Effective and Stable Senomorphic Apigenin Delivery System Obtained by Supercritical Carbon Dioxide Processing
Abstract
1. Introduction
2. Results
2.1. Preparation of the Systems and Solubility Studies
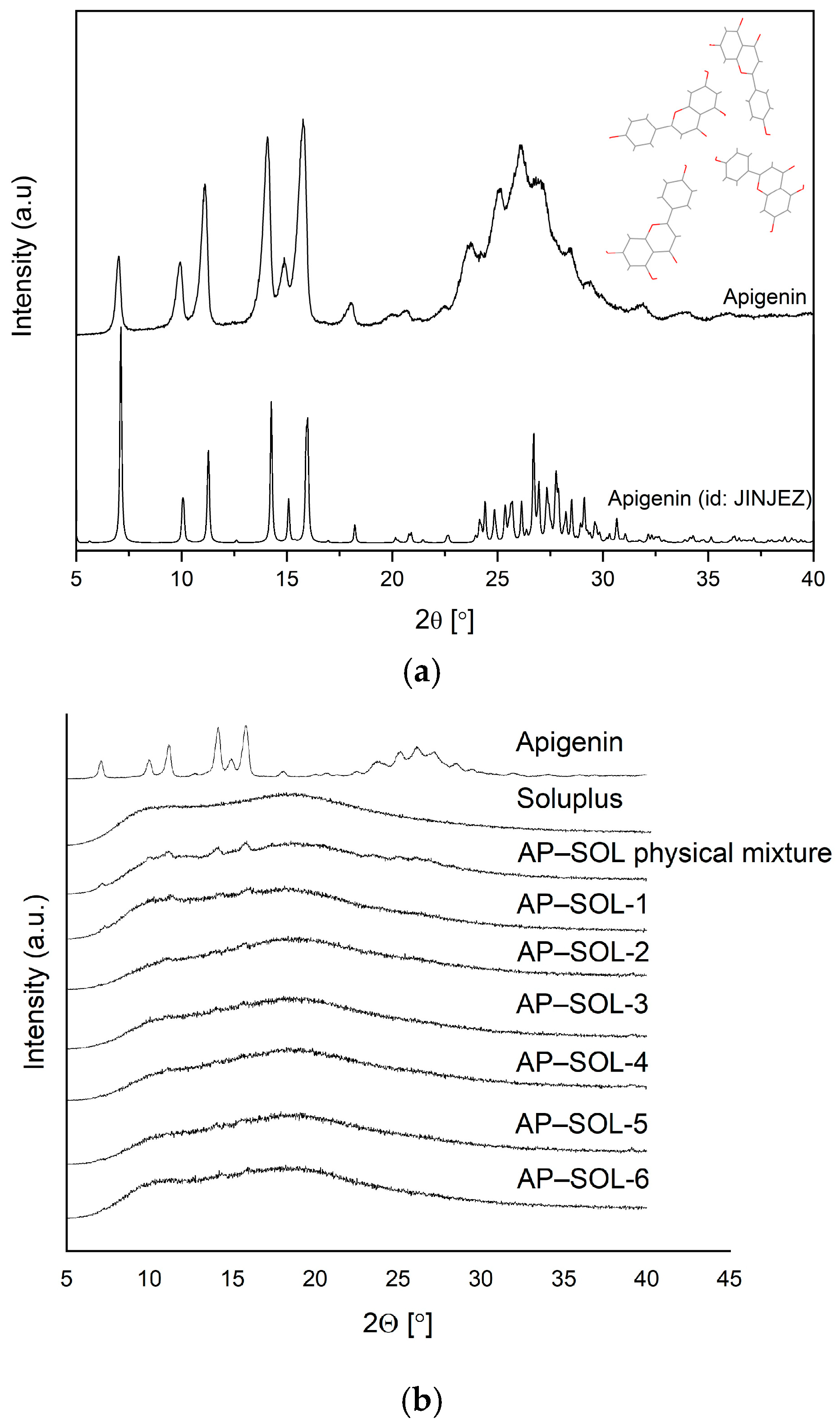
2.2. X-Ray Powder Diffraction
2.3. Fourier-Transform Infrared Spectroscopy
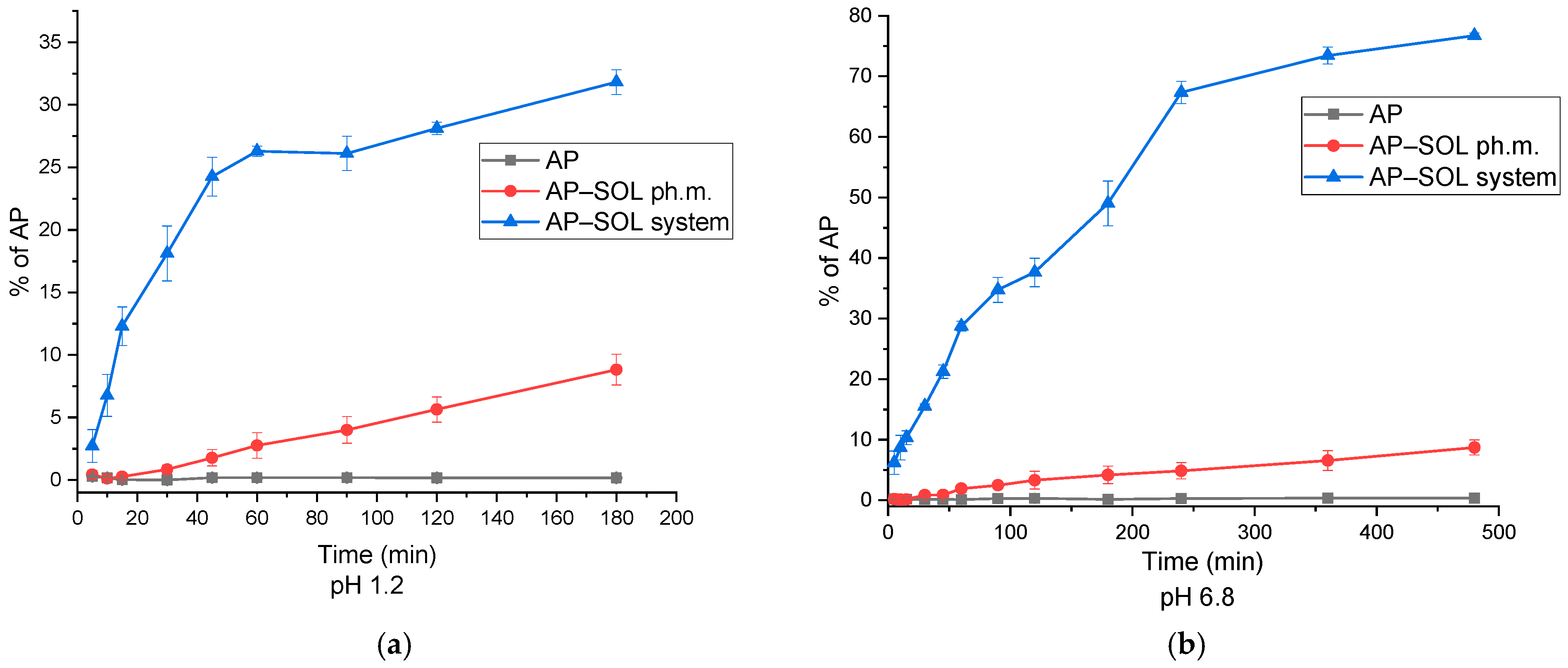
2.4. The Dissolution-Rate Studies
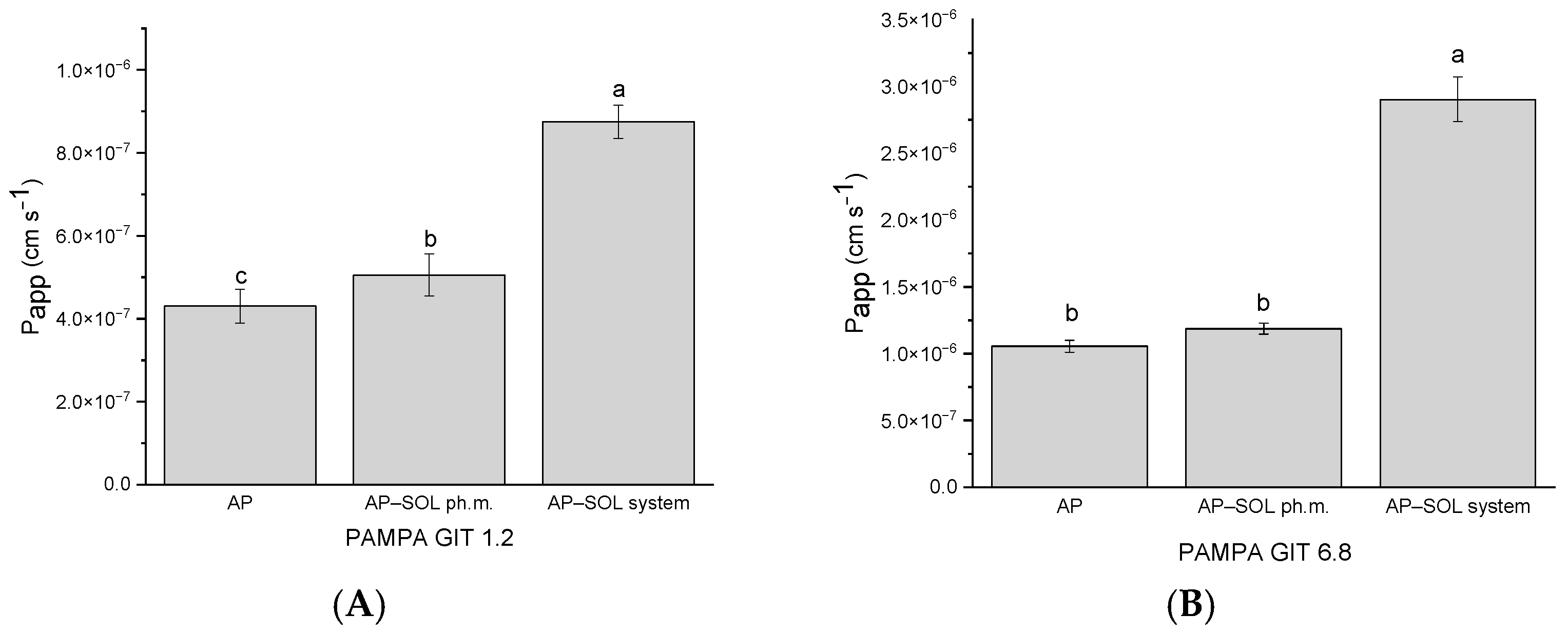
2.5. In Vitro Parallel Artificial Membrane Permeability Assay
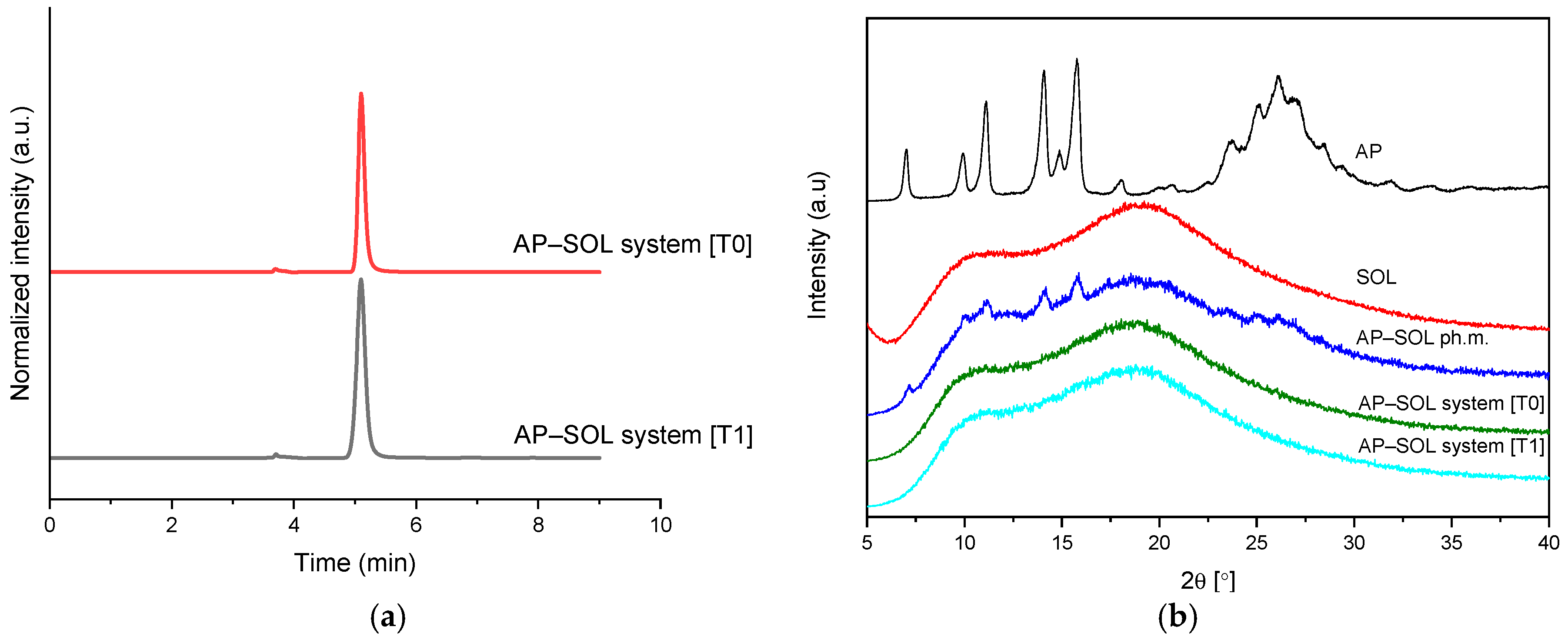
2.6. Stability Studies
2.7. Antioxidant Activity
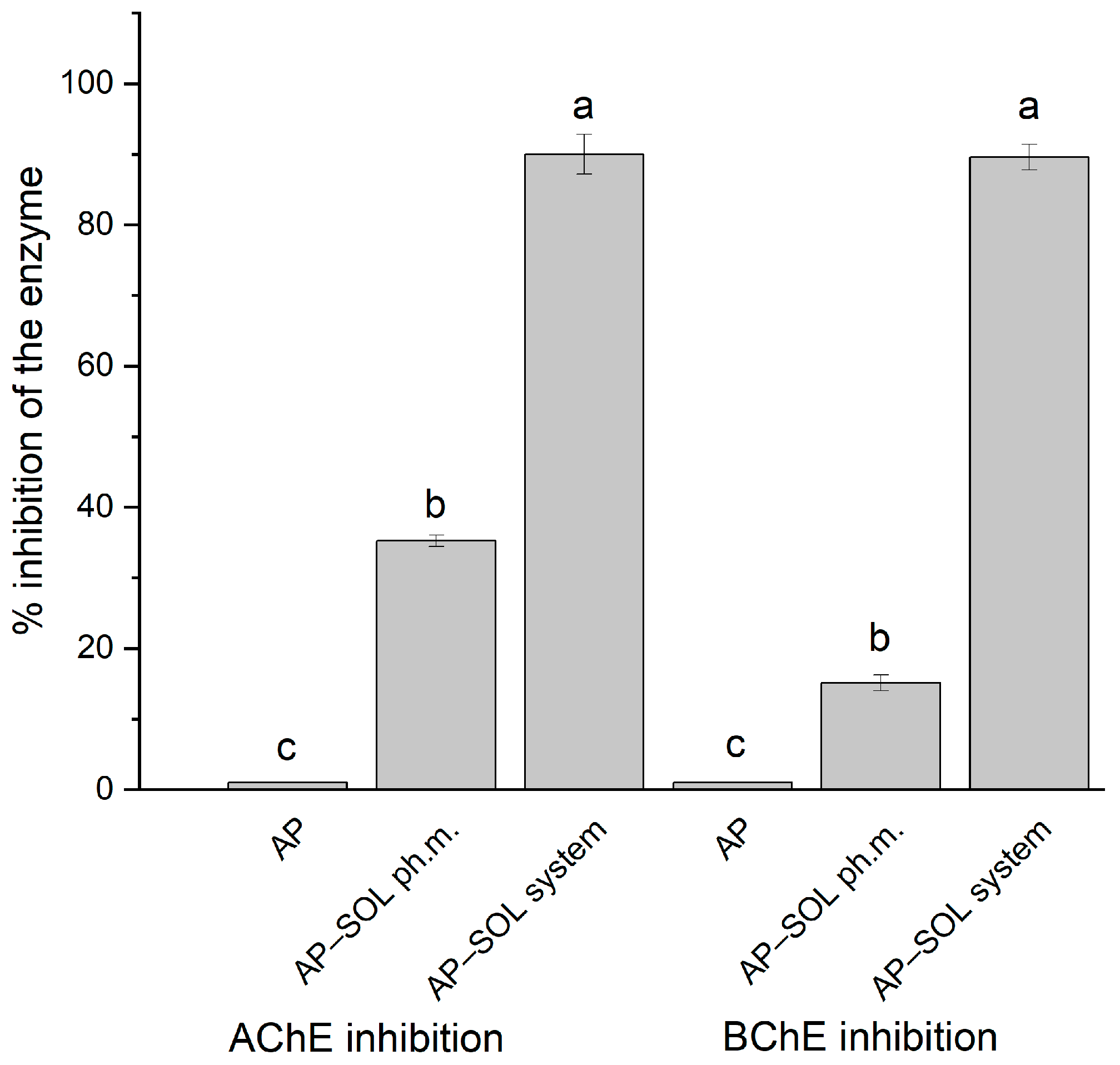
2.8. Anticholinesterase Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Systems and Solubility Studies
4.3. X-Ray Powder Diffraction
4.4. Fourier-Transform Infrared Spectroscopy
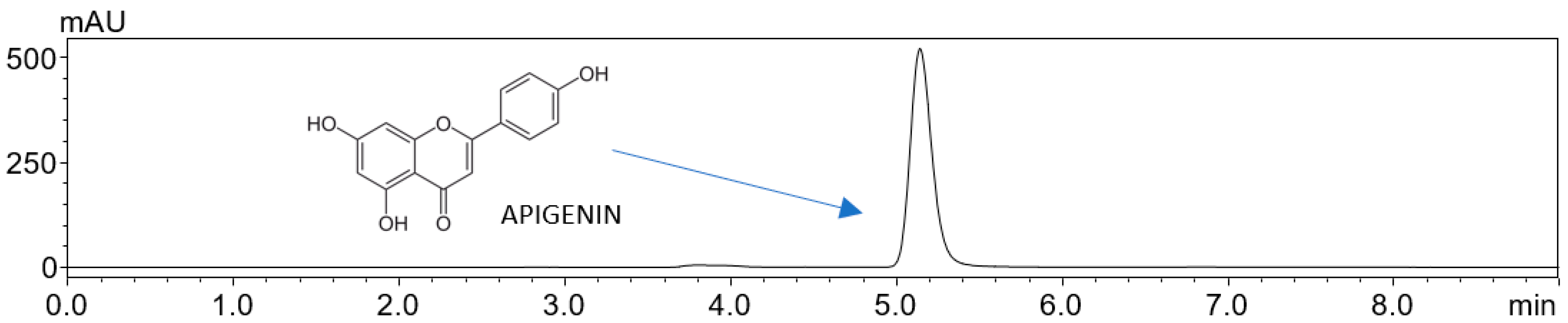
4.5. High-Performance Liquid Chromatography
4.6. The Dissolution-Rate Studies
4.7. In Vitro Parallel Artificial Membrane Permeability Assay
4.8. Stability Studies
4.9. Antioxidant Activity
4.10. Anticholinesterase Activity
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| Aβ | Amyloid-β |
| AChE | Acetylcholinesterase |
| AMPK | AMP-activated protein kinase |
| ANOVA | Analysis of Variance |
| AP | Apigenin |
| ATR-FT-IR/FT-IR | (Attenuated Total Reflectance) Fourier-Transform Infrared (Spectroscopy) |
| BBB | Blood–Brain Barrier |
| BChE | Butyrylcholinesterase |
| CCDC | Cambridge Crystallographic Data Centre |
| CIF | Crystallographic Information File |
| CUPRAC | Cupric (ion) Reducing Antioxidant Capacity |
| DFT | Density Functional Theory |
| DMSO | Dimethyl Sulfoxide |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GIT | Gastrointestinal Tract |
| HPLC-DAD/UHPLC-DAD | (Ultra-)High-Performance Liquid Chromatography with Diode Array Detector |
| HP-α-CD | 2-Hydroxypropyl-α-cyclodextrin |
| HP-β-CD | 2-Hydroxypropyl-β-cyclodextrin |
| HP-γ-CD | 2-Hydroxypropyl-γ-cyclodextrin |
| HPMC | Hydroxypropyl Methylcellulose |
| Kollidon 30 | Polyvinylpyrrolidone K30 |
| Kollidon VA64 | Vinylpyrrolidone–Vinyl Acetate Copolymer |
| Neusilin US2 | Magnesium aluminometasilicate |
| ORAC | Oxygen Radical Absorbance Capacity |
| PAMPA | Parallel Artificial Membrane Permeability Assay |
| Papp | Apparent Permeability Coefficient |
| PEG 6000 | Polyethylene Glycol 6000 |
| PVA | Polyvinyl Alcohol |
| PVP-co-vinyl acetate | Poly(1-vinylpyrrolidone-co-vinyl acetate) |
| PVP K25F | Polyvinylpyrrolidone K25 |
| ROS | Reactive Oxygen Species |
| SASP | Senescence-Associated Secretory Phenotype |
| scCO2 | Supercritical Carbon Dioxide |
| SOL | Soluplus® (polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer) |
| TRKB | Tropomyosin receptor kinase B |
| XRPD | X-ray Powder Diffraction |
References
- Hong, C.; Sun, L.; Liu, G.; Guan, B.; Li, C.; Luo, Y. Response of Global Health Towards the Challenges Presented by Population Aging. China CDC Wkly. 2023, 5, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular Senescence: The Good, the Bad and the Unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Saliev, T.; Singh, P.B. Targeting Senescence: A Review of Senolytics and Senomorphics in Anti-Aging Interventions. Biomolecules 2025, 15, 860. [Google Scholar] [CrossRef]
- Tavenier, J.; Nehlin, J.O.; Houlind, M.B.; Rasmussen, L.J.; Tchkonia, T.; Kirkland, J.L.; Andersen, O.; Rasmussen, L.J.H. Fisetin as a Senotherapeutic Agent: Evidence and Perspectives for Age-Related Diseases. Mech. Ageing Dev. 2024, 222, 111995. [Google Scholar] [CrossRef]
- Zoico, E.; Nori, N.; Darra, E.; Tebon, M.; Rizzatti, V.; Policastro, G.; De Caro, A.; Rossi, A.P.; Fantin, F.; Zamboni, M. Senolytic Effects of Quercetin in an in Vitro Model of Pre-Adipocytes and Adipocytes Induced Senescence. Sci. Rep. 2021, 11, 23237. [Google Scholar] [CrossRef]
- Ryan, P.; Lee, J. In Vitro Senescence and Senolytic Functional Assays. Biomater. Sci. 2025, 13, 3509–3531. [Google Scholar] [CrossRef]
- Górnicka, J.; Mika, M.; Wróblewska, O.; Siudem, P.; Paradowska, K. Methods to Improve the Solubility of Curcumin from Turmeric. Life 2023, 13, 207. [Google Scholar] [CrossRef]
- Atanacković, M.T.; Gojković-Bukarica, L.C.; Cvejić, J.M. Improving the Low Solubility of Resveratrol. BMC Pharmacol. Toxicol. 2012, 13, A25. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of Quercetin: Problems and Promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Jeong, H.-M.; Kang, H.-N.; Lee, Y.-R.; Kim, E.-A.; Lee, E.-H.; Shim, J.-H. Improved Low Water Solubility of Fisetin by Enzymatic Encapsulation Reaction Using Cycloamylose Produced by Cyclodextrin Glucanotransferase. Process Biochem. 2023, 130, 138–146. [Google Scholar] [CrossRef]
- DeRango-Adem, E.F.; Blay, J. Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front. Pharmacol. 2021, 12, 681477. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Q.; Jiang, Z.; Sun, R.; Liu, S.; Kirkland, J.L.; Zhang, W.; Sun, Y. Repurposing the Plant-Derived Compound Apigenin for Senomorphic Effect in Antiaging Pipelines. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Q.; Jiang, Z.; Sun, R.; Wang, Q.; Liu, S.; Luan, X.; Campisi, J.; Kirkland, J.L.; Zhang, W.; et al. Targeting Senescence with Apigenin Improves Chemotherapeutic Efficacy and Ameliorates Age-Related Conditions in Mice. Adv. Sci. 2025, 12, 2412950. [Google Scholar] [CrossRef]
- Perrott, K.M.; Wiley, C.D.; Desprez, P.-Y.; Campisi, J. Apigenin Suppresses the Senescence-Associated Secretory Phenotype and Paracrine Effects on Breast Cancer Cells. Geroscience 2017, 39, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gutiérrez, A.; Sendros, J.; Noya, T.; González, M.C. Apigenin and Phloretin Combination for Skin Aging and Hyperpigmentation Regulation. Cosmetics 2024, 11, 128. [Google Scholar] [CrossRef]
- Ali, D.; Okla, M.; Abuelreich, S.; Vishnubalaji, R.; Ditzel, N.; Hamam, R.; Kowal, J.M.; Sayed, A.; Aldahmash, A.; Alajez, N.M.; et al. Apigenin and Rutaecarpine Reduce the Burden of Cellular Senescence in Bone Marrow Stromal Stem Cells. Front. Endocrinol. 2024, 15, 1360054. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Mandal, M. Oxidative Stress Triggered by Naturally Occurring Flavone Apigenin Results in Senescence and Chemotherapeutic Effect in Human Colorectal Cancer Cells. Redox Biol. 2015, 5, 153–162. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Wu, C.-Y.; Peng, H.-H.; Voisin, L.; Perfettini, J.-L.; Ko, Y.-F.; Young, J.D. Emerging Use of Senolytics and Senomorphics against Aging and Chronic Diseases. Med. Res. Rev. 2020, 40, 2114–2131. [Google Scholar] [CrossRef]
- Ge, Y.; Zhou, M.; Chen, C.; Wu, X.; Wang, X. Role of AMPK Mediated Pathways in Autophagy and Aging. Biochimie 2022, 195, 100–113. [Google Scholar] [CrossRef]
- Xie, C.; Shi, Y.; Chen, Z.; Zhou, X.; Luo, P.; Hong, C.; Tian, N.; Wu, Y.; Zhou, Y.; Lin, Y.; et al. Apigenin Alleviates Intervertebral Disc Degeneration via Restoring Autophagy Flux in Nucleus Pulposus Cells. Front. Cell Dev. Biol. 2021, 9, 787278. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Alam, W.; Rocca, C.; Khan, H.; Hussain, Y.; Aschner, M.; De Bartolo, A.; Amodio, N.; Angelone, T.; Cheang, W.S. Current Status and Future Perspectives on Therapeutic Potential of Apigenin: Focus on Metabolic-Syndrome-Dependent Organ Dysfunction. Antioxidants 2021, 10, 1643. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Bakhoda, M.R.; Bahmanpour, Z.; Ilkhani, K.; Zarrabi, A.; Makvandi, P.; Khan, H.; Mazaheri, S.; Darvish, M.; Mirzaei, H. Apigenin as Tumor Suppressor in Cancers: Biotherapeutic Activity, Nanodelivery, and Mechanisms With Emphasis on Pancreatic Cancer. Front. Chem. 2020, 8, 829. [Google Scholar] [CrossRef]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective Effects of Apigenin against Inflammation, Neuronal Excitability and Apoptosis in an Induced Pluripotent Stem Cell Model of Alzheimer’s Disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef]
- Liu, W.; Kong, S.; Xie, Q.; Su, J.; Li, W.; Guo, H.; Li, S.; Feng, X.; Su, Z.; Xu, Y.; et al. Protective Effects of Apigenin against 1-Methyl-4-Phenylpyridinium Ion-induced Neurotoxicity in PC12 Cells. Int. J. Mol. Med. 2015, 35, 739–746. [Google Scholar] [CrossRef]
- Chiu, Y.-J.; Teng, Y.-S.; Chen, C.-M.; Sun, Y.-C.; Hsieh-Li, H.M.; Chang, K.-H.; Lee-Chen, G.-J. A Neuroprotective Action of Quercetin and Apigenin through Inhibiting Aggregation of Aβ and Activation of TRKB Signaling in a Cellular Experiment. Biomol. Ther. 2023, 31, 285–297. [Google Scholar] [CrossRef]
- Ling, C.; Lei, C.; Zou, M.; Cai, X.; Xiang, Y.; Xie, Y.; Li, X.; Huang, D.; Wang, Y. Neuroprotective Effect of Apigenin against Cerebral Ischemia/Reperfusion Injury. J. Int. Med. Res. 2020, 48, 0300060520945859. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.-L.; Liu, R.; Li, X.-X.; Li, J.-F.; Zhang, L. Neuroprotective, Anti-Amyloidogenic and Neurotrophic Effects of Apigenin in an Alzheimer’s Disease Mouse Model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kong, S.; Chen, W.; Dai, Z.; Lin, T.; Su, J.; Li, S.; Xie, Q.; Su, Z.; Xu, Y.; et al. Apigenin Mediated Protection of OGD-Evoked Neuron-like Injury in Differentiated PC12 Cells. Neurochem. Res. 2014, 39, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Rusdin, A.; Mohd Gazzali, A.; Ain Thomas, N.; Megantara, S.; Aulifa, D.L.; Budiman, A.; Muchtaridi, M. Advancing Drug Delivery Paradigms: Polyvinyl Pyrolidone (PVP)-Based Amorphous Solid Dispersion for Enhanced Physicochemical Properties and Therapeutic Efficacy. Polymers 2024, 16, 286. [Google Scholar] [CrossRef]
- Dudek, M.K.; Trzeciak, K.; Tajber, L.; Zając, J.; Kaźmierski, S.; Pindelska, E.; Makowski, T.; Svyntkivska, M.; Potrzebowski, M.J. A New Look at the Mechanism of Cocrystal Formation and Coformers Exchange in Processes Forced by Mechanical and/or Thermal Stimuli—Ex Situ and in Situ Studies of Low-Melting Eutectic Mixtures. Chemistry 2023, 30, e202302138. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, Y.; Li, X.; Xiong, Y.; Peng, Y.; Wang, C.; Zhuo, L.; Jiang, W.; Lu, X.; Wang, Z. Development of O-Aminobenzamide Salt Derivatives for Improving Water Solubility and Anti-Undifferentiated Gastric Cancer. Front. Pharmacol. 2023, 14, 1118397. [Google Scholar] [CrossRef] [PubMed]
- Shamsher, E.; Khan, R.S.; Davis, B.M.; Dine, K.; Luong, V.; Somavarapu, S.; Cordeiro, M.F.; Shindler, K.S. Nanoparticles Enhance Solubility and Neuroprotective Effects of Resveratrol in Demyelinating Disease. Neurotherapeutics 2023, 20, 1138–1153. [Google Scholar] [CrossRef]
- Markeev, V.B.; Tishkov, S.V.; Vorobei, A.M.; Parenago, O.O.; Blynskaya, E.V.; Alekseev, K.V.; Marakhova, A.I.; Vetcher, A.A. Modeling of the Aqueous Solubility of N-Butyl-N-Methyl-1-Phenylpyrrolo [1,2-a] Pyrazine-3-Carboxamide: From Micronization to Creation of Amorphous-Crystalline Composites with a Polymer. Polymers 2023, 15, 4136. [Google Scholar] [CrossRef]
- Rana, A.A.; Yusaf, A.; Shahid, S.; Usman, M.; Ahmad, M.; Aslam, S.; Al-Hussain, S.A.; Zaki, M.E.A. Unveiling the Role of Nonionic Surfactants in Enhancing Cefotaxime Drug Solubility: A UV-Visible Spectroscopic Investigation in Single and Mixed Micellar Formulations. Pharmaceuticals 2023, 16, 1663. [Google Scholar] [CrossRef]
- Tretyakova, I.S.; Rychkov, D.A.; Kil’met’ev, A.S.; Lomovskiy, I.O. Computational Study of Chemical Phenol Glycosylation Mechanism in the Gas Phase for Modeling Direct Glycoconjugate Formation in Raw Plant Material. Comput. Theor. Chem. 2023, 1225, 114182. [Google Scholar] [CrossRef]
- Hozhabr Araghi, S.; Amalraj, J.; Sadeghi Googheri, M.; Pyarasani, R.D.; Sadeghi Googheri, M.S. An In-Silico Study to Gain a Comprehensive Understanding of the Effects of Glucosylation on Quercetin Properties. Comput. Theor. Chem. 2023, 1220, 113981. [Google Scholar] [CrossRef]
- Pudipeddi, M.; Serajuddin, A.T.M. Trends in Solubility of Polymorphs. J. Pharm. Sci. 2005, 94, 929–939. [Google Scholar] [CrossRef]
- Wang, C.; Rosbottom, I.; Turner, T.D.; Laing, S.; Maloney, A.G.P.; Sheikh, A.Y.; Docherty, R.; Yin, Q.; Roberts, K.J. Molecular, Solid-State and Surface Structures of the Conformational Polymorphic Forms of Ritonavir in Relation to Their Physicochemical Properties. Pharm. Res. 2021, 38, 971–990. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Blattner, S.M.; Hirsh, D.; Hoffmann, R.; Luebbert, C.; Schaefer, K. Development of Ternary Amorphous Solid Dispersions Manufactured by Hot-Melt Extrusion and Spray-Drying—Comparison of In Vitro and In Vivo Performance. Mol. Pharm. 2024, 21, 1309–1320. [Google Scholar] [CrossRef]
- Almeida, H.; Teixeira, N.; Sarmento, B.; Vasconcelos, T. Freeze-Drying Cycle Optimization of an Amorphous Solid Dispersion of Resveratrol. Eur. J. Pharm. Sci. 2024, 200, 106855. [Google Scholar] [CrossRef]
- Wang, M.; Gong, J.; Rades, T.; Martins, I.C.B. Amorphization of Different Furosemide Polymorphic Forms during Ball Milling: Tracking Solid-to-Solid Phase Transformations. Int. J. Pharm. 2023, 648, 123573. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Qiu, Z.; Huang, D.; Lu, T.; Zhang, Z.J.; Luo, D.; Pan, P.; Zhang, L.; Liu, Y.; Guan, S.; et al. Enhancement of the Apparent Solubility and Bioavailability of Tadalafil Nanoparticles via Antisolvent Precipitation. Eur. J. Pharm. Sci. 2019, 128, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Sher, M.; Hussain, I.; Khan, F.A.; Afridi, H.H.; Sher, W.M.; Gulfam, N.; Khalil, M.S.; Aman, A.; Sulaiman, M.; Ahmad, Z. Effect of Silymarin Particle Size on Its Solubility and Oral Bioavailability, the Enhancement of Pharmacological Actions. J. Popl. Ther. Clin. Pharmacol. 2023, 30, 2527–2560. [Google Scholar] [CrossRef]
- Wdowiak, K.; Pietrzak, R.; Tykarska, E.; Cielecka-Piontek, J. Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities. Molecules 2023, 28, 3848. [Google Scholar] [CrossRef]
- Garbiec, E.; Rosiak, N.; Tykarska, E.; Zalewski, P.; Cielecka-Piontek, J. Sinapic Acid Co-Amorphous Systems with Amino Acids for Improved Solubility and Antioxidant Activity. Int. J. Mol. Sci. 2023, 24, 5533. [Google Scholar] [CrossRef] [PubMed]
- Garbiec, E.; Rosiak, N.; Zalewski, P.; Tajber, L.; Cielecka-Piontek, J. Genistein Co-Amorphous Systems with Amino Acids: An Investigation into Enhanced Solubility and Biological Activity. Pharmaceutics 2023, 15, 2653. [Google Scholar] [CrossRef]
- Quodbach, J.; Preis, E.; Karkossa, F.; Winck, J.; Finke, J.H.; Steiner, D. Novel Strategies for the Formulation of Poorly Water-Soluble Drug Substances by Different Physical Modification Strategies with a Focus on Peroral Applications. Pharmaceuticals 2025, 18, 1089. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zografi, G. Characteristics and Significance of the Amorphous State in Pharmaceutical Systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef]
- Sun, C.C. Decoding Powder Tabletability: Roles of Particle Adhesion and Plasticity. J. Adhes. Sci. Technol. 2011, 25, 483–499. [Google Scholar] [CrossRef]
- Fu, X.; Huck, D.; Makein, L.; Armstrong, B.; Willen, U.; Freeman, T. Effect of Particle Shape and Size on Flow Properties of Lactose Powders. Particuology 2012, 10, 203–208. [Google Scholar] [CrossRef]
- da Costa, N.F.; Daniels, R.; Fernandes, A.I.; Pinto, J.F. Downstream Processing of Amorphous and Co-Amorphous Olanzapine Powder Blends. Pharmaceutics 2022, 14, 1535. [Google Scholar] [CrossRef]
- Mah, P.T.; Novakovic, D.; Saarinen, J.; Van Landeghem, S.; Peltonen, L.; Laaksonen, T.; Strachan, C.J.; Isomäki, A. Elucidation of Compression-Induced Surface Crystallization in Amorphous Tablets Using Sum Frequency Generation (SFG) Microscopy. Pharm. Res. 2017, 34, 957–970. [Google Scholar] [CrossRef]
- Davis, D.A.; Miller, D.A.; Santitewagun, S.; Zeitler, J.A.; Su, Y.; Williams, R.O. Formulating a Heat- and Shear-Labile Drug in an Amorphous Solid Dispersion: Balancing Drug Degradation and Crystallinity. Int. J. Pharm. X 2021, 3, 100092. [Google Scholar] [CrossRef]
- Martynek, D.; Ridvan, L.; Sivén, M.; Šoóš, M. Stability and Recrystallization of Amorphous Solid Dispersions Prepared by Hot-Melt Extrusion and Spray Drying. Int. J. Pharm. 2025, 672, 125331. [Google Scholar] [CrossRef]
- Sip, S.; Rosiak, N.; Sip, A.; Żarowski, M.; Hojan, K.; Cielecka-Piontek, J. A Fisetin Delivery System for Neuroprotection: A Co-Amorphous Dispersion Prepared in Supercritical Carbon Dioxide. Antioxidants 2024, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Pápay, Z.E.; Kállai-Szabó, N.; Ludányi, K.; Klebovich, I.; Antal, I. Development of Oral Site-Specific Pellets Containing Flavonoid Extract with Antioxidant Activity. Eur. J. Pharm. Sci. 2016, 95, 161–169. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and Characterization of an Apigenin-Phospholipid Phytosome (APLC) for Improved Solubility, in Vivo Bioavailability, and Antioxidant Potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef]
- Wu, W.; Zu, Y.; Wang, L.; Wang, L.; Wang, H.; Li, Y.; Wu, M.; Zhao, X.; Fu, Y. Preparation, Characterization and Antitumor Activity Evaluation of Apigenin Nanoparticles by the Liquid Antisolvent Precipitation Technique. Drug Deliv. 2017, 24, 1713–1720. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, L.; Meng, L.; Wang, J.; Zhai, G. Design and Evaluation of a Self-Microemulsifying Drug Delivery System for Apigenin. Drug Dev. Ind. Pharm. 2012, 39, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Stiani, S.N.; Rusdiana, T.; Subarnas, A. Improving Solubility and Dissolution of a Natural Product Apigenin via Preparation of Solid Dispersion by Hot Melt Extrusion. Int. J. Appl. Pharm. 2021, 13, 47–52. [Google Scholar] [CrossRef]
- Rosiak, N.; Tykarska, E.; Miklaszewski, A.; Pietrzak, R.; Cielecka-Piontek, J. Enhancing the Solubility and Dissolution of Apigenin: Solid Dispersions Approach. Int. J. Mol. Sci. 2025, 26, 566. [Google Scholar] [CrossRef]
- Dourado, N.S.; Souza, C.d.S.; de Almeida, M.M.A.; Bispo da Silva, A.; dos Santos, B.L.; Silva, V.D.A.; De Assis, A.M.; da Silva, J.S.; Souza, D.O.; Costa, M.d.F.D.; et al. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in Vitro Models of Neuroinflammation Associated With Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 119. [Google Scholar] [CrossRef]
- Charrière, K.; Schneider, V.; Perrignon-Sommet, M.; Lizard, G.; Benani, A.; Jacquin-Piques, A.; Vejux, A. Exploring the Role of Apigenin in Neuroinflammation: Insights and Implications. Int. J. Mol. Sci. 2024, 25, 5041. [Google Scholar] [CrossRef]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of Apigenin as a Potent Antioxidant with Emphasis on Bioavailability, Metabolism, Action Mechanism and in Vitro and in Vivo Studies: A Review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef]
- Makadia, J.; Seaton, C.C.; Li, M. Apigenin Cocrystals: From Computational Prescreening to Physicochemical Property Characterization. Cryst. Growth Des. 2023, 23, 3480–3495. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Liu, D.; Gao, Y.; Qian, S. Preparation of Apigenin Nanocrystals Using Supercritical Antisolvent Process for Dissolution and Bioavailability Enhancement. Eur. J. Pharm. Sci. 2013, 48, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Sadeghi, F.; Nokhodchi, A.; Varshosaz, J.; Afrasiabi Garekani, H. Preparation and Characterization of Celecoxib Dispersions in Soluplus®: Comparison of Spray Drying and Conventional Methods. Iran. J. Pharm. Res. 2015, 14, 35–50. [Google Scholar] [PubMed]
- Omer, A.B.; Fatima, F.; Ahmed, M.M.; Aldawsari, M.F.; Alalaiwe, A.; Anwer, K.; Mohammed, A.A. Enhanced Apigenin Dissolution and Effectiveness Using Glycyrrhizin Spray-Dried Solid Dispersions Filled in 3D-Printed Tablets. Biomedicines 2023, 11, 3341. [Google Scholar] [CrossRef]
- Skakunova, K.D.; Rychkov, D.A. Low Temperature and High-Pressure Study of Bending L-Leucinium Hydrogen Maleate Crystals. Crystals 2021, 11, 1575. [Google Scholar] [CrossRef]
- Katsyuba, S.A.; Spicher, S.; Gerasimova, T.P.; Grimme, S. Fast and Accurate Quantum Chemical Modeling of Infrared Spectra of Condensed-Phase Systems. J. Phys. Chem. B 2020, 124, 6664–6670. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, W.; Chen, J.; Wang, H.; Zhang, S.; Xiong, S. The Synergetic Effects of Nonpolar and Polar Protic Solvents on the Properties of Felodipine and Soluplus in Solutions, Casting Films, and Spray-Dried Solid Dispersions. J. Pharm. Sci. 2018, 107, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.A.; Neau, S.H. Investigation of the in Vitro Performance Difference of Drug-Soluplus® and Drug-PEG 6000 Dispersions When Prepared Using Spray Drying or Lyophilization. Saudi Pharm. J. 2017, 25, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Aditha, S.K.; Rattan, T.; Kamisetti, V. Aceclofenac-Soluplus® Nanocomposites for Increased Bioavailability. Soft Nanosci. Lett. 2015, 5, 13–20. [Google Scholar] [CrossRef]
- Lan, Y.; Ali, S.; Langley, N. Characterization of Soluplus® by FTIR and Raman Spectroscopy. In Proceedings of the CRS 2010 Annual Conference, Portland, OR, USA, 10–14 July 2010. [Google Scholar]
- Rosiak, N.; Wdowiak, K.; Tykarska, E.; Cielecka-Piontek, J. Amorphous Solid Dispersion of Hesperidin with Polymer Excipients for Enhanced Apparent Solubility as a More Effective Approach to the Treatment of Civilization Diseases. Int. J. Mol. Sci. 2022, 23, 15198. [Google Scholar] [CrossRef]
- Karavas, E.; Koutris, E.; Papadopoulos, A.G.; Sigalas, M.P.; Nanaki, S.; Papageorgiou, G.Z.; Achilias, D.Z.; Bikiaris, D.N. Application of Density Functional Theory in Combination with FTIR and DSC to Characterise Polymer Drug Interactions for the Preparation of Sustained Release Formulations between Fluvastatin and Carrageenans. Int. J. Pharm. 2014, 466, 211–222. [Google Scholar] [CrossRef]
- Jia, S.; Ning, S.; Leng, Y.; Jing, Q.; Xu, Z.; Ren, F. Stabilizing Effect of Soluplus on Erlotinib Metastable Crystal Form in Microparticles and Amorphous Solid Dispersions. Polymers 2022, 14, 1241. [Google Scholar] [CrossRef]
- Lu, J.; Cuellar, K.; Hammer, N.I.; Jo, S.; Gryczke, A.; Kolter, K.; Langley, N.; Repka, M.A. Solid-State Characterization of Felodipine–Soluplus Amorphous Solid Dispersions. Drug Dev. Ind. Pharm. 2016, 42, 485–496. [Google Scholar] [CrossRef]
- Fischer, H.; Kansy, M.; Avdeef, A.; Senner, F. Permeation of Permanently Positive Charged Molecules through Artificial Membranes--Influence of Physico-Chemical Properties. Eur. J. Pharm. Sci. 2007, 31, 32–42. [Google Scholar] [CrossRef]
- Fossatelli, L.; Maroccia, Z.; Fiorentini, C.; Bonucci, M. Resources for Human Health from the Plant Kingdom: The Potential Role of the Flavonoid Apigenin in Cancer Counteraction. Int. J. Mol. Sci. 2024, 25, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics Classification and Intestinal Absorption Study of Apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia (Ph. Eur.), 11th ed.; Supplement 11.8; Council of Europe: Strasbourg, France, 2025. [Google Scholar]
- Zhang, Z.; Cui, C.; Wei, F.; Lv, H. Improved Solubility and Oral Bioavailability of Apigenin via Soluplus/Pluronic F127 Binary Mixed Micelles System. Drug Dev. Ind. Pharm. 2017, 43, 1276–1282. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zu, Y.; Wang, L.; Deng, Y.; Wu, M.; Wang, H. Enhanced Solubility and Bioavailability of Apigenin via Preparation of Solid Dispersions of Mesoporous Silica Nanoparticles. Iran. J. Pharm. Res. 2019, 18, 168–182. [Google Scholar] [PubMed]
- Wu, W.; Zu, Y.; Zhao, X.; Zhang, X.; Wang, L.; Li, Y.; Wang, L.; Zhang, Y.; Lian, B. Solubility and Dissolution Rate Improvement of the Inclusion Complex of Apigenin with 2-Hydroxypropyl-β-Cyclodextrin Prepared Using the Liquid Antisolvent Precipitation and Solvent Removal Combination Methods. Drug Dev. Ind. Pharm. 2017, 43, 1366–1377. [Google Scholar] [CrossRef]
- Ang, S.-S.; Thoo, Y.Y.; Siow, L.F. Encapsulation of Hydrophobic Apigenin into Small Unilamellar Liposomes Coated with Chitosan Through Ethanol Injection and Spray Drying. Food Bioproc. Technol. 2023, 17, 424–439. [Google Scholar] [CrossRef]
- Rosiak, N.; Tykarska, E.; Cielecka-Piontek, J. Amorphous Pterostilbene Delivery Systems Preparation—Innovative Approach to Preparation Optimization. Pharmaceutics 2023, 15, 1231. [Google Scholar] [CrossRef]
- Gurunath, S.; Pradeep Kumar, S.; Basavaraj, N.K.; Patil, P.A. Amorphous Solid Dispersion Method for Improving Oral Bioavailability of Poorly Water-Soluble Drugs. J. Pharm. Res. 2013, 6, 476–480. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, Y.-W.; Tin, Y.-Y.; Soe, M.-T.-P.; Ko, B.-H.; Park, S.-J.; Lee, J.-W. Recent Technologies for Amorphization of Poorly Water-Soluble Drugs. Pharmaceutics 2021, 13, 1318. [Google Scholar] [CrossRef]
- Koromili, M.; Kapourani, A.; Barmpalexis, P. Preparation and Evaluation of Amorphous Solid Dispersions for Enhancing Luteolin’s Solubility in Simulated Saliva. Polymers 2023, 15, 169. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Zhao, R.; Yang, M.; Liu, W.; Dai, Q.; Bao, X.; Chen, Y.; Ma, J. The Amorphous Solid Dispersion of Chrysin in Plasdone® S630 Demonstrates Improved Oral Bioavailability and Antihyperlipidemic Performance in Rats. Pharmaceutics 2023, 15, 2378. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rawat, M.S.M.; Semalty, A.; Semalty, M. Quercetin-Phospholipid Complex: An Amorphous Pharmaceutical System in Herbal Drug Delivery. Curr. Drug Discov. Technol. 2012, 9, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Alhajri, A.; Alshehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef]
- Waheed, A.; Zameer, S.; Ashrafi, K.; Ali, A.; Sultana, N.; Aqil, M.; Sultana, Y.; Iqbal, Z. Insights into Pharmacological Potential of Apigenin through Various Pathways on a Nanoplatform in Multitude of Diseases. Curr. Pharm. Des. 2023, 29, 1326–1340. [Google Scholar] [CrossRef]
- Zulkifli, M.S.A.; Ismail, A.; Loh, S.P.; Jailani, F.; Kassim, N.K. Intestinal Permeability and Transport of Apigenin across Caco-2 Cell Monolayers. J. Food Bioact. 2019, 7, 48–55. [Google Scholar] [CrossRef]
- Sánchez-Marzo, N.; Pérez-Sánchez, A.; Ruiz-Torres, V.; Martínez-Tébar, A.; Castillo, J.; Herranz-López, M.; Barrajón-Catalán, E. Antioxidant and Photoprotective Activity of Apigenin and Its Potassium Salt Derivative in Human Keratinocytes and Absorption in Caco-2 Cell Monolayers. Int. J. Mol. Sci. 2019, 20, 2148. [Google Scholar] [CrossRef]
- Sato, H.; Sangfuang, M.; Nontakham, J.; Junyaprasert, V.B.; Teeranachaideekul, V.; Morakul, B. Enhancement of in Vitro Transcellular Absorption and in Vivo Oral Bioavailability of Apigenin by Self-Nanoemulsifying Drug Delivery Systems. Sci. Rep. 2024, 14, 32148. [Google Scholar] [CrossRef]
- Patole, V.; Gaikwad, P.; Kharat, S.; Jadhav, P.; Deshkar, S.; Giram, P. Green Tea Extract Solid Dispersion Pellets with Enhanced Permeability for Hyperlipidemia. Future Pharmacol. 2023, 3, 708–730. [Google Scholar] [CrossRef]
- Guembe-Michel, N.; Nguewa, P.; González-Gaitano, G. Soluplus®-Based Pharmaceutical Formulations: Recent Advances in Drug Delivery and Biomedical Applications. Int. J. Mol. Sci. 2025, 26, 1499. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, J.D.; Valdés, A.; Gallego, R.; Suárez-Montenegro, Z.J.; Alarcón, M.; Ibañez, E.; Alvarez-Rivera, G.; Cifuentes, A. Blood-Brain Barrier Permeability Study of Potential Neuroprotective Compounds Recovered From Plants and Agri-Food by-Products. Front. Nutr. 2022, 9, 924596. [Google Scholar] [CrossRef]
- Könczöl, Á.; Rendes, K.; Dékány, M.; Müller, J.; Riethmüller, E.; Balogh, G.T. Blood-Brain Barrier Specific Permeability Assay Reveals N-Methylated Tyramine Derivatives in Standardised Leaf Extracts and Herbal Products of Ginkgo Biloba. J. Pharm. Biomed. Anal. 2016, 131, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Ayatollahi, S.A.; As’Habi, M.A.; Kobarfard, F.; Khoramjouy, M.; Boroujeni, F.N.; Faizi, M.; Ghassempour, A. Investigating the Neuroprotective Effects of Dracocephalum Moldavica Extract and Its Effect on Metabolomic Profile of Rat Model of Sporadic Alzheimer’s Disease. Heliyon 2025, 11, e42412. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-Y.; Tsai, M.-S.; Hsu, L.-C.; Lin, S.-W.; Liang, P.-H. Traversal of the Blood–Brain Barrier by Cleavable l-Lysine Conjugates of Apigenin. J. Agric. Food Chem. 2018, 66, 8124–8131. [Google Scholar] [CrossRef] [PubMed]
- Garbiec, E.; Rosiak, N.; Sip, S.; Zalewski, P.; Cielecka-Piontek, J. Curcumin Solubility and Bioactivity Enhancement Through Amorphization with Tryptophan via Supercritical Fluid Technology. Int. J. Mol. Sci. 2025, 26, 855. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, W.; Ye, J.; Hao, H.; Zhou, J.; Wang, R.; Liu, Y. A Novel Matrix Dispersion Based on Phospholipid Complex for Improving Oral Bioavailability of Baicalein: Preparation, in Vitro and in Vivo Evaluations. Drug Deliv. 2017, 24, 720–728. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Monteiro, M.; Resende, D.; Braga, S.S.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Inclusion Complex of Resveratrol with γ-Cyclodextrin as a Functional Ingredient for Lemon Juices. Foods 2020, 10, 16. [Google Scholar] [CrossRef]
- Bayliss, N.; Schmidt, B.V.K.J. Hydrophilic Polymers: Current Trends and Visions for the Future. Prog. Polym. Sci. 2023, 147, 101753. [Google Scholar] [CrossRef]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics 2023, 15, 976. [Google Scholar] [CrossRef]
- Chakrabarti, C.; Pillai, S.A.; Kuperkar, K.; Ray, D.; Aswal, V.K.; Bahadur, P. Phase Behaviour and Characterization of Micelles of Graft Copolymer Soluplus® and Non-Ionic Surfactant Solutol® HS15: A Detailed Comparison in the Presence of Additives. J. Mol. Liq. 2022, 349, 118158. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The Use of Poly(N-Vinyl Pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef]
- Padín-González, E.; Lancaster, P.; Bottini, M.; Gasco, P.; Tran, L.; Fadeel, B.; Wilkins, T.; Monopoli, M.P. Understanding the Role and Impact of Poly (Ethylene Glycol) (PEG) on Nanoparticle Formulation: Implications for COVID-19 Vaccines. Front. Bioeng. Biotechnol. 2022, 10, 882363. [Google Scholar] [CrossRef]
- Stasiłowicz, A.; Tykarska, E.; Lewandowska, K.; Kozak, M.; Miklaszewski, A.; Kobus-Cisowska, J.; Szymanowska, D.; Plech, T.; Jenczyk, J.; Cielecka-Piontek, J. Hydroxypropyl-β-Cyclodextrin as an Effective Carrier of Curcumin—Piperine Nutraceutical System with Improved Enzyme Inhibition Properties. J. Enzym. Inhib. Med. Chem. 2020, 35, 1811–1821. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.P.G.A.; Petersson, G.A.; Nakatsuji, H.J.W.C.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009.
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High Throughput Artificial Membrane Permeability Assay for Blood-Brain Barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E.; Altun, M. Total Antioxidant Capacity Assay of Human Serum Using Copper(II)-Neocuproine as Chromogenic Oxidant: The CUPRAC Method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]



| Compound | Carrier | Solubility (μg/mL) |
|---|---|---|
| Apigenin | - | <1 b |
| Soluplus (SOL) | 6455.4 ± 27.2 a | |
| Neusilin US2 | 1.3 ± 0.1 b | |
| PEG 6000 | 1.5 ± 0.1 b | |
| HPMC | 10.4 ± 0.4 b | |
| Polyvinyl alcohol | 4.8 ± 0.3 b | |
| PVP covinyl acetate | 5.2 ± 0.2 b | |
| Vivapharm PVP K25F | 1.4 ± 0.1 b | |
| Kollidon 30 | <1 b | |
| Kollidon VA64 | 9.6 ± 0.4 b | |
| HP-α-CD | <1 b | |
| HP-β-CD | 1.0 ± 0.1 b | |
| HP-γ-CD | 2.9 ± 0.2 b |
| AP | Sol [cm−1] | AP–Sol System [cm−1] | Band Assignment |
|---|---|---|---|
| 428 | |||
| 453 | δCCC | ||
| 473 | * | δCOC + δCCC + δOCC | |
| 501 | γCCOC + γOCCC | ||
| 579 | γOCCC | ||
| 633 | γHOC + τHCCC | ||
| 669 | γOCCC | ||
| 692 | δCCC + γOCCC | ||
| 737 | τHCCC + γOCCC | ||
| 806 | τHCCC | ||
| 827 | τCCCC | ||
| 908 | τHCCC + τCCCC | ||
| 1018 | 1022 | no information in the literature | |
| 1053 | 1049 | no information in the literature | |
| 1148 | 1152 | no information in the literature | |
| 1177 | 1182, | δHOC + δHCC | |
| 1196 | no information in the literature | ||
| 1221 | * | νCC + δHOC + δHCC | |
| 1234 | 1236 | νCOC in the ether groups | |
| 1244 | * | δHCC + νCC | |
| 1269 | * | νOC + δHCC | |
| 1352 | 1354 | no information in the literature | |
| 1369 | 1364 | O(C)O or NH | |
| 1400 | * | δHOC + δHCC | |
| 1495 | 1503, | δHCC + νCC | |
| 1557 | νCC + δHOC | ||
| 1587 | * | νCC + νOC | |
| 1605 | * | νOC + νCC | |
| 1651 | * | νOC + νCC | |
| 2862 | 2859 | νCH |
| DPPH | ABTS | CUPRAC | |
|---|---|---|---|
| AP | <0.1% c | <0.1% b | <0.01 c |
| AP–SOL ph.m. | 12.3 ± 0.9% b | 85.2 ± 2.4% a | 0.51 ± 0.03 b |
| AP–SOL system | 27.9 ± 0.7% a | 84.9 ± 3.1% a | >1.00 a |
| IC50/IC0.5 | 27.7 ± 0.6 mg/mL | 59.0 ± 3.1 μg/mL | 0.91 ± 0.02 mg/mL |
| Trolox IC50/IC0.5 | 92.04 ± 1.37 μg/mL | 118.72 ± 3.87 μg/mL | 56.15 ± 0.79 μg/mL |
| Name | Temperature (°C) | Pressure (PSI) |
|---|---|---|
| AP–SOL-1 | 50 | 5000 |
| AP–SOL-2 | 65 | 5000 |
| AP–SOL-3 | 80 | 5000 |
| AP–SOL-4 | 50 | 6500 |
| AP–SOL-5 | 65 | 6500 |
| AP–SOL-6 | 80 | 6500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiłowicz-Krzemień, A.; Rosiak, N.; Racaniello, G.F.; Denora, N.; Cielecka-Piontek, J. Effective and Stable Senomorphic Apigenin Delivery System Obtained by Supercritical Carbon Dioxide Processing. Int. J. Mol. Sci. 2025, 26, 8126. https://doi.org/10.3390/ijms26178126
Stasiłowicz-Krzemień A, Rosiak N, Racaniello GF, Denora N, Cielecka-Piontek J. Effective and Stable Senomorphic Apigenin Delivery System Obtained by Supercritical Carbon Dioxide Processing. International Journal of Molecular Sciences. 2025; 26(17):8126. https://doi.org/10.3390/ijms26178126
Chicago/Turabian StyleStasiłowicz-Krzemień, Anna, Natalia Rosiak, Giuseppe Francesco Racaniello, Nunzio Denora, and Judyta Cielecka-Piontek. 2025. "Effective and Stable Senomorphic Apigenin Delivery System Obtained by Supercritical Carbon Dioxide Processing" International Journal of Molecular Sciences 26, no. 17: 8126. https://doi.org/10.3390/ijms26178126
APA StyleStasiłowicz-Krzemień, A., Rosiak, N., Racaniello, G. F., Denora, N., & Cielecka-Piontek, J. (2025). Effective and Stable Senomorphic Apigenin Delivery System Obtained by Supercritical Carbon Dioxide Processing. International Journal of Molecular Sciences, 26(17), 8126. https://doi.org/10.3390/ijms26178126











