CRISPR/Cas-Mediated Optimization of Soybean Shoot Architecture for Enhanced Yield
Abstract
1. Introduction
2. Evolution of CRISPR/Cas Technology
2.1. From Prokaryotic Immunity to Programmable Nucleases
2.2. Diversifying the CRISPR Toolbox
2.3. Adaptation to Plant Genomes
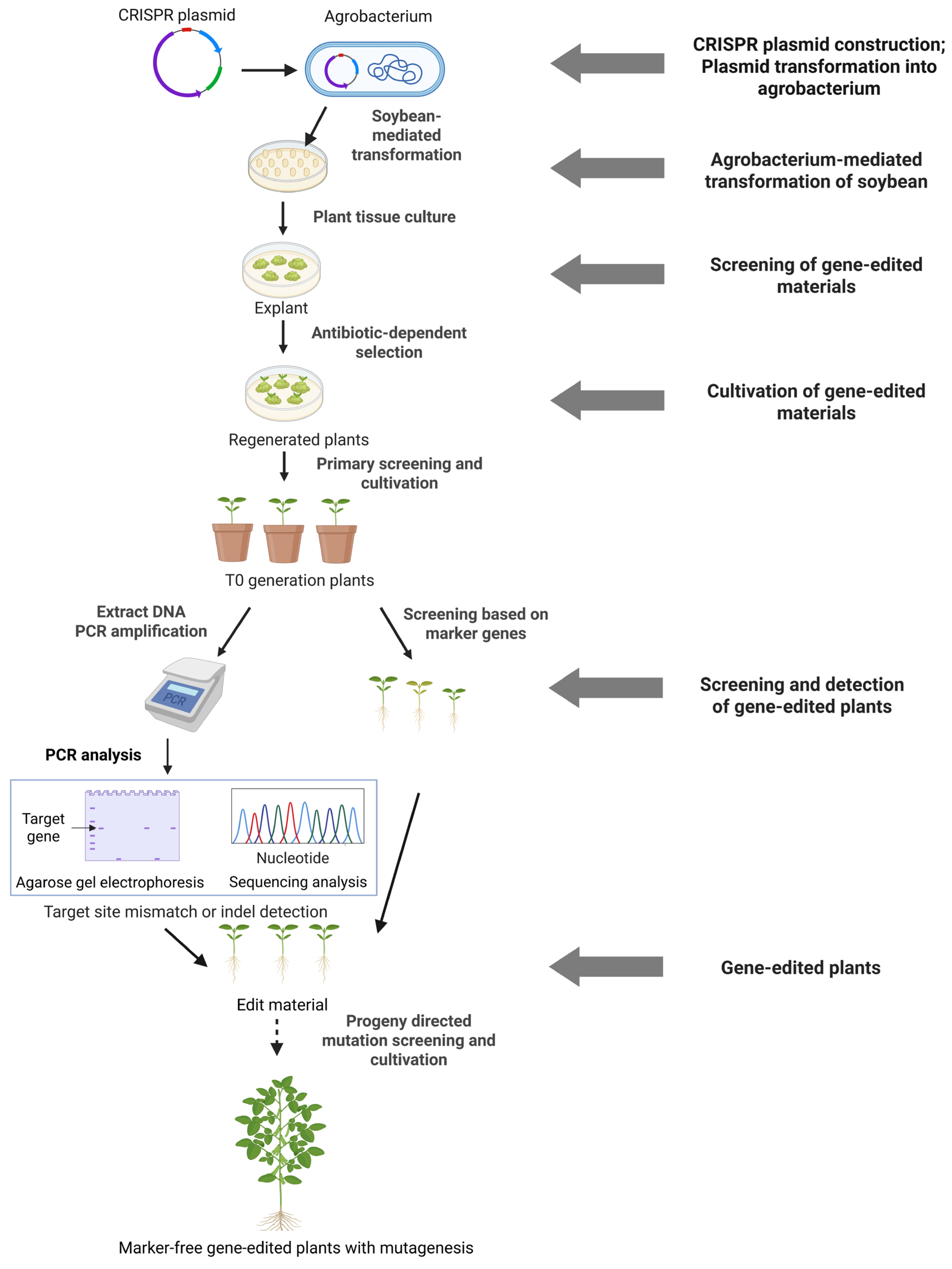
3. Applications of CRISPR/Cas in Crop Improvement
4. Broad Applications of CRISPR/Cas in Soybean Improvement
| Trait Category | Target Gene(s) | Modification | Key Phenotypic Outcome(s) | Reference |
|---|---|---|---|---|
| Abiotic Stress Resistance | ||||
| Drought resistance | GmHdz4 | Knockout | Enhanced drought tolerance | Zhong et al. [83] |
| Salt tolerance | GmAITR2, GmAITR3, GmAITR4, GmAITR5, GmAITR6 | Multiplex knockout | Enhanced salt tolerance (germination, seedling, and field) | Wang et al. [84] |
| Salt tolerance/quality improvement | GmCG-1, GmCG-2, GmCG-3 | Knockdown | Reduced β-conglycinin; increased protein and sulfur-amino acids; enhanced salt tolerance (germination/seedling) | Yang et al. [85] |
| Multiple stress tolerance | GmARM | Knockout | Enhanced tolerance to salt, alkali, and pathogens | Luo et al. [95] |
| Biotic Stress Resistance | ||||
| Soybean mosaic virus resistance | GmF3H1 GmF3H2 GmFNSII-1 | Multiplex knockout | Enhanced resistance to soybean mosaic virus | Zhang et al. [87] |
| Powdery mildew resistance | GmMLO02 GmMLO19 GmMLO20 GmMLO23 | Multiplex knockout | Enhanced powdery mildew resistance | Bui et al. [86] |
| Chewing insects resistance | GmUGT | Knockout | Enhanced resistance to cotton bollworm and armyworm | Zhang et al. [88] |
| Root rot disease resistance | GmTAP1 | Knockout | Enhanced resistance to multiple Phytophthora sojae biotypes | Liu et al. [96] |
| Soybean cyst nematode resistance | GmSNAP02 | Knockout | Enhanced resistance to cyst nematodes | Usovsky et al. [97] |
| Insect resistance/flowering time regulation | GmCDPK38 | Knockout | Delayed flowering and enhanced resistance to Spodoptera litura | Li et al. [98] |
| Soybean Quality Improvement | ||||
| Increased Oil Content | GmSWEET10a, GmSWEET10b | Gene editing (informed by AlphaFold) | Increased oil content | Wang et al. [92] |
| GmSFAR4a, GmSFAR4b | Knockout | Increased oil content | Liao et al. [99] | |
| Removal of beany flavor | GmLox1, GmLox2, GmLox3 | Multiplex knockout | Elimination of beany flavor | Wang et al. [90] |
| Reduced soy allergenicity | GmP34, GmP34h1, GmP34h2 | Multiplex knockout | Reduced GmP34 allergen potential | Baek et al. [100] |
| Reduce anti-nutritional factors | GmRS2 GmRS3 | Multiplex knockout | Reduced raffinose family oligosaccharides | Cao et al. [91] |
| Other | ||||
| Pod shattering resistance | GmPDH1 | Knockout | Increased pod-shattering resistance | Zhang et al. [101] |
| Increased seed size | GmEOD1 | Knockout | Increased seed size and hundred-seed weight | Yu, et al. [93] |
| Herbicide tolerance | GmALS1, GmALS3 | Base editing (C-to-T) | Increased herbicide resistance without yield penalty | Niu et al. [102] |
| Enhanced symbiotic nitrogen fixation | GmRIC1, GmRIC2 | Knockout | Increased nodule number; balanced C/N allocation | Zhong, et al. [94] |
5. CRISPR/Cas Applications in Soybean Shoot Architectural Improvement
5.1. Precision Engineering of Node Number
5.2. Precision Engineering of Internode Length
5.3. Precision Editing of Branching Patterns
5.4. Precision Editing of Leaf Structure and Petiole Angle
6. Challenges and Future Directions
6.1. Future Directions of CRISPR in Soybean Plant Architecture Regulation
6.2. Technical Challenges of CRISPR in Soybean
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, H.; Li, D.; Yang, R.; Zhang, L.; Zhang, Y.; Liu, X.; Kong, X.; Sun, J. GSK3 phosphorylates and regulates the Green Revolution protein Rht-B1b to reduce plant height in wheat. Plant Cell 2023, 35, 1970–1983. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, W.; Yamauchi, T.; Tsuda, K. Genetic basis controlling rice plant architecture and its modification for breeding. Breed. Sci. 2023, 73, 3–45. [Google Scholar] [CrossRef]
- Song, L.; Liu, J.; Cao, B.; Liu, B.; Zhang, X.; Chen, Z.; Dong, C.; Liu, X.; Zhang, Z.; Wang, W.; et al. Reducing brassinosteroid signalling enhances grain yield in semi-dwarf wheat. Nature 2023, 617, 118–124. [Google Scholar] [CrossRef]
- Jian, C.; Hao, P.; Hao, C.; Liu, S.; Mao, H.; Song, Q.; Zhou, Y.; Yin, S.; Hou, J.; Zhang, W.; et al. The miR319/TaGAMYB3 module regulates plant architecture and improves grain yield in common wheat (Triticum aestivum). New Phytol. 2022, 235, 1515–1530. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, X.; Gong, G.; Zhang, G.; Zhao, H.; Zheng, Z.; Wang, C.; Zhu, H.; Huang, J.; Li, Z.; et al. qGLF5 from Oryza rufipogon Griff. improves kernel shape, plant architecture, and yield in rice. Theor. Appl. Genet. 2023, 136, 225. [Google Scholar] [CrossRef]
- Liu, X.; Ji, P.; Liao, J.; Duan, X.; Luo, Z.; Yu, X.; Jiang, C.J.; Xu, C.; Yang, H.; Peng, B.; et al. CRISPR/Cas knockout of the NADPH oxidase gene OsRbohB reduces ROS overaccumulation and enhances heat stress tolerance in rice. Plant Biotechnol. J. 2025, 23, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, S.; Liang, Y.; Zhang, R.; Liu, L.; Qin, P.; Zhang, Z.; Wang, Y.; Zhou, J.; Tang, X.; et al. Loss-function mutants of OsCKX gene family based on CRISPR-Cas systems revealed their diversified roles in rice. Plant Genome 2023, 16, e20283. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, P.; Gui, J. Advancements of CRISPR-Mediated Base Editing in Crops and Potential Applications in Populus. Int. J. Mol. Sci. 2024, 25, 8314. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Singh, A.K.; Behera, T.K. CRISPR/Cas genome editing in tomato improvement: Advances and applications. Front. Plant Sci. 2023, 14, 1121209. [Google Scholar] [CrossRef]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef]
- Jansen, R.; van Embden, J.D.A.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef]
- Mojica, F.J.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 2017, 37, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Slaymaker, I.M.; Gao, L.; Zetsche, B.; Scott, D.A.; Yan, W.X.; Zhang, F. Rationally engineered Cas9 nucleases with improved specificity. Science 2016, 351, 84–88. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Ren, J.; Meng, X.; Hu, F.; Liu, Q.; Cao, Y.; Li, H.; Yan, C.; Li, J.; Wang, K.; Yu, H.; et al. Expanding the scope of genome editing with SpG and SpRY variants in rice. Sci. China Life Sci. 2021, 64, 1784–1787. [Google Scholar] [CrossRef]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Ye, L.; Qin, L.; Liu, X.; He, Y.; Wang, J.; Chen, R.; Lu, Y. CRISPR-Act3.0: Expanding the scope of genome regulation in plants. Nat. Plants 2021, 7, 1024–1036. [Google Scholar]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef]
- Xiang, W.; Lin, X.; Yang, Y.; Huang, L.; Chen, Y.; Chen, J.; Liu, L. Cas12h is a crRNA-guided DNA nickase that can be utilized for precise gene editing. Cell Rep. 2025, 44, 115718. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.T.; Kellner, M.J.; Regev, A.; et al. RNA targeting with CRISPR-Cas13. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Rajan, V.; Prykhozhij, S.V.; Berman, J.N.; Ignacimuthu, S. Insert, remove or replace: A highly advanced genome editing system using CRISPR/Cas9. Biochim. Biophys. Acta. 2016, 1863, 2333–2344. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, D.R.; Ramírez-Solís, R.; Garza-Elizondo, M.A.; Garza-Rodríguez, M.D.L.; Barrera-Saldaña, H.A. Genome editing: A perspective on the application of CRISPR/Cas9 to study human diseases (Review). Int. J. Mol. Med. 2019, 43, 1559–1574. [Google Scholar] [CrossRef]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.S.; Li, C.; Nguyen, H.; Liu, B.; et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef]
- Wang, W.; Simmonds, J.; Pan, Q.; Davidson, D.; He, F.; Battal, A.; Akhunova, A.; Trick, H.N.; Uauy, C.; Akhunov, E. Gene editing and mutagenesis reveal inter-cultivar differences and additivity in the contribution of TaGW2 homoeologues to grain size and weight in wheat. TAG Theor. Appl. Genet. 2018, 131, 2463–2475. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Tsai, S.Q.; Prew, M.S.; Nguyen, N.T.; Welch, M.M.; Lopez, J.M.; McCaw, Z.R.; Aryee, M.J.; Joung, J.K. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat. Biotechnol. 2016, 34, 869–874. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Hur, J.K.; Been, K.W.; Yoon, S.H.; Kim, J.S. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat. Biotechnol. 2016, 34, 863–868, Erratum in Nat. Biotechnol. 2016, 34, 888. https://doi.org/10.1038/nbt0816-888a. [Google Scholar] [CrossRef] [PubMed]
- Hillary, V.E.; Ignacimuthu, S.; Ceasar, S.A. Potential of CRISPR/Cas system in the diagnosis of COVID-19 infection. Expert Rev. Mol. Diagn. 2021, 21, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Yin, J.; Yuan, S.; Ou, L.; Liu, M.; Zhang, W.; Hu, J. Comprehensive assessment of miniature CRISPR-Cas12f nucleases for gene disruption. Nat. Commun. 2022, 13, 5623. [Google Scholar] [CrossRef]
- Endo, A.; Masafumi, M.; Kaya, H.; Toki, S. Efficient targeted mutagenesis of rice and tobacco genomes using Cpf1 from Francisella novicida. Sci. Rep. 2016, 6, 38169. [Google Scholar] [CrossRef]
- Ming, M.; Ren, Q.; Pan, C.; He, Y.; Zhang, Y.; Liu, S.; Zhong, Z.; Wang, J.; Malzahn, A.A.; Wu, J.; et al. CRISPR–Cas12b enables efficient plant genome engineering. Nat. Plants 2020, 6, 202–208. [Google Scholar] [CrossRef]
- Smargon, A.A.; Cox, D.B.T.; Pyzocha, N.K.; Zheng, K.; Slaymaker, I.M.; Gootenberg, J.S.; Abudayyeh, O.A.; Essletzbichler, P.; Shmakov, S.; Makarova, K.S.; et al. Cas13b Is a Type VI-B CRISPR-Associated RNA-Guided RNase Differentially Regulated by Accessory Proteins Csx27 and Csx28. Mol. Cell 2017, 65, 618–630.e7. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zou, J.; Hussain, A.; Jia, R.; Fan, Y.; Liu, J.; Nie, X.; Zhang, X.; Jin, S. Systemic evaluation of various CRISPR/Cas13 orthologs for knockdown of targeted transcripts in plants. Genome Biol. 2024, 25, 307. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788, Erratum in Nat. Rev. Genet. 2018, 19, 801. https://doi.org/10.1038/s41576-018-0068-0. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Huang, X. Base editors: A powerful tool for generating animal models of human diseases. Cell Stress 2018, 2, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, X.H.; Li, D.L. The “new favorite” of gene editing technology-single base editors. Yi Chuan 2017, 39, 1115–1121. [Google Scholar]
- Zong, Y.; Wang, Y.; Li, C.; Zhang, R.; Chen, K.; Ran, Y.; Qiu, J.L.; Wang, D.; Gao, C. Author Correction: Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat. Biotechnol. 2025, 43, 1011. [Google Scholar] [CrossRef]
- Fan, T.; Cheng, Y.; Wu, Y.; Liu, S.; Tang, X.; He, Y.; Liao, S.; Zheng, X.; Zhang, T.; Qi, Y.; et al. High performance TadA-8e derived cytosine and dual base editors with undetectable off-target effects in plants. Nat. Commun. 2024, 15, 5103. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef]
- Kim, H.K.; Yu, G.; Park, J.; Min, S.; Lee, S.; Yoon, S.; Kim, H.H. Predicting the efficiency of prime editing guide RNAs in human cells. Nat. Biotechnol. 2021, 39, 198–206, Erratum in Nat. Biotechnol. 2024, 42, 529. https://doi.org/10.1038/s41587-024-02159-6. [Google Scholar] [CrossRef]
- Xu, R.; Liu, X.; Li, J.; Qin, R.; Wei, P. Identification of herbicide resistance OsACC1 mutations via in planta prime-editing-library screening in rice. Nat. Plants 2021, 7, 888–892. [Google Scholar] [CrossRef]
- Liang, R.; Wang, S.; Cai, Y.; Li, Z.; Li, K.M.; Wei, J.; Sun, C.; Zhu, H.; Chen, K.; Gao, C. Circular RNA-mediated inverse prime editing in human cells. Nat. Commun. 2025, 16, 5057. [Google Scholar] [CrossRef]
- Yang, C.; Fang, Q.; Li, M.; Zhang, J.; Li, R.; Zhou, T.; Wang, K.; Deng, J.; Wang, X.; Huang, C.; et al. Prime editor with rational design and AI-driven optimization for reverse editing window and enhanced fidelity. Nat. Commun. 2025, 16, 5144. [Google Scholar] [CrossRef]
- Karlson, C.K.S.; Mohd-Noor, S.N.; Nolte, N.; Tan, B.C. CRISPR/dCas9-Based Systems: Mechanisms and Applications in Plant Sciences. Plants 2021, 10, 2055. [Google Scholar] [CrossRef]
- Chen, B.; Gilbert, L.A.; Cimini, B.A.; Schnitzbauer, J.; Zhang, W.; Li, G.W.; Park, J.; Blackburn, E.H.; Weissman, J.S.; Qi, L.S.; et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 2013, 155, 1479–1491. [Google Scholar] [CrossRef]
- Adamson, B.; Smogorzewska, A.; Sigoillot, F.D.; King, R.W.; Elledge, S.J. A genome-wide homologous recombination screen identifies the RNA-binding protein RBMX as a component of the DNA-damage response. Nat. Cell Biol. 2012, 14, 318–328. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Y.; Zheng, J.; Zhai, H.; He, S.; Zhang, H.; Zhao, N.; Liu, Q.; Gao, S. Haploid induction in sweet potato by activating the AP2/ERF family transcription factor IbBBM. Plant Biotechnol. J. 2025, 23, 3113–3115. [Google Scholar] [CrossRef]
- Bai, M.; Yuan, J.; Kuang, H.; Gong, P.; Li, S.; Zhang, Z.; Liu, B.; Sun, J.; Yang, M.; Yang, L.; et al. Generation of a multiplex mutagenesis population via pooled CRISPR-Cas9 in soya bean. Plant Biotechnol. J. 2020, 18, 721–731. [Google Scholar] [CrossRef]
- Liu, H.J.; Jian, L.; Xu, J.; Zhang, Q.; Zhang, M.; Jin, M.; Peng, Y.; Yan, J.; Han, B.; Liu, J.; et al. High-Throughput CRISPR/Cas9 Mutagenesis Streamlines Trait Gene Identification in Maize. Plant Cell 2020, 32, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Sukegawa, S.; Endo, M.; Hara, N.; Nureki, O.; Saika, H.; Toki, S. Systemic delivery of engineered compact AsCas12f by a positive-strand RNA virus vector enables highly efficient targeted mutagenesis in plants. Front. Plant Sci. 2024, 15, 1454554. [Google Scholar] [CrossRef]
- Pan, C.; Li, G.; Malzahn, A.A.; Cheng, Y.; Leyson, B.; Sretenovic, S.; Gurel, F.; Coleman, G.D.; Qi, Y. Boosting plant genome editing with a versatile CRISPR-Combo system. Nat. Plants 2022, 8, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J.; et al. Morphogenic Regulators Baby boom and Wuschel Improve Monocot Transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shi, L.; Liang, X.; Zhao, P.; Wang, W.; Liu, J.; Chang, Y.; Hiei, Y.; Yanagihara, C.; Du, L.; et al. The gene TaWOX5 overcomes genotype dependency in wheat genetic transformation. Nat. Plants 2022, 8, 110–117, Erratum in Nat. Plants 2022, 8, 717–720. https://doi.org/10.1038/s41477-022-01173-3. [Google Scholar] [CrossRef]
- Subburaj, S.; Zanatta, C.B.; Nunn, J.A.L.; Hoepers, A.M.; Nodari, R.O.; Agapito-Tenfen, S.Z. A DNA-Free Editing Platform for Genetic Screens in Soybean via CRISPR/Cas9 Ribonucleoprotein Delivery. Front. Plant Sci. 2022, 13, 939997. [Google Scholar] [CrossRef]
- Mahas, A.; Ali, Z.; Tashkandi, M.; Mahfouz, M.M. Virus-Mediated Genome Editing in Plants Using the CRISPR/Cas9 System. Methods Mol. Biol. 2019, 1917, 311–326. [Google Scholar]
- Vu, T.V.; Sivankalyani, V.; Kim, E.J.; Doan, D.T.H.; Tran, M.T.; Kim, J.; Sung, Y.W.; Park, M.; Kang, Y.J.; Kim, J.Y. Highly efficient homology-directed repair using CRISPR/Cpf1-geminiviral replicon in tomato. Plant Biotechnol. J. 2020, 18, 2133–2143. [Google Scholar] [CrossRef]
- Dahan-Meir, T.; Filler-Hayut, S.; Melamed-Bessudo, C.; Bocobza, S.; Czosnek, H.; Aharoni, A.; Levy, A.A. Efficient in planta gene targeting in tomato using geminiviral replicons and the CRISPR/Cas9 system. Plant J. 2018, 95, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xie, H.; Song, M.; Lu, J.; Ma, P.; Huang, B.; Wang, M.; Tian, Y.; Chen, F.; Peng, J.; et al. Cut-dip-budding delivery system enables genetic modifications in plants without tissue culture. Innovation 2022, 4, 100345. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Bhattacharjee, O.; Mandal, D.; Sen, M.K.; Dey, D.; Dasgupta, A.; Kazi, T.A.; Gupta, R.; Sinharoy, S.; Acharya, K.; et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci. 2019, 232, 116636. [Google Scholar] [CrossRef]
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas System: Recent Advances and Future Prospects for Genome Editing. Trends Plant Sci. 2019, 24, 1102–1125. [Google Scholar] [CrossRef]
- Manghwar, H.; Li, B.; Ding, X.; Hussain, A.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas Systems in Genome Editing: Methodologies and Tools for sgRNA Design, Off-Target Evaluation, and Strategies to Mitigate Off-Target Effects. Adv. Sci. 2020, 7, 1902312. [Google Scholar] [CrossRef]
- Chakrabarti, A.M.; Henser-Brownhill, T.; Monserrat, J.; Poetsch, A.R.; Luscombe, N.M.; Scaffidi, P. Target-Specific Precision of CRISPR-Mediated Genome Editing. Mol. Cell 2019, 73, 699–713.e6. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- He, F.; Wang, C.; Sun, H.; Tian, S.; Zhao, G.; Liu, C.; Wan, C.; Guo, J.; Huang, X.; Zhan, G.; et al. Simultaneous editing of three homoeologues of TaCIPK14 confers broad-spectrum resistance to stripe rust in wheat. Plant Biotechnol. J. 2023, 21, 354–368. [Google Scholar] [CrossRef]
- Wang, H.; Chen, M.; Zhang, D.; Meng, X.; Yan, J.; Chu, J.; Li, J.; Yu, H. Shaping rice Green Revolution traits by engineering ATG immediate upstream 5’-UTR sequences of OsSBI and OsHTD1. Plant Biotechnol. J. 2024, 22, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Je, B.I.; Xu, F.; Wu, Q.; Liu, L.; Meeley, R.; Gallagher, J.P.; Corcilius, L.; Payne, R.J.; Bartlet, M.E.; Jackson, D. The CLAVATA receptor FASCIATED EAR2 responds to distinct CLE peptides by signaling through two downstream effectors. Elife 2018, 7, e35673. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Toro, D.M.; de Folter, S.; Alvarez-Venegas, R. CRISPR/dCas12a-mediated activation of SlPAL2 enhances tomato resistance against bacterial canker disease. PLoS ONE 2025, 20, e0320436. [Google Scholar] [CrossRef]
- Yao, T.; Yuan, G.; Lu, H.; Liu, Y.; Zhang, J.; Tuskan, G.A.; Muchero, W.; Chen, J.G.; Yang, X. CRISPR/Cas9-based gene activation and base editing in Populus. Hortic. Res. 2023, 10, uhad085. [Google Scholar] [CrossRef]
- Jacobs, T.B.; LaFayette, P.R.; Schmitz, R.J.; Parrott, W.A. Targeted genome modifications in soybean with CRISPR/Cas9. BMC Biotechnol. 2015, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, L.; Liu, X.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. CRISPR/Cas9-Mediated Genome Editing in Soybean Hairy Roots. PLoS ONE 2015, 10, e0136064. [Google Scholar] [CrossRef]
- Sun, X.; Hu, Z.; Chen, R.; Jiang, Q.; Song, G.; Zhang, H.; Xi, Y. Targeted mutagenesis in soybean using the CRISPR-Cas9 system. Sci. Rep. 2015, 5, 10342. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Hong, W.; Shu, Y.; Li, J.; Liu, L.; Chen, X.; Islam, F.; Zhou, W.; Tang, G. CRISPR/Cas9 mediated gene-editing of GmHdz4 transcription factor enhances drought tolerance in soybean (Glycine max [L.] Merr.). Front. Plant Sci. 2022, 13, 988505. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xun, H.; Wang, W.; Ding, X.; Tian, H.; Hussain, S.; Dong, Q.; Li, Y.; Cheng, Y.; Wang, C.; et al. Mutation of GmAITR Genes by CRISPR/Cas9 Genome Editing Results in Enhanced Salinity Stress Tolerance in Soybean. Front. Plant Sci. 2021, 12, 779598. [Google Scholar] [CrossRef]
- Yang, R.; Ma, Y.; Yang, Z.; Pu, Y.; Liu, M.; Du, J.; Xu, Z.; Xu, Z.; Zhang, S.; Zhang, H.; et al. Knockdown of β-conglycinin α’ and α subunits alters seed protein composition and improves salt tolerance in soybean. Plant J. 2024, 120, 1488–1507. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.; Le, H.; Ta, D.T.; Nguyen, C.X.; Le, N.T.; Tran, T.T.; Van Nguyen, P.; Stacey, G.; Stacey, M.G.; Pham, N.B.; et al. Enhancing powdery mildew resistance in soybean by targeted mutation of MLO genes using the CRISPR/Cas9 system. BMC Plant Biol. 2023, 23, 533. [Google Scholar] [CrossRef]
- Zhang, P.; Du, H.; Wang, J.; Pu, Y.; Yang, C.; Yan, R.; Yang, H.; Cheng, H.; Yu, D. Multiplex CRISPR/Cas9-mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnol. J. 2020, 18, 1384–1395. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Chen, L.; Shen, X.; Yang, H.; Fang, Y.; Ouyang, W.; Mai, S.; Chen, H.; Chen, S.; et al. CRISPR/Cas9-Mediated Targeted Mutagenesis of GmUGT Enhanced Soybean Resistance Against Leaf-Chewing Insects Through Flavonoids Biosynthesis. Front. Plant Sci. 2022, 13, 802716. [Google Scholar] [CrossRef]
- Do, P.T.; Nguyen, C.X.; Bui, H.T.; Tran, L.T.N.; Stacey, G.; Gillman, J.D.; Zhang, Z.J.; Stacey, M.G. Demonstration of highly efficient dual gRNA CRISPR/Cas9 editing of the homeologous GmFAD2-1A and GmFAD2-1B genes to yield a high oleic, low linoleic and α-linolenic acid phenotype in soybean. BMC Plant Biol. 2019, 19, 311. [Google Scholar] [CrossRef]
- Wang, J.; Kuang, H.; Zhang, Z.; Yan, L.; Guo, Y.; Zhu, S.; Chen, S. Generation of seed lipoxygenase-free soybean using CRISPR/Cas9. Crop J. 2020, 8, 432–439. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Z.; Ma, H.; Liu, T.; Ji, J.; Duan, K. Multiplex CRISPR/Cas9-mediated raffinose synthase gene editing reduces raffinose family oligosaccharides in soybean. Front. Plant Sci. 2022, 13, 1048967. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Wang, S.; Wang, X.; Li, S.; Gong, P.; Bai, M.; Paul, A.; Tvedt, N.; Ren, H.; et al. AlphaFold-Guided Bespoke Gene Editing Enhances Field-Grown Soybean Oil Contents. Adv. Sci. 2025, 12, e2500290. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhao, J.; Chen, L.; Wu, T.; Jiang, B.; Xu, C.; Cai, Y.; Dong, J.; Han, T.; Sun, S.; et al. CRISPR/Cas9-Mediated Targeted Mutagenesis of GmEOD1 Enhances Seed Size of Soybean. Agronomy 2023, 13, 2359. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, J.; Shi, X.; Bai, M.; Yuan, C.; Cai, C.; Wang, N.; Zhu, X.; Kuang, H.; Wang, X.; et al. Genetically optimizing soybean nodulation improves yield and protein content. Nat. Plants 2024, 10, 736–742. [Google Scholar] [CrossRef]
- Luo, T.; Ma, C.; Fan, Y.; Qiu, Z.; Li, M.; Tian, Y.; Shang, Y.; Liu, C.; Cao, Q.; Peng, Y.; et al. CRISPR-Cas9-mediated editing of GmARM improves resistance to multiple stresses in soybean. Plant Sci. 2024, 346, 112147. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ji, J.; Cheng, Y.; Zhang, S.; Wang, Z.; Duan, K.; Wang, Y. CRISPR/Cas9-mediated editing of GmTAP1 confers enhanced resistance to Phytophthora sojae in soybean. J. Integr. Plant Biol. 2023, 65, 1609–1612. [Google Scholar] [CrossRef]
- Usovsky, M.; Gamage, V.A.; Meinhardt, C.G.; Dietz, N.; Triller, M.; Basnet, P.; Gillman, J.D.; Bilyeu, K.D.; Song, Q.; Dhital, B.; et al. Loss-of-function of an α-SNAP gene confers resistance to soybean cyst nematode. Nat. Commun. 2023, 14, 7629. [Google Scholar] [CrossRef]
- Li, X.; Hu, D.; Cai, L.; Wang, H.; Liu, X.; Du, H.; Yang, Z.; Zhang, H.; Hu, Z.; Huang, F.; et al. CALCIUM- DEPENDENT PROTEIN KINASE38 regulates flowering time and common cutworm resistance in soybean. Plant Physiol. 2022, 190, 480–499. [Google Scholar] [CrossRef]
- Liao, W.; Guo, R.; Li, J.; Liu, N.; Jiang, L.; Whelan, J.; Shou, H. CRISPR/Cas9-mediated mutagenesis of SEED FATTY ACID REDUCER genes significantly increased seed oil content in soybean. Plant Cell Physiol. 2025, 66, 273–284. [Google Scholar] [CrossRef]
- Baek, D.; Jin, B.J.; Park, M.S.; Cha, Y.J.; Han, T.H.; Jang, Y.N.; Bin Kim, S.; Shim, S.I.; Chung, J.-I.; Chun, H.J.; et al. CRISPR/Cas9-mediated simultaneous targeting of GmP34 and its homologs produces T-DNA-free soybean mutants with reduced allergenic potential. Front. Plant Sci. 2025, 16, 1612747. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Kuang, H.; Hou, Z.; Gong, P.; Bai, M.; Zhou, S.; Yao, X.; Song, S.; Yan, L.; et al. Elimination of an unfavorable allele conferring pod shattering in an elite soybean cultivar by CRISPR/Cas9. aBIOTECH 2022, 3, 110–114. [Google Scholar] [CrossRef]
- Niu, Q.; Xie, H.; Cao, X.; Song, M.; Wang, X.; Li, S.; Pang, K.; Zhang, Y.; Zhu, J.K.; Zhu, J. Engineering soybean with high levels of herbicide resistance with a Cas12-SF01-based cytosine base editor. Plant Biotechnol. J. 2024, 22, 2435–2437. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Bian, Y.; Luo, X.; Jia, C.; Hao, Q.; Tian, X.; Cao, Q.; Chen, W.; Ma, W.; Ni, Z.; et al. Dissecting pleiotropic functions of the wheat Green Revolution gene Rht-B1b in plant morphogenesis and yield formation. Development 2023, 150, dev201601. [Google Scholar] [CrossRef]
- Hou, Z.; Huang, H.; Wang, Y.; Chen, L.; Yue, L.; Liu, B.; Kong, F.; Yang, H. Molecular Regulation of Shoot Architecture in Soybean. Plant Cell Environ. 2024. [Google Scholar] [CrossRef]
- Kim, J.H.; Scaboo, A.; Pantalone, V.; Li, Z.; Bilyeu, K. Utilization of Plant Architecture Genes in Soybean to Positively Impact Adaptation to High Yield Environments. Front. Plant Sci. 2022, 13, 891587. [Google Scholar] [CrossRef]
- Lin, X.; Liu, B.; Weller, J.L.; Abe, J.; Kong, F. Molecular mechanisms for the photoperiodic regulation of flowering in soybean. J. Integr. Plant Biol. 2021, 63, 981–994. [Google Scholar] [CrossRef]
- Wu, Y.M.; Ma, Y.J.; Wang, M.; Zhou, H.; Gan, Z.M.; Zeng, R.F.; Ye, L.X.; Zhou, J.J.; Zhang, J.Z.; Hu, C.G. Mobility of FLOWERING LOCUS T protein as a systemic signal in trifoliate orange and its low accumulation in grafted juvenile scions. Hortic. Res. 2022, 9, uhac056. [Google Scholar] [CrossRef]
- Nan, H.; Cao, D.; Zhang, D.; Li, Y.; Lu, S.; Tang, L.; Yuan, X.; Liu, B.; Kong, F. GmFT2a and GmFT5a redundantly and differentially regulate flowering through interaction with and upregulation of the bZIP transcription factor GmFDL19 in soybean. PLoS ONE 2014, 9, e97669. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, R.; Nan, H.; Harigai, K.; Dong, L.; Zhu, J.; Lu, S.; Xu, M.; Yamagishi, N.; Yoshikawa, N.; Liu, B.; et al. Functional divergence between soybean FLOWERING LOCUS T orthologues FT2a and FT5a in post-flowering stem growth. J. Exp. Bot. 2019, 70, 3941–3953. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, L.; Chen, L.; Wu, T.; Liu, L.; Sun, S.; Wu, C.; Yao, W.; Jiang, B.; Yuan, S.; et al. Mutagenesis of GmFT2a and GmFT5a mediated by CRISPR/Cas9 contributes for expanding the regional adaptability of soybean. Plant Biotechnol. J. 2020, 18, 298–309. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, L.; Liu, X.; Guo, C.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. CRISPR/Cas9-mediated targeted mutagenesis of GmFT2a delays flowering time in soya bean. Plant Biotechnol. J. 2018, 16, 176–185. [Google Scholar] [CrossRef]
- Yue, L.; Li, X.; Fang, C.; Chen, L.; Yang, H.; Yang, J.; Chen, Z.; Nan, H.; Chen, L.; Zhang, Y.; et al. FT5a interferes with the Dt1-AP1 feedback loop to control flowering time and shoot determinacy in soybean. J. Integr. Plant Biol. 2021, 63, 1004–1020. [Google Scholar] [CrossRef]
- Cheng, Q.; Dong, L.; Su, T.; Li, T.; Gan, Z.; Nan, H.; Lu, S.; Fang, C.; Kong, L.; Li, H.; et al. CRISPR/Cas9-mediated targeted mutagenesis of GmLHY genes alters plant height and internode length in soybean. BMC Plant Biol. 2019, 19, 562. [Google Scholar] [CrossRef]
- Chen, L.; Nan, H.; Kong, L.; Yue, L.; Yang, H.; Zhao, Q.; Fang, C.; Li, H.; Cheng, Q.; Lu, S.; et al. Soybean AP1 homologs control flowering time and plant height. J. Integr. Plant Biol. 2020, 62, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.; Chen, H.; Chen, L.; Chen, S.; Hao, Q.; Guo, W.; Qiu, D.; Shan, Z.; Yang, Z.; Yuan, S.; et al. CRISPR/Cas9-mediated targeted mutagenesis of GmSPL9 genes alters plant architecture in soybean. BMC Plant Biol. 2019, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Hedden, P. The Current Status of Research on Gibberellin Biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Guo, Y.; Ou, L.; Hong, H.; Wang, J.; Liu, Z.X.; Guo, B.; Zhang, L.; Qiu, L. Identification of the dwarf gene GmDW1 in soybean (Glycine max L.) by combining mapping-by-sequencing and linkage analysis. Theor. Appl. Genet. 2018, 131, 1001–1016. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.W.; Wong, F.L.; Luk, C.Y.; Chung, C.Y.; Yung, W.S.; Wang, Z.; Xie, M.; Song, S.; Chung, G.; et al. Increased copy number of gibberellin 2-oxidase 8 genes reduced trailing growth and shoot length during soybean domestication. Plant J. 2021, 107, 1739–1755. [Google Scholar] [CrossRef]
- Lyu, X.; Cheng, Q.; Qin, C.; Li, Y.; Xu, X.; Ji, R.; Mu, R.; Li, H.; Zhao, T.; Liu, J.; et al. GmCRY1s modulate gibberellin metabolism to regulate soybean shade avoidance in response to reduced blue light. Mol. Plant 2021, 14, 298–314. [Google Scholar] [CrossRef]
- Ji, R.; Xu, X.; Liu, J.; Zhao, T.; Li, H.; Zhai, J.; Liu, B. Induced Mutation in GmCOP1b Enhances the Performance of Soybean under Dense Planting Conditions. Int. J. Mol. Sci. 2022, 23, 5394. [Google Scholar] [CrossRef]
- Zhao, W.; Zeng, D.; Zhao, C.; Han, D.; Li, S.; Wen, M.; Liang, X.; Zhang, X.; Liu, Z.; Ali, S.; et al. Identification of QTLs and key genes enhancing lodging resistance in soybean through chemical and physical trait analysis. Plants 2024, 13, 3470. [Google Scholar] [CrossRef]
- Li, S.; Sun, Z.; Sang, Q.; Qin, C.; Kong, L.; Huang, X.; Liu, H.; Su, T.; Li, H.; He, M.; et al. Soybean reduced internode 1 determines internode length and improves grain yield at dense planting. Nat. Commun. 2023, 14, 7939. [Google Scholar] [CrossRef]
- Qin, C.; Li, Y.H.; Li, D.; Zhang, X.; Kong, L.; Zhou, Y.; Lyu, X.; Ji, R.; Wei, X.; Cheng, Q.; et al. PH13 improves soybean shade traits and enhances yield for high-density planting at high latitudes. Nat. Commun. 2023, 14, 6813. [Google Scholar] [CrossRef]
- Xiang, X.; Yang, H.; Yuan, X.; Dong, X.; Mai, S.; Zhang, Q.; Chen, L.; Cao, D.; Chen, H.; Guo, W.; et al. CRISPR/Cas9-mediated editing of GmDWF1 brassinosteroid biosynthetic gene induces dwarfism in soybean. Plant Cell Rep. 2024, 43, 116. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, S.; Lu, Q.; Li, S.; Zhao, S.; Zheng, X.; Zhou, Q.; Zhang, W.; Li, J.; Wang, L.; et al. UV-B irradiation-activated E3 ligase GmILPA1 modulates gibberellin catabolism to increase plant height in soybean. Nat. Commun. 2023, 14, 6262. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Chen, L.; Yang, X.; Yang, H.; Liu, S.; Kou, K.; Fan, L.; Zhang, Z.; Duan, Z.; Yuan, Y.; et al. Natural variation of Dt2 determines branching in soybean. Nat. Commun. 2022, 13, 6429. [Google Scholar] [CrossRef]
- Virdi, K.S.; Sreekanta, S.; Dobbels, A.; Haaning, A.; Jarquin, D.; Stupar, R.M.; Lorenz, A.J.; Muehlbauer, G.J. Branch angle and leaflet shape are associated with canopy coverage in soybean. Plant Genome 2023, 16, e20304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, L.; Ke, M.; Gao, Z.; Tu, T.; Huang, L.; Chen, J.; Guan, Y.; Huang, X.; Chen, X. GmPIN1-mediated auxin asymmetry regulates leaf petiole angle and plant architecture in soybean. J. Integr. Plant Biol. 2022, 64, 1325–1338. [Google Scholar] [CrossRef]
- Feng, L.; Raza, M.A.; Li, Z.; Chen, Y.; Khalid, M.H.B.; Du, J.; Liu, W.; Wu, X.; Song, C.; Yu, L.; et al. The Influence of Light Intensity and Leaf Movement on Photosynthesis Characteristics and Carbon Balance of Soybean. Front. Plant Sci. 2019, 9, 1952. [Google Scholar] [CrossRef]
- Tsukaya, H. Mechanism of leaf-shape determination. Annu. Rev. Plant Biol. 2006, 57, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Ma, Y.; Wu, S.; Liu, Z.; Wang, Z.; Yang, R.; Hu, G.; Zhou, Z.; Yu, H.; Zhang, M.; et al. Genome-wide association studies dissect the genetic networks underlying agronomical traits in soybean. Genome Biol. 2017, 18, 161. [Google Scholar] [CrossRef]
- Cai, Z.; Xian, P.; Cheng, Y.; Ma, Q.; Lian, T.; Nian, H.; Ge, L. CRISPR/Cas9-mediated gene editing of GmJAGGED1 increased yield in the low-latitude soybean variety Huachun 6. Plant Biotechnol. J. 2021, 19, 1898–1900. [Google Scholar] [CrossRef]
- Gao, J.; Yang, S.; Cheng, W.; Fu, Y.; Leng, J.; Yuan, X.; Jiang, N.; Ma, J.; Feng, X. GmILPA1, Encoding an APC8-like Protein, Controls Leaf Petiole Angle in Soybean. Plant Physiol. 2017, 174, 1167–1176. [Google Scholar] [PubMed]
- Liu, B.; Watanabe, S.; Uchiyama, T.; Kong, F.; Kanazawa, A.; Xia, Z.; Nagamatsu, A.; Arai, M.; Yamada, T.; Kitamura, K.; et al. The soybean stem growth habit gene Dt1 is an ortholog of Arabidopsis TERMINAL FLOWER1. Plant Physiol. 2010, 153, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Liu, Y.; Guo, D.; Fan, R.; Liu, Y.; Xu, K.; Zhu, J.; Quan, L.; Lu, W.; Bai, X.; et al. CRISPR/Cas9-mediated targeted mutation of the E1 decreases photoperiod sensitivity, alters stem growth habits, and decreases branch number in soybean. Front. Plant Sci. 2022, 13, 1066820. [Google Scholar] [CrossRef]
- Wu, S.Q.; Chen, L.; Guo, M.W.; Cai, Y.P.; Gao, Y.; Yuan, S.; Sun, S.; Zhang, Y.X.; Hou, W.S.; Han, T.F. CRISPR/Cas9-mediated knockout of E4 gene promotes maturation in soybean. Oil Crop Sci. 2024, 9, 170–179. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Cai, Y.; Su, Q.; Chen, Y.; Li, M.; Hou, W. A novel MORN-motif type gene GmMRF2 controls flowering time and plant height of soybean. Int. J. Biol. Macromol. 2023, 245, 125464. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, L.; Liu, X.; Yao, W.; Hou, W. GmNF-YC4 delays soybean flowering and maturation by directly repressing GmFT2a and GmFT5a expression. J. Integr. Plant Biol. 2024, 66, 1370–1384. [Google Scholar] [CrossRef]
- Xie, H.; Su, F.; Niu, Q.; Geng, L.; Cao, X.; Song, M.; Dong, J.; Zheng, Z.; Guo, R.; Zhang, Y.; et al. Knockout of miR396 genes increases seed size and yield in soybean. J. Integr. Plant Biol. 2024, 66, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, H.; Fang, Y.; Guo, W.; Chen, H.; Zhang, X.; Dai, W.; Chen, S.; Hao, Q.; Yuan, S.; et al. Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway. Plant Biotechnol. J. 2021, 19, 702–716. [Google Scholar] [CrossRef]
- Li, J.; Ni, Q.; He, G.; Huang, J.; Chao, H.; Li, S.; Chen, M.; Hu, G.; Whelan, J.; Shou, H. SoyOD: An Integrated Soybean Multi-omics Database for Mining Genes and Biological Research. Genom. Proteom. Bioinform. 2025, 22, qzae080. [Google Scholar] [CrossRef]
- Cervantes-Pérez, S.A.; Thibivilliers, S.; Amini, S.; Pelletier, J.M.; Meyer, I.; Xu, H.; Tennant, S.; Ma, P.; Sprueill, C.M.; Farmer, A.D.; et al. Tabula Glycine: The whole-soybean single-cell resolution transcriptome atlas. bioRxiv 2024. [Google Scholar]
- Fan, J.; Shen, Y.; Chen, C.; Chen, X.; Yang, X.; Liu, H.; Chen, R.; Liu, S.; Zhang, B.; Zhang, M.; et al. A large-scale integrated transcriptomic atlas for soybean organ development. Mol. Plant 2025, 18, 669–689. [Google Scholar] [CrossRef]
- Haidar, S.; Hooker, J.; Lackey, S.; Elian, M.; Puchacz, N.; Szczyglowski, K.; Marsolais, F.; Golshani, A.; Cober, E.R.; Samanfar, B. Harnessing Multi-Omics Strategies and Bioinformatics Innovations for Advancing Soybean Improvement: A Comprehensive Review. Plants 2024, 13, 2714. [Google Scholar] [CrossRef]
- Khatun, M.M.; Khan, I.; Borphukan, B.; Das, K.C.; Khan, H.; Islam, M.R.; Reddy, M.K.; Salimullah, M. Characterization of the novel SmsHSP24.1 Promoter: Unveiling its abiotic stress-inducible expression in transgenic Eggplant (Solanum melongena L.). bioRxiv 2024. [Google Scholar] [CrossRef]
- Wang, L.; O’Conner, S.; Tanvir, R.; Zheng, W.; Cothron, S.; Towery, K.; Li, L. CRISPR/Cas9-based editing of NF-YC4 promoters yields high-protein rice and soybean. New Phytol. 2024, 245, 1805–1807. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Li, Y.; Zhang, R.; Yang, J.; Ma, H.; Min, L.; Zhang, X. Reversing anther thermotolerance by manipulating the cis-elements in the promoter of a high-temperature upregulated gene Casein Kinase I in upland cotton. Sci. China Life Sci. 2025, 68, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Li, S.; Shi, Z.; Zou, Y.; Zhang, Y.; Huang, X.; Yang, D.; Yang, Y.; Li, Z.; Xu, C. Engineering source-sink relations by prime editing confers heat-stress resilience in tomato and rice. Cell 2025, 188, 530–549.e20. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2021, 463, 178–183, Erratum in Nature 2010, 465, 120. https://doi.org/10.1038/nature08957. [Google Scholar] [CrossRef] [PubMed]
- Kou, K.; Yang, H.; Li, H.; Fang, C.; Chen, L.; Yue, L.; Nan, H.; Kong, L.; Li, X.; Wang, F.; et al. A functionally divergent SOC1 homolog improves soybean yield and latitudinal adaptation. Curr. Biol. 2022, 32, 1728–1742. [Google Scholar] [CrossRef]
- Ma, R.; Huang, W.; Hu, Q.; Tian, G.; An, J.; Fang, T.; Liu, J.; Hou, J.; Zhao, M.; Sun, L. Tandemly duplicated MYB genes are functionally diverged in the regulation of anthocyanin biosynthesis in soybean. Plant Physiol. 2024, 194, 2549–2563. [Google Scholar] [CrossRef]
- Jung, W.J.; Park, S.J.; Cha, S.; Kim, K. Factors affecting the cleavage efficiency of the CRISPR-Cas9 system. Anim. Cells Syst. 2024, 28, 75–83. [Google Scholar] [CrossRef]
- Liang, R.; He, Z.; Zhao, K.T.; Zhu, H.; Hu, J.; Liu, G.; Gao, Q.; Liu, M.; Zhang, R.; Qiu, J.L.; et al. Prime editing using CRISPR-Cas12a and circular RNAs in human cells. Nat. Biotechnol. 2024, 42, 1867–1875, Erratum in Nat. Biotechnol. 2024, 42, 1921–1922. https://doi.org/10.1038/s41587-024-02160-z. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhong, Z.; Tang, X.; Yang, S.; Zhang, Y.; Wang, S.; Liu, Y.; Zhang, Y.; Zheng, X.; Zhang, Y.; et al. Advancing PAM-less genome editing in soybean using CRISPR-SpRY. Hortic. Res. 2024, 11, uhae160. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Sretenovic, S.; Liu, S.; Tang, X.; Huang, L.; He, Y.; Liu, L.; Guo, Y.; Zhong, Z.; Liu, G.; et al. PAM-less plant genome editing using a CRISPR-SpRY toolbox. Nat. Plants 2021, 7, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cong, Y.; Liu, Y.; Wang, T.; Shuai, Q.; Chen, N.; Gai, J.; Li, Y. Optimization of Agrobacterium-Mediated Transformation in Soybean. Front. Plant Sci. 2017, 8, 246. [Google Scholar] [CrossRef]
- Kong, J.; Martin-Ortigosa, S.; Finer, J.; Orchard, N.; Gunadi, A.; Batts, L.A.; Thakare, D.; Rush, B.; Schmitz, O.; Stuiver, M.; et al. Overexpression of the Transcription Factor GROWTH-REGULATING FACTOR5 Improves Transformation of Dicot and Monocot Species. Front. Plant Sci. 2020, 11, 572319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cheng, P.; Liu, Y.; Liu, C.; Hu, Z.; Xin, D.; Wu, X.; Yang, M.; Chen, Q. A highly efficient soybean transformation system using GRF3-GIF1 chimeric protein. J. Integr. Plant Biol. 2025, 67, 3–6. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Li, X.; Zhang, R.; Li, J. Virus-Induced Gene Editing and Its Applications in Plants. Int. J. Mol. Sci. 2022, 23, 10202. [Google Scholar] [CrossRef]
- Gong, Z.; Cheng, M.; Botella, J.R. Non-GM Genome Editing Approaches in Crops. Front. Genome Ed. 2021, 3, 817279. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, X.; Liu, H.; Li, Z. Highly efficient DNA-free plant genome editing using virally delivered CRISPR-Cas9. Nat. Plants 2020, 6, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Na, R.; Nowak, J.S.; Qiu, Y.; Lu, Q.S.; Yang, C.; Marsolais, F.; Tian, L. Development of a Csy4-processed guide RNA delivery system with soybean-infecting virus ALSV for genome editing. BMC Plant Biol. 2021, 21, 419. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhong, Z.; Wu, Z.; Wang, Y.; Zhang, Y.; Chen, Y.; Jacobsen, S.E. Genome editing in plants using the compact editor CasΦ. Proc. Natl. Acad. Sci. USA 2023, 120, e2216822120. [Google Scholar] [CrossRef]
- Yuan, G.; Lu, H.; De, K.; Hassan, M.M.; Liu, Y.; Li, Y.; Muchero, W.; Abraham, P.E.; Tuskan, G.A.; Yang, X. An Intein-Mediated Split-nCas9 System for Base Editing in Plants. ACS Synth. Biol. 2022, 11, 2513–2517. [Google Scholar] [CrossRef]


| Tool | Enzyme(s) | Guide RNA | Target Type | PAM or PFS Site | Advantages | Disadvantages | Application Examples |
|---|---|---|---|---|---|---|---|
| CRISPR–Cas9 | Cas9 | sgRNA | dsDNA | PAM: typically 5′-NGG-3′ (SpCas9); varies by Cas9 variant | Simple design; high efficiency; multiplex capability for editing multiple targets; broad applicability [13] | Restricted by PAM; potentially high on-/off-target effects in some organisms [30,31] | Enhancing broad-spectrum resistance in rice varieties Kitaake, IR64, and Ciherang-Sub1 [32]; adjusting TaGW2 dosage to increase wheat grain weight [33] |
| CRISPR–Cas12a | Cas12a (Cpf1) | crRNA | dsDNA/ssDNA | PAM: 5′-(T)TTN-3′ | No need for tracrRNA; lower off-target rate; distinct recognition site from Cas9 [34,35] | Dependent on host DNA repair mechanisms; frequently results in small deletions (<100 bp) [36,37] | Site-directed mutagenesis of OsDL and OsALS genes in rice [38] |
| CRISPR-Cas12b | Cas12b (C2c1) | crRNA + tracrRNA or sgRNA | dsDNA/ssDNA | PAM: 5′-TTN-3′(species-dependent) | Higer temperature tolerance; compact size; high targeting specificity [39] | Less characterized; may require elevated temperatures for optimal activity | Enhancing broad-spectrum resistance in rice using AaCas12b-mediated editing of OsEPFL9 and OsGS3 [39] |
| CRISPR–Cas13 | Cas13a/Cas13b (C2c2) | crRNA | ssRNA | PFS: 3′ non-G (bacteria); N/A in eukaryotes | PAM-independent; cleaves RNA only; high editing efficiency [29] | Potential nonspecific cleavage of bystander RNAs (collateral effect) [40] | Enhancing cotton resistance to Tobacco Mosaic Virus (TMV) [41] |
| Base Editing | dCas9 or nCas9 + deaminase | sgRNA | ssDNA | PAM: typically 5′-NGG-3′ (SpCas9); varies by Cas9 variant | No DSBs or donor template needed; avoids indels; enables precise C→T or A→G base conversion [42] | Limited editing window and base substitution types (only purine→purine or pyrimidine→pyrimidine) [43,44] | Efficient C→T base editing in rice, wheat, and maize [45]; herbicide tolerance in rice via miRNA target site editing [46] |
| Prime Editing | nCas9 + M-MLV RT | pegRNA | ssDNA within R-loop generated by pegRNA-nCas9 complex | PAM: typically 5′-NGG-3′ (SpCas9); alternative PAMs available with engineered variants | Enables all 12 base substitutions and small indels without DSBs or donor DNA [26,47] | Editing efficiency is relatively low and context-dependent; pegRNA design and MMR activity influence outcomes [26,48] | Herbicide resistance in rice via saturation mutagenesis of OsACC1 using prime-editing libraries [49] |
| Reverse Prime Editing | nCas9-D10A–M-MLV RT ± Rep-X helicase | rpegRNA/pegRNA | Chromosomal DNA (upstream of nick site) | PAM: typically 5′-NGG-3′ (SpCas9); alternative PAMs available with engineered variants | Improves efficiency and fidelity; reduces DSBs and indels [50,51] | Still in early development; editing window design is complex; broad applicability yet to be validated | Expanding editing scope in human cells through reverse prime editing at protein-coding loci such as BRCA1 and RPE65 [50] |
| dCas9 system | dCas9 | sgRNA | dsDNA | PAM: typically 5′-NGG-3′ (SpCas9); alternative PAMs available with engineered variants | Lacks nuclease activity; genome remains intact; compatible with CRISPRa/i and live-cell imaging via fusion proteins [52,53] | Complex off-target effects may interfere with screening accuracy [54] | Induction of haploid formation in sweet potato via IbBBM activation using dCas9-based activation system [55] |
| Gene | Functional Validation | Key Phenotypes | Citation |
|---|---|---|---|
| Dt1 | Natural variation | Controls stem growth habit; delays vegetative-to-reproductive phase transition | Liu et al. [135] |
| Dt2 | Natural variation | Modulates branch number | Liang et al. [127] |
| DW1 | EMS-induced mutation | Dwarf phenotype; shortened internodes | Li et al. [118] |
| E1 | CRISPR/Cas9 knockout | Reduces photoperiod sensitivity; determinate stem; fewer branches | Wan et al. [136] |
| E4 | CRISPR/Cas9 knockout | Promotes early maturation; reduces height and node number | Wu et al. [137] |
| GmCRY1s | CRISPR/Cas9 knockout; Overexpression | Represses stem elongation; enhances lodging resistance and branching | Lyu et al. [120] |
| GmDWF1 | CRISPR/Cas9 knockout | Dwarf plants with stable node number; more pods in field trials | Xiang et al. [125] |
| GmGA2OX8 | Overexpression; Copy number variation | Reduces shoot length and trailing growth | Wang et al. [119] |
| GmILPA1 | Natural mutant (UV-B responsive) | Reduced height under UV-B; shorter internodes and petioles | Sun et al. [126] |
| GmJAG1 | CRISPR/Cas9 knockout | Narrower leaves; increased 3- and 4-seeded pods; yield improvement | Cai et al. [133] |
| GmLHY | CRISPR/Cas9 knockout | Reduced height and internode length via GA pathway | Cheng et al. [113] |
| GmMRF2 | Overexpression | Earlier flowering in LD; taller plants in both LD and SD | Zhang et al. [138] |
| GmNF-YC4 | CRISPR/Cas9 knockout | Early flowering and maturity; adapted to higher latitudes | Cai et al. [139] |
| GmPIN1 | CRISPR/Cas9 knockout | Compact architecture; reduced petiole angle; higher yield at density | Zhang et al. [129] |
| miR396 | CRISPR/Cas12SF01 knockout | Larger seeds and increased yield in specific regions | Xie et al. [140] |
| PH13 | CRISPR/Cas9 knockout; Overexpression | Enhanced shade tolerance and yield under high-density planting | Qin et al. [124] |
| RIN1 | CRISPR/Cas9 knockout; γ-ray mutant | Shorter internodes; increased yield at high density; early flowering | Li et al. [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Yuan, X.; Han, B.; Guo, W.; Chen, H. CRISPR/Cas-Mediated Optimization of Soybean Shoot Architecture for Enhanced Yield. Int. J. Mol. Sci. 2025, 26, 7925. https://doi.org/10.3390/ijms26167925
Li N, Yuan X, Han B, Guo W, Chen H. CRISPR/Cas-Mediated Optimization of Soybean Shoot Architecture for Enhanced Yield. International Journal of Molecular Sciences. 2025; 26(16):7925. https://doi.org/10.3390/ijms26167925
Chicago/Turabian StyleLi, Nianao, Xi Yuan, Bei Han, Wei Guo, and Haifeng Chen. 2025. "CRISPR/Cas-Mediated Optimization of Soybean Shoot Architecture for Enhanced Yield" International Journal of Molecular Sciences 26, no. 16: 7925. https://doi.org/10.3390/ijms26167925
APA StyleLi, N., Yuan, X., Han, B., Guo, W., & Chen, H. (2025). CRISPR/Cas-Mediated Optimization of Soybean Shoot Architecture for Enhanced Yield. International Journal of Molecular Sciences, 26(16), 7925. https://doi.org/10.3390/ijms26167925






