Google Scholar]
- Ren, C.; Cheng, L.; Sun, Y.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Lactic Acid Bacteria Secrete Toll like Receptor 2 Stimulating and Macrophage Immunomodulating Bioactive Factors. J. Funct. Foods 2020, 66, 103783. [Google Scholar] [CrossRef]
- Ren, C.; Dokter-Fokkens, J.; Figueroa Lozano, S.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Lactic Acid Bacteria May Impact Intestinal Barrier Function by Modulating Goblet Cells. Mol. Nutr. Food Res. 2018, 62, 1700572. [Google Scholar] [CrossRef]
- Venegas-Ortega, M.G.; Flores-Gallegos, A.C.; Martínez-Hernández, J.L.; Aguilar, C.N.; Nevárez-Moorillón, G.V. Production of Bioactive Peptides from Lactic Acid Bacteria: A Sustainable Approach for Healthier Foods. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1039–1051. [Google Scholar] [CrossRef]
- Haandrikman, A.J.; Kok, J.; Venema, G. Lactococcal Proteinase Maturation Protein PrtM Is a Lipoprotein. J. Bacteriol. 1991, 173, 4517–4525. [Google Scholar] [CrossRef]
- Holck, A.; Næs, H. Cloning, Sequencing and Expression of the Gene Encoding the Cell-Envelope-Associated Proteinase from Lactobacillus paracasei subsp. Paracasei NCDO 151. Microbiology 1992, 138, 1353–1364. [Google Scholar] [CrossRef]
- Siezen, R.J. Multi-Domain, Cell-Envelope Proteinases of Lactic Acid Bacteria. In Lactic Acid Bacteria: Genetics, Metabolism and Applications, Proceedings of the Sixth Symposium on Lactic Acid Bacteria: Genetics, Metabolism and Applications, Veldhoven, The Netherlands, 19–23 September 1999; Konings, W.N., Kuipers, O.P., In ’t Veld, J.H.J.H., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 139–155. ISBN 978-94-017-2027-4. [Google Scholar]
- Kieliszek, M.; Pobiega, K.; Piwowarek, K.; Kot, A.M. Characteristics of the Proteolytic Enzymes Produced by Lactic Acid Bacteria. Molecules 2021, 26, 1858. [Google Scholar] [CrossRef]
- Doeven, M.K.; Kok, J.; Poolman, B. Specificity and Selectivity Determinants of Peptide Transport in Lactococcus lactis and Other Microorganisms. Mol. Microbiol. 2005, 57, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Tellez, A.; Corredig, M.; Brovko, L.Y.; Griffiths, M.W. Characterization of Immune-Active Peptides Obtained from Milk Fermented by Lactobacillus helveticus. J. Dairy Res. 2010, 77, 129–136. [Google Scholar] [CrossRef]
- Kok, J.; van Gijtenbeek, L.A.; de Jong, A.; van der Meulen, S.B.; Solopova, A.; Kuipers, O.P. The Evolution of Gene Regulation Research in Lactococcus lactis. FEMS Microbiol. Rev. 2017, 41, S220–S243. [Google Scholar] [CrossRef] [PubMed]
- de Ruyter, P.G.; Kuipers, O.P.; de Vos, W.M. Controlled Gene Expression Systems for Lactococcus lactis with the Food-Grade Inducer Nisin. Appl. Environ. Microbiol. 1996, 62, 3662–3667. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Montalbán-López, M.; Masuda, Y.; Kuipers, O.P. Zirex: A Novel Zinc-Regulated Expression System for Lactococcus lactis. Appl. Environ. Microbiol. 2013, 79, 4503–4508. [Google Scholar] [CrossRef]
- Miyoshi, A.; Jamet, E.; Commissaire, J.; Renault, P.; Langella, P.; Azevedo, V. A Xylose-Inducible Expression System for Lactococcus lactis. FEMS Microbiol. Lett. 2004, 239, 205–212. [Google Scholar] [CrossRef]
- Linares, D.M.; Alvarez-Sieiro, P.; del Rio, B.; Ladero, V.; Redruello, B.; Martin, M.C.; Fernandez, M.; Alvarez, M.A. Implementation of the Agmatine-Controlled Expression System for Inducible Gene Expression in Lactococcus lactis. Microb. Cell Factories 2015, 14, 208. [Google Scholar] [CrossRef]
- Leenhouts, K.; Buist, G.; Bolhuis, A.; Ten Berge, A.; Kiel, J.; Mierau, I.; Dabrowska, M.; Venema, G.; Kok, J. A General System for Generating Unlabelled Gene Replacements in Bacterial Chromosomes. Mol. Gen. Genet. MGG 1996, 253, 217–224. [Google Scholar] [CrossRef]
- Solem, C.; Defoor, E.; Jensen, P.R.; Martinussen, J. Plasmid pCS1966, a New Selection/Counterselection Tool for Lactic Acid Bacterium Strain Construction Based on the oroP Gene, Encoding an Orotate Transporter from Lactococcus lactis. Appl. Environ. Microbiol. 2008, 74, 4772–4775. [Google Scholar] [CrossRef] [PubMed]
- Van Pijkeren, J.-P.; Britton, R.A. High Efficiency Recombineering in Lactic Acid Bacteria. Nucleic Acids Res. 2012, 40, e76. [Google Scholar] [CrossRef]
- Barrangou, R.; van Pijkeren, J.-P. Exploiting CRISPR–Cas Immune Systems for Genome Editing in Bacteria. Curr. Opin. Biotechnol. 2016, 37, 61–68. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S. Multigene Editing in the Escherichia coli Genome via the CRISPR-Cas9 System. Appl. Environ. Microbiol. 2015, 81, 2506–2514, Erratum in Appl. Environ. Microbiol. 2016, 82, e01181-16. [Google Scholar] [CrossRef]
- Selle, K.; Klaenhammer, T.R.; Barrangou, R. CRISPR-Based Screening of Genomic Island Excision Events in Bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 8076–8081. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.-T.; Seo, S.-O.; Lynn, P.; Lu, T.; Jin, Y.-S.; Blaschek, H.P. Bacterial Genome Editing with CRISPR-Cas9: Deletion, Integration, Single Nucleotide Modification, and Desirable “Clean” Mutant Selection in Clostridium beijerinckii as an Example. ACS Synth. Biol. 2016, 5, 721–732. [Google Scholar] [CrossRef]
- Westbrook, A.W.; Moo-Young, M.; Chou, C.P. Development of a CRISPR-Cas9 Tool Kit for Comprehensive Engineering of Bacillus subtilis. Appl. Environ. Microbiol. 2016, 82, 4876–4895. [Google Scholar] [CrossRef]
- Oh, J.-H.; van Pijkeren, J.-P. CRISPR–Cas9-Assisted Recombineering in Lactobacillus reuteri. Nucleic Acids Res. 2014, 42, e131. [Google Scholar] [CrossRef] [PubMed]
- Berlec, A.; Škrlec, K.; Kocjan, J.; Olenic, M.; Štrukelj, B. Single Plasmid Systems for Inducible Dual Protein Expression and for CRISPR-Cas9/CRISPRi Gene Regulation in Lactic Acid Bacterium Lactococcus lactis. Sci. Rep. 2018, 8, 1009. [Google Scholar] [CrossRef]
- Framson, P.E.; Nittayajarn, A.; Merry, J.; Youngman, P.; Rubens, C.E. New Genetic Techniques for Group B Streptococci: High-Efficiency Transformation, Maintenance of Temperature-Sensitive pWV01 Plasmids, and Mutagenesis with Tn917. Appl. Environ. Microbiol. 1997, 63, 3539–3547. [Google Scholar] [CrossRef]
- Song, X.; Huang, H.; Xiong, Z.; Ai, L.; Yang, S. CRISPR-Cas9D10A Nickase-Assisted Genome Editing in Lactobacillus casei. Appl. Environ. Microbiol. 2017, 83, e01259-17, Erratum in Appl. Environ. Microbiol. 2018, 84, e00146-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yi, Y.; Lü, X. CRISPR/Cas9-Based Genome Editing Platform for Companilactobacillus crustorum to Reveal the Molecular Mechanism of Its Probiotic Properties. J. Agric. Food Chem. 2021, 69, 15279–15289. [Google Scholar] [CrossRef]
- Goh, Y.J.; Barrangou, R. Portable CRISPR-Cas9N System for Flexible Genome Engineering in Lactobacillus acidophilus, Lactobacillus gasseri, and Lactobacillus paracasei. Appl. Environ. Microbiol. 2021, 87, e02669-20. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.A.; Wilke, T.; Erdmann, R. Efficacy of Bacteriocin-Containing Cell-Free Culture Supernatants from Lactic Acid Bacteria to Control Listeria Monocytogenes in Food. Int. J. Food Microbiol. 2011, 146, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.-M.; Park, S.-K.; Khan, F.; Kang, M.-G.; Lee, J.-H.; Kim, Y.-M. An Approach to Extend the Shelf Life of Ribbonfish Fillet Using Lactic Acid Bacteria Cell-Free Culture Supernatant. Food Control 2021, 123, 107731. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, Y.; Luo, X.; Chen, X.; Wang, G. Impact of Cell-Free Supernatant of Lactic Acid Bacteria on Staphylococcus aureus Biofilm and Its Metabolites. Front. Vet. Sci. 2023, 10, 1184989. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Zhai, G.; Fu, S.; Xia, Y.; Hu, B.; Cai, X.; Zhang, Y.; Li, Y.; Deng, Z.; et al. A Cell-Free Platform Based on Nisin Biosynthesis for Discovering Novel Lanthipeptides and Guiding Their Overproduction In Vivo. Adv. Sci. 2020, 7, 2001616. [Google Scholar] [CrossRef]
- O’Donnell, S.T.; Ross, R.P.; Stanton, C. The Progress of Multi-Omics Technologies: Determining Function in Lactic Acid Bacteria Using a Systems Level Approach. Front. Microbiol. 2020, 10, 3084. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, L.; Zhang, H.; Wang, Y.; Liu, J.; Wang, Z.; Mu, D.; Wu, X.; Li, X. Multi-Omics Analysis of Metabolic Differences in Rape Bee Pollen Fermented by Single and Mixed Lactic Acid Bacterial Strains. Food Biosci. 2024, 62, 105401. [Google Scholar] [CrossRef]
- Shi, H.; An, F.; Lin, H.; Li, M.; Wu, J.; Wu, R. Advances in Fermented Foods Revealed by Multi-Omics: A New Direction toward Precisely Clarifying the Roles of Microorganisms. Front. Microbiol. 2022, 13, 1044820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, Y.; Liu, Z.; Su, M.; Wu, Z.; Zhang, H.; Zhang, C.; Xu, X. Insights into Characteristic Metabolites and Potential Bioactive Peptides Profiles of Fresh Cheese Fermented with Three Novel Probiotics Based Metabolomics and Peptidomics. Food Chem. X 2024, 21, 101147. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wei, G.; Chen, F.; Ma, Q.; Huang, A. Integrated Metabolomics and Peptidomics to Delineate Characteristic Metabolites in Milk Fermented with Novel Lactiplantibacillus plantarum L3. Food Chem. X 2023, 18, 100732. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Gao, X.; Pan, D.; Hua, Y.; He, J.; Liu, Z.; Dang, Y. Advances in the Stability Challenges of Bioactive Peptides and Improvement Strategies. Curr. Res. Food Sci. 2022, 5, 2162–2170. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An Overview of Fermentation in the Food Industry—Looking Back from a New Perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef]

| NO | Sequence a | Start b | End b | Bioactivity c | References |
|---|---|---|---|---|---|
| 1 | KVLILA | 2 | 7 | ACE-I | [61] |
| 2 | RELEELNVPGEIVESLSSSEESITRINK | 16 | 43 | Immuno-R | [19,21,43,62,63,64,65,66,67] |
| 3 | RELEELNVPGEIVESLSSSEESITR | 16 | 40 | Immuno-R | [18,20,22,68] |
| 4 | LNVPGEIVE | 21 | 29 | ACE-I | [69] |
| 5 | VPGEIVE | 23 | 29 | DPP-IV-I | [70] |
| 6 | RINKK | 40 | 44 | ACE-I; Anti-M | [71,72] |
| 7 | RINK | 40 | 43 | Anti-M | [71] |
| 8 | INKKI | 41 | 45 | Immuno-R; Anti-C | [73,74] |
| 9 | NKKI | 42 | 45 | Anti-M | [71] |
| 10 | DELQDKIHPFAQTQSLVYPFPGPIPNS | 58 | 84 | ACE-I | [75] |
| 11 | DKIHPF | 62 | 67 | ACE-I | [69] |
| 12 | KIHPFAQTQSLVYP | 63 | 76 | ACE-I | [76] |
| 13 | IHPFAQTQ | 64 | 71 | PEP-I | [77] |
| 14 | IHPFAQTQSLVYP | 64 | 76 | ACE-I | [76] |
| 15 | HPFAQTQSLVYP | 65 | 76 | ACE-I | [76] |
| 16 | FAQTQSLVYP | 67 | 76 | ACE-I | [76] |
| 17 | AQTQSLVYP | 68 | 76 | ACE-I | [76] |
| 18 | QTQSLVYP | 69 | 76 | ACE-I | [76] |
| 19 | TQSLVYP | 70 | 76 | ACE-I | [76] |
| 20 | QSLVYP | 71 | 76 | ACE-I | [76] |
| 21 | SLVYP | 72 | 76 | ACE-I | [76] |
| 22 | LVYPFPGPIPNSLPQNIPP | 73 | 91 | ACE-I | [78,79,80] |
| 23 | LVYPFPGPIPNSLPQN | 73 | 88 | ACE-I | [81] |
| 24 | LVYPFPGPIPNSLPQ | 73 | 87 | PEP-I | [53] |
| 25 | LVYPFPGP | 73 | 80 | ACE-I | [78] |
| 26 | LVYPFP | 73 | 78 | ACE-I | [82] |
| 27 | LVYP | 73 | 76 | ACE-I | [76] |
| 28 | LVY | 73 | 75 | ACE-I | [83] |
| 29 | VYPFPGPIP | 74 | 82 | PEP-I | [77] |
| 30 | VYPFPGPI | 74 | 81 | PEP-I | [77] |
| 31 | VYPFPGPIPN | 74 | 83 | ACE-I | [84] |
| 32 | VYPFPGPIPNSLPQNIPP | 74 | 91 | ACE-I | [79] |
| 33 | VYPFPG | 74 | 79 | ACE-I | [85] |
| 34 | VYP | 74 | 76 | ACE-I | [76,85] |
| 35 | YPFPGPIP | 75 | 82 | Opioid; ACE-I | [86,87] |
| 36 | YPFPGPIPNSL | 75 | 85 | Opioid | [88,89] |
| 37 | YPFPGPI | 75 | 81 | Opioid; Satiety-Ic; Immuno-R; Anxiolytic; Anti-C | [25,26,27,28,90,91,92,93,94,95,96,97,98,99] |
| 38 | YPFPG | 75 | 79 | Opioid; PNO; IM-Ic; Immuno-R; L&M-Ip | [25,31,33,34,91,92] |
| 39 | YPFPGP | 75 | 80 | Opioid; DPP-IV-I | [25,70,90,100] |
| 40 | YPFP | 75 | 78 | Opioid; Anti-C | [90,99,100,101] |
| 41 | YPFPGPIPN | 75 | 83 | ACE-I; DPP-IV-I; Opioid | [84,102,103,104] |
| 42 | YPFPGPIPNSLPQ | 75 | 87 | Opioid | [102] |
| 43 | YPFPGPIPNSLPQNIPPLTQT | 75 | 95 | Opioid | [102] |
| 44 | PFPGPI | 76 | 81 | Cathepsin B-I | [90] |
| 45 | FPGPIPN | 77 | 83 | DPP-IV-I | [104] |
| 46 | PGPIPN | 78 | 83 | Immuno-R; Anti-C | [105,106,107,108,109,110] |
| 47 | NSLP | 83 | 86 | ACE-I | [111] |
| 48 | SLPQN | 84 | 88 | ACE-I | [72] |
| 49 | LPQNIPPL | 85 | 92 | DPP-IV-I | [104] |
| 50 | LPQNIPPLT | 85 | 93 | DPP-IV-I | [70] |
| 51 | LPQNIPP | 85 | 91 | DPP-IV-I | [104] |
| 52 | LPQNIPPLTQTPVVVPPFLQPEVMGVSK | 85 | 112 | ACE-I | [75] |
| 53 | LPQ | 85 | 87 | DPP-IV-I | [104] |
| 54 | PQNIPPL | 86 | 92 | DPP-IV-I | [104] |
| 55 | NIPPLTQTPV | 88 | 97 | ACE-I | [69] |
| 56 | IPPLTQT | 89 | 95 | DPP-IV-I | [70] |
| 57 | IPP | 89 | 91 | ACE-I | [49] |
| 58 | LTQTPVVVPPF | 92 | 102 | ACE-I | [80,112] |
| 59 | TQTPVVVPPFLQPE | 93 | 106 | Anti-O (DPPH) | [52] |
| 60 | TPVVVPPFLQP | 95 | 105 | ACE-I | [85] |
| 61 | VVVPPF | 97 | 102 | ACE-I | [80] |
| 62 | VVPP | 98 | 101 | ACE-I | [61] |
| 63 | VPP | 99 | 101 | ACE-I; Anti-Infla; Boneloss-R | [49,72,113,114,115,116,117,118,119,120,121] |
| 64 | FLQP | 102 | 105 | ACE-I; DPP-IV-I | [111,122] |
| 65 | LQP | 103 | 105 | ACE-I | [51] |
| 66 | LQPE | 103 | 106 | Hypolipidemic | [55] |
| 67 | GVSKVKEAMAPKHKEMPFPKYPVEPFTESQ | 109 | 138 | Opioid; MUC-Ic | [123,124,125,126] |
| 68 | VKEAMAPK | 113 | 120 | Anti-O (LA/Lox; LA/AAPH; Hpode/Hb); Anti-M | [40,127] |
| 69 | EAMAPKHK | 115 | 122 | Anti-M | [40] |
| 70 | EAMAPK | 115 | 120 | Anti-M | [40] |
| 71 | MAP | 117 | 119 | ACE-I | [51,111] |
| 72 | HKEMPFPK | 121 | 128 | Anti-M | [40,128,129] |
| 73 | EMPFPK | 123 | 128 | MUC-Ic; Anti-M; Anti-M; Brad-P | [40,124,130,131] |
| 74 | MPFPKYPVEP | 124 | 133 | ACE-I | [132] |
| 75 | KYPVEPFTESQSLTL | 128 | 142 | ACE-I | [75] |
| 76 | KYP | 128 | 130 | ACE-I | [111] |
| 77 | YPVEPF | 129 | 134 | Opioid; DPP-IV-I; MUC-Ic | [70,102,124,133] |
| 78 | YPVEPFTE | 129 | 136 | ACE-I; Brad-P | [131] |
| 79 | VEP | 131 | 133 | ACE-I | [111] |
| 80 | HLPLP | 140 | 144 | ACE-I | [76,134,135] |
| 81 | LPLP | 143 | 146 | ACE-I | [76] |
| 82 | VENLHLPLPLL | 145 | 155 | ACE-I | [136] |
| 83 | ENLHLPLPLL | 146 | 155 | ACE-I | [136] |
| 84 | NLHLP | 147 | 151 | ACE-I | [76] |
| 85 | NLHLPLPLL | 147 | 155 | ACE-I | [136] |
| 86 | LHLP | 148 | 151 | ACE-I | [76] |
| 87 | LHLPLPL | 148 | 154 | ACE-I | [80] |
| 88 | LHLPLP | 148 | 153 | ACE-I | [80] |
| 89 | LPLPLL | 150 | 155 | DPP-IV-I | [70] |
| 90 | LPLPL | 150 | 154 | DPP-IV-I | [70,137] |
| 91 | LPL | 150 | 152 | DPP-IV-I | [137] |
| 92 | LQSW | 155 | 158 | ACE-I | [138] |
| 93 | QSWMHQPHQ | 156 | 164 | ACE-I | [139] |
| 94 | HQP | 163 | 165 | ACE-I | [111] |
| 95 | PLP | 165 | 167 | ACE-I | [76] |
| 96 | LPP | 166 | 168 | ACE-I | [111] |
| 97 | PPQSVLSLSQSKVLPVPQ | 173 | 190 | ACE-I | [75] |
| 98 | SQSKVLPVPQ | 181 | 190 | ACE-I | [132] |
| 99 | SQSKVLPVPQK | 181 | 191 | Hypolipidemic | [57] |
| 100 | SKVLPVPQ | 183 | 190 | ACE-I | [75] |
| 101 | KVLPVPQK | 184 | 191 | Anti-O (LA/Lox; LA/AAPH; Hpode/Hb) | [127] |
| 102 | KVLPVPQ | 184 | 190 | ACE-I | [138] |
| 103 | KVLPVP | 184 | 189 | ACE-I | [138,140] |
| 104 | VLPVPQ | 185 | 190 | Hypolipidemic | [55] |
| 105 | VLPVPQK | 185 | 191 | Anti-O (LA/Lox; LA/AAPH; Hpode/Hb); Anti-M; Anti-apo | [40,127,141] |
| 106 | VLPVPQKAVPYPQR | 185 | 198 | Anti-M | [40] |
| 107 | LPVPQ | 186 | 190 | DPP-IV-I | [70] |
| 108 | LPVP | 186 | 189 | DPP-IV-I | [133] |
| 109 | AVPYPQR | 192 | 198 | ACE-I; Anti-O (LA/Lox; LA/AAPH; Hpode/Hb); Anti-M | [40,127,130,142] |
| 110 | AVPYP | 192 | 196 | ACE-I | [143] |
| 111 | AVP | 192 | 194 | ACE-I | [143] |
| 112 | VPYPQ | 193 | 197 | Anti-O (ORAC) | [144] |
| 113 | PYPQ | 194 | 197 | Anti-O (ABTS) | [145] |
| 114 | PYP | 194 | 196 | ACE-I | [143] |
| 115 | PQR | 196 | 198 | ACE-I | [146] |
| 116 | RDMPIQAF | 198 | 205 | ACE-I | [75] |
| 117 | DMPIQAFLLYQEPVLGPVR | 199 | 217 | Anti-Infla | [147] |
| 118 | IQA | 202 | 204 | ACE-I | [111] |
| 119 | AFL | 204 | 206 | ACE-I | [148] |
| 120 | LLYQEPVLGPVRGPFPIIV | 206 | 224 | ACE-I | [75] |
| 121 | LLY | 206 | 208 | Immuno-R | [149] |
| 122 | LYQEPVLGPVRGPFPIIV | 207 | 224 | Immuno-R | [150] |
| 123 | YQEPVLGPVRGPFPI | 208 | 222 | Anti-M | [39] |
| 124 | YQEPVLGPVRGPFPIIV | 208 | 224 | ACE-I; Anti-M; Immuno-R | [39,75,151,152] |
| 125 | YQEPVLGPVR | 208 | 217 | ACE-I; Anti-O (ABTS&ORAC); Anti-Infla; Anti-Co | [54,153,154] |
| 126 | YQEPVL | 208 | 213 | ACE-I | [72,130] |
| 127 | QEPVL | 209 | 213 | Anti-Infla; Immuno-R | [44,155] |
| 128 | QEPV | 209 | 212 | Immuno-R | [155] |
| 129 | QEPVLGPVRGPFP | 209 | 221 | Hypoglycemic | [56] |
| 130 | QEPVLGPVRGPFPIIV | 209 | 224 | ACE-I | [153] |
| 131 | EPVLGPVRGP | 210 | 219 | Cyto-M | [156] |
| 132 | EPVLGPVRGPFP | 210 | 221 | ACE-I | [132] |
| 133 | VLGP | 212 | 215 | ACE-I; DPP-IV-I | [111,122] |
| 134 | VLGPVRGPFP | 212 | 221 | ACE-I | [80] |
| 135 | LGP | 213 | 215 | ACE-I | [157,158] |
| 136 | VRGPFPIIV | 216 | 224 | ACE-I | [80] |
| 137 | VRGPFP | 216 | 221 | ACE-I | [159] |
| 138 | GPFPIIV | 218 | 224 | ACE-I | [160,161] |
| 139 | GPFPI | 218 | 222 | Cathepsin B-I | [162] |
| 140 | PFP | 219 | 221 | ACE-I | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Cheng, L. Unlocking Casein Bioactivity: Lactic Acid Bacteria and Molecular Strategies for Peptide Release. Int. J. Mol. Sci. 2025, 26, 8119. https://doi.org/10.3390/ijms26178119
Huang C, Cheng L. Unlocking Casein Bioactivity: Lactic Acid Bacteria and Molecular Strategies for Peptide Release. International Journal of Molecular Sciences. 2025; 26(17):8119. https://doi.org/10.3390/ijms26178119
Chicago/Turabian StyleHuang, Chenxi, and Lianghui Cheng. 2025. "Unlocking Casein Bioactivity: Lactic Acid Bacteria and Molecular Strategies for Peptide Release" International Journal of Molecular Sciences 26, no. 17: 8119. https://doi.org/10.3390/ijms26178119
APA StyleHuang, C., & Cheng, L. (2025). Unlocking Casein Bioactivity: Lactic Acid Bacteria and Molecular Strategies for Peptide Release. International Journal of Molecular Sciences, 26(17), 8119. https://doi.org/10.3390/ijms26178119









