The Antioxidant Potential of Black Tea Polyphenols in Heavy Metal Toxicity: An In Vitro Perspective
Abstract
1. Introduction
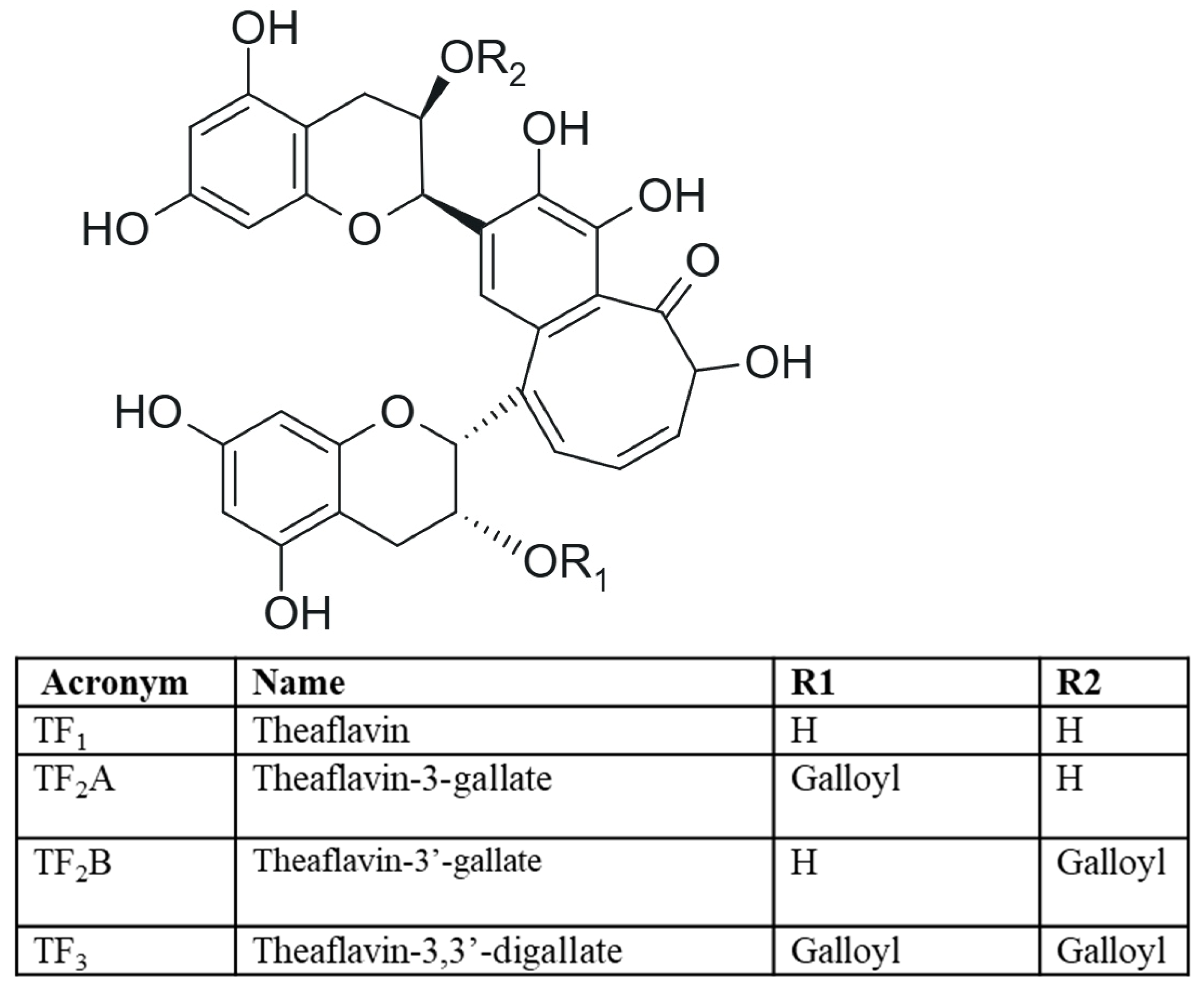
1.1. Theaflavins
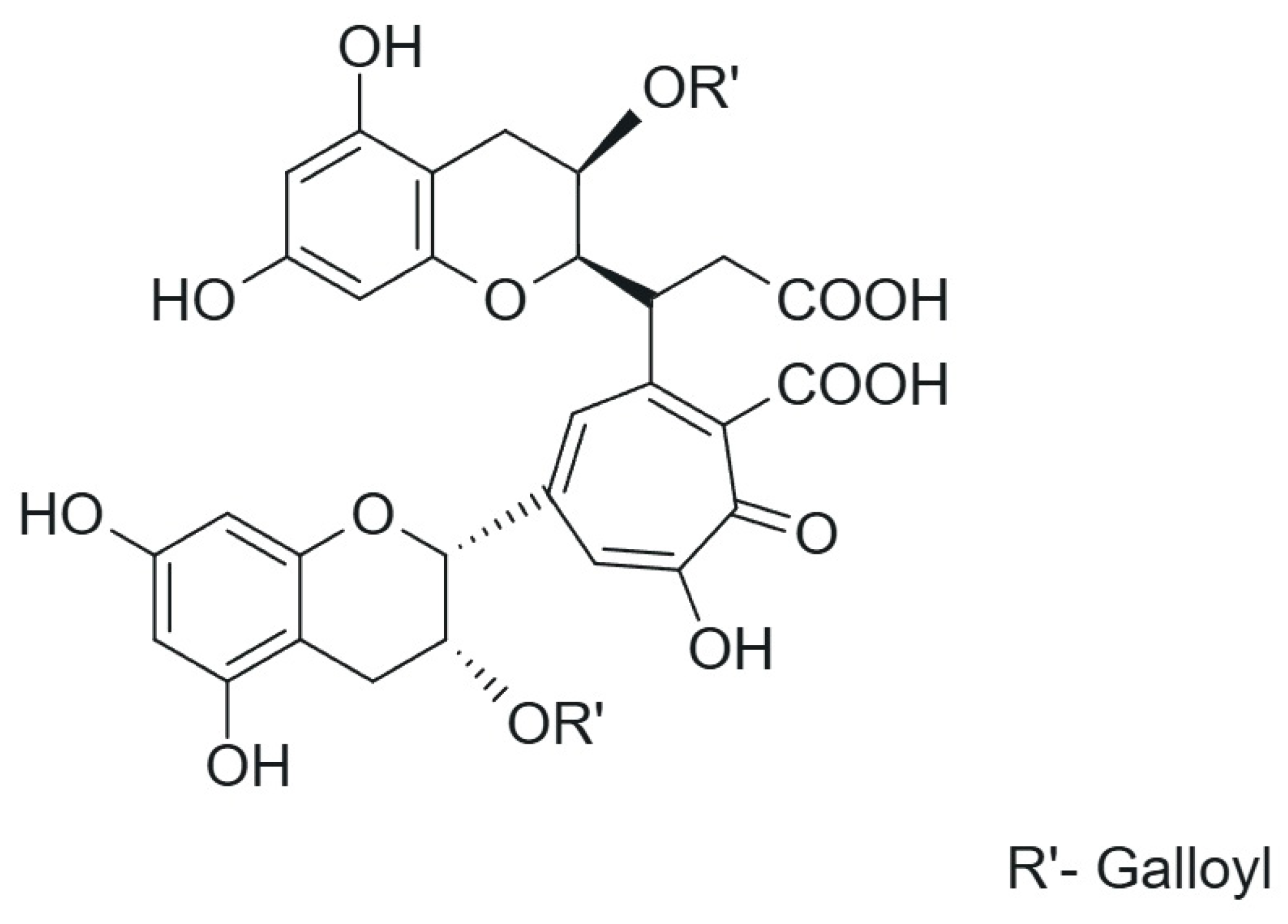
1.2. Thearubigins
1.3. Protective Effects of TFs and TRs
2. The Effects of Heavy Metals on Oxidative Stress
2.1. Cadmium
2.2. Arsenic
2.3. Lead
2.4. Mercury
3. Effects of Black Tea Components on Heavy Metal Toxicity
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koch, W. Theaflavins, thearubigins, and theasinensins. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S., Asakawa, Y., Eds.; Springer: Singapore, 2020; pp. 975–1003. [Google Scholar] [CrossRef]
- Li, S.; Lo, C.Y.; Pan, M.H.; Lai, C.S.; Ho, C.T. Black tea: Chemical analysis and stability. Food Funct. 2012, 4, 10–18. [Google Scholar] [CrossRef]
- Arun, S.D.; Minal, M.K.; Karibasappa, G.N.; Prashanth, V.K.; Girija, A.D.; Harish, C.J. Comparative assessment of antibacterial efficacy of aqueous extract of commercially available black, green, and lemon tea: An in vitro study. Int. J. Health Sci. 2017, 11, 42–46. [Google Scholar]
- Yi, T.; Zhu, L.; Peng, W.L.; He, X.C.; Chen, H.L.; Li, J.; Yu, T.; Liang, Z.T.; Zhao, Z.Z.; Chen, H.B. Comparison of ten major constituents in seven types of processed tea using HPLC-DAD-MS followed by principal component and hierarchical cluster analysis. LWT Food Sci. Technol. 2015, 62, 194–201. [Google Scholar] [CrossRef]
- Dey, A.; Gomes, A.; Dasgupta, S.C. Black Tea (Camellia sinensis) extract induced prenatal and postnatal toxicity in experimental albino rats. Pharmacogn. Mag. 2017, 13, 769–774. [Google Scholar] [CrossRef]
- Liang, Y.; Lu, J.; Zhang, L.; Wu, S.; Wu, Y. Estimation of black tea quality by analysis of chemical composition and colour difference of tea infusions. Food Chem. 2003, 80, 283–290. [Google Scholar] [CrossRef]
- Sharma, V.; Rao, L.J.M. A thought on the biological activities of black tea. Crit. Rev. Food Sci. Nutr. 2009, 49, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Leenen, R.; Roodenburg, A.J.C.; Tijburg, L.B.M.; Wiseman, S.A. A single dose of tea with or without milk increases plasma antioxidant activity in humans. Eur. J. Clin. Nutr. 2000, 54, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Khanom, R.A.; Mahmood, S.; Tanmy, T.T. In vitro studies on antioxidant activity of black tea or Camellia sinensis. Sch. Acad. Sci. Publ. 2013, 1, 50–54. [Google Scholar] [CrossRef]
- Yoshino, K.; Hara, Y.; Sano, M.; Tomita, I. Antioxidative effects of black tea theaflavins and thearubigin on lipid peroxidation of rat liver homogenates induced by tert-butyl hydroperoxide. Biol. Pharm. Bull. 1994, 17, 146–149. [Google Scholar] [CrossRef]
- Łuczaj, W.; Skrzydlewska, E. Antioxidative properties of black tea. Prev. Med. 2005, 40, 910–918. [Google Scholar] [CrossRef]
- He, H.F. Research progress on theaflavins: Efficacy, formation, and preparation. Food Nutr. Res. 2017, 61, 1344521. [Google Scholar] [CrossRef]
- Engelhardt, U.H. Tea chemistry—What do and what don’t we know?—A micro review. Food Res. Int. 2020, 132, 109120. [Google Scholar] [CrossRef]
- Su, Y.L.; Xu, J.Z.; Ng, C.H.; Leung, L.K.; Huang, Y.; Chen, Z.Y. Antioxidant activity of tea theaflavins and methylated catechins in canola oil. J. Am. Oil Chem. Soc. 2004, 81, 269–274. [Google Scholar] [CrossRef]
- Yang, Z.; Tu, Y.; Xia, H.; Jie, G.; Chen, X.; He, P. Suppression of free-radicals and protection against H2O2-induced oxidative damage in HPF-1 cell by oxidized phenolic compounds present in black tea. Food Chem. 2007, 105, 1349–1356. [Google Scholar] [CrossRef]
- Leung, L.K.; Su, Y.; Chen, R.; Zhang, Z.; Huang, Y.; Chen, Z.Y. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J. Nutr. 2001, 131, 2248–2251. [Google Scholar] [CrossRef]
- Skotnicka, M.; Chorostowska-Wynimko, J.; Jankun, J.; Skrzypczak-Jankun, E. The black tea bioactivity: An overview. Cent. J. Immunol. 2011, 36, 284–292. [Google Scholar]
- Halder, J.; Bhaduri, A.N. Protective role of black tea against oxidative damage of human red blood cells. Biochem. Biophys. Res. Commun. 1998, 244, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Bhaduri, A. Black tea is a powerful chemopreventor of reactive oxygen and nitrogen species: Comparison with its individual catechin constituents and green tea. Biochem. Biophys. Res. Commun. 2001, 284, 173–178. [Google Scholar] [CrossRef]
- Yoshida, H.; Ishikawa, T.; Hosoai, H.; Suzukawa, M.; Ayaori, M.; Hisada, T.; Sawada, S.; Yonemura, A.; Higashi, K.; Ito, T.; et al. Inhibitory effect of tea flavonoids on the ability of cells to oxidize low density lipoprotein. Biochem. Pharmacol. 1999, 58, 1695–1703. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, S.; Li, J.; Xiong, L.; Tian, L.; Liu, J.; Huang, J.; Liu, Z. Neuroprotective Effects of Theaflavins Against Oxidative Stress-Induced Apoptosis in PC12 Cells. Neurochem. Res. 2016, 41, 3364–3372. [Google Scholar] [CrossRef]
- Feng, Q.; Torii, Y.; Uchida, K.; Nakamura, Y.; Hara, Y.; Osawa, T. Black tea polyphenols, theaflavins, prevent cellular DNA damage by inhibiting oxidative stress and suppressing cytochrome P450 1A1 in cell cultures. J. Agric. Food Chem. 2002, 50, 213–220. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Li, J.; Zhang, Z.; Wang, Y.; Qiu, C.; Sun, Y.; Pan, C. Research progress on the functions and biosynthesis of theaflavins. Food Chem. 2024, 450, 139285. [Google Scholar] [CrossRef]
- Lahiry, L.; Saha, B.; Chakraborty, J.; Bhattacharyya, S.; Chattopadhyay, S.; Banerjee, S.; Choudhuri, T.; Mandal, D.; Bhattacharyya, A.; Sa, G.; et al. Contribution of p53-mediated Bax transactivation in theaflavin-induced mammary epithelial carcinoma cell apoptosis. Apoptosis 2008, 13, 771–781. [Google Scholar] [CrossRef]
- Lahiry, L.; Saha, B.; Chakraborty, J.; Adhikary, A.; Mohanty, S.; Hossain, D.M.S.; Banerjee, S.; Das, K.; Sa, G.; Das, T. Theaflavins target Fas/caspase-8 and Akt/pBad pathways to induce apoptosis in p53-mutated human breast cancer cells. Carcinogenesis 2010, 31, 259–268. [Google Scholar] [CrossRef]
- Bhattacharya, U.; Halder, B.; Mukhopadhyay, S.; Giri, A.K. Role of oxidation-triggered activation of JNK and p38 MAPK in black tea polyphenols induced apoptotic death of A375 cells. Cancer Sci. 2009, 100, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Y.; Liao, J.; Li, C.; Chung, J.; Yurkow, E.J.; Ho, C.T.; Yang, C.S. Effect of black and green tea polyphenols on c-jun phosphorylation and H2O2 production in transformed and non-transformed human bronchial cell lines: Possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis 2000, 21, 2035–2039. [Google Scholar] [CrossRef]
- Nagai, E.; Iwai, M.; Koketsu, R.; Sogabe, R.; Morimoto, R.; Suzuki, Y.; Ohta, Y.; Okuno, Y.; Ohshima, A.; Enomoto, T.; et al. Inhibition of influenza virus replication by adlay tea. J. Sci. Food Agric. 2017, 98, 1899–1905. [Google Scholar] [CrossRef]
- Chen, C.N.; Lin, C.P.C.; Huang, K.K.; Chen, W.C.; Hsieh, H.P.; Liang, P.H.; Hsu, J.T.A. Inhibition of SARS-CoV 3C-like protease activity by theaflavin-3,3′-digallate (TF3). Evid. Based Complement. Altern. Med. 2005, 2, 209–215. [Google Scholar] [CrossRef]
- Clark, K.J.; Grant, P.G.; Sarr, A.B.; Belakere, J.R.; Swaggerty, C.L.; Phillips, T.D.; Woode, G.N. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Vet. Microbiol. 1998, 63, 147–157. [Google Scholar] [CrossRef]
- Zuo, G.; Li, Z.; Chen, L.; Xu, X. Activity of compounds from Chinese herbal medicine Rhodiola kirilowii (Regel) Maxim against HCV NS3 serine protease. Antiviral Res. 2007, 76, 86–92. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Tan, S.; Jin, H.; Qiu, J.; Mao, Q.; Li, R.; Xia, C.; Jiang, Z.H.; Jiang, S.; et al. A natural theaflavins preparation inhibits HIV-1 infection by targeting the entry step: Potential applications for preventing HIV-1 infection. Fitoterapia 2012, 83, 348–355. [Google Scholar] [CrossRef]
- Zu, M.; Yang, F.; Zhou, W.; Liu, A.; Du, G.; Zheng, L. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antiviral Res. 2012, 94, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Gosslau, A.; En Jao, D.L.; Huang, M.T.; Ho, C.T.; Evans, D.; Rawson, N.E.; Chen, K.Y. Effects of the black tea polyphenol theaflavin-2 on apoptotic and inflammatory pathways in vitro and in vitro. Mol. Nutr. Food Res. 2011, 55, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chaudhuri, T.; Seth, P.; Ganguly, D.K.; Giri, A.K. Antimutagenic effects of black tea (World blend) and its two active polyphenols theaflavins and thearubigins in Salmonella assays. Phyther. Res. 2002, 16, 655–661. [Google Scholar] [CrossRef]
- Long, P.; Rakariyatham, K.; Ho, C.T.; Zhang, L. Thearubigins: Formation, structure, health benefit and sensory property. Trends Food Sci. Technol. 2023, 133, 37–48. [Google Scholar] [CrossRef]
- Truong, V.L.; Jeong, W.S. Cellular defensive mechanisms of tea polyphenols: Structure-activity relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H. Assessments of antioxidant effect of black tea extract and its rationals by erythrocyte haemolysis assay, plasma oxidation assay and cellular antioxidant activity (CAA) assay. J. Funct. Foods 2014, 18, 1095–1105. [Google Scholar] [CrossRef]
- Imran, A.; Butt, M.S.; Xiao, H.; Imran, M.; Rauf, A.; Mubarak, M.S.; Ramadan, M.F. Inhibitory effect of black tea (Camellia sinensis) theaflavins and thearubigins against HCT 116 colon cancer cells and HT 460 lung cancer cells. J. Food Biochem. 2019, 43, e12822. [Google Scholar] [CrossRef]
- Bhattacharya, U.; Mukhopadhyay, S.; Giri, A.K. Comparative antimutagenic and anticancer activity of three fractions of black tea polyphenols thearubigins. Nutr. Cancer 2011, 63, 1122–1132. [Google Scholar] [CrossRef]
- Halder, B.; Bhattacharya, U.; Mukhopadhyay, S.; Giri, A.K. Molecular mechanism of black tea polyphenols induced apoptosis in human skin cancer cells: Involvement of Bax translocation and mitochondria mediated death cascade. Carcinogenesis 2008, 29, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, P.; Yi, S.; Wang, C. Thearubigin regulates the production of Nrf2 and alleviates LPS-induced acute lung injury in neonatal rats. 3 Biotech 2019, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Ai, Z.; Liu, S.; Zhang, H.; Chen, Y.; Wang, Y.; Ni, D. Study on mechanism of low bioavailability of black tea theaflavins by using Caco-2 cell monolayer. Drug Deliv. 2021, 28, 1737–1747. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Moreno-Rojas, J.M.; Brindani, N.; Del Rio, D.; Lean, M.E.J.; Hara, Y.; Crozier, A. Bioavailability of black tea theaflavins: Absorption, metabolism, and colonic catabolism. J. Agric. Food Chem. 2017, 65, 5365–5374. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, N. Unraveling the structure of the black tea thearubigins. Arch. Biochem. Biophys. 2010, 501, 37–51. [Google Scholar] [CrossRef]
- Kuhnert, N.; Drynan, W.; Obuchowicz, J.; Clifford, M.N.; Witt, M. Mass spectrometric characterization of black tea thearubigins leading to an oxidative cascade hypothesis for thearubigin formation. Rapid Commun. Mass Spectrom. 2010, 24, 3387–3404. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy Metals: Toxicity and Human Health Effects. Arch. Toxicol. 2024, 99, 153–209. [Google Scholar]
- Sodhi, K.K.; Mishra, L.C.; Singh, C.K.; Kumar, M. Perspective on the heavy metal pollution and recent remediation strategies. Curr. Res. Microb. Sci. 2022, 3, 100166. [Google Scholar] [CrossRef]
- Kiran, B.R.; Sharma, R. Effect of heavy metals: An overview. Mater. Today Proc. 2021, 51, 880–885. [Google Scholar] [CrossRef]
- Zwolak, I. Epigallocatechin gallate for management of heavy metal-induced oxidative stress: Mechanisms of action, efficacy, and concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef]
- Cannino, G.; Ferruggia, E.; Luparello, C.; Rinaldi, A.M. Cadmium and mitochondria. Mitochondrion 2009, 9, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shu, Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int. J. Mol. Sci. 2015, 16, 1484–1494. [Google Scholar] [CrossRef]
- Wang, S.; Ren, X.; Hu, X.; Zhou, L.; Zhang, C.; Zhang, M. Cadmium-induced apoptosis through reactive oxygen species-mediated mitochondrial oxidative stress and the JNK signaling pathway in TM3 cells, a model of mouse Leydig cells. Toxicol. Appl. Pharmacol. 2019, 368, 37–48. [Google Scholar] [CrossRef]
- Medda, N.; De, S.K.; Maiti, S. Different mechanisms of arsenic related signaling in cellular proliferation, apoptosis and neo-plastic transformation. Ecotoxicol. Environ. Saf. 2021, 208, 111752. [Google Scholar] [CrossRef]
- Shi, H.; Shi, X.; Liu, K.J. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Biochem. 2004, 255, 67–78. [Google Scholar] [CrossRef]
- Medda, N.; Patra, R.; Ghosh, T.K.; Maiti, S. Neurotoxic mechanism of arsenic: Synergistic effect of mitochondrial instability, oxidative stress, and hormonal-neurotransmitter impairment. Biol. Trace Elem. Res. 2020, 198, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of coptisine against oxidative stress-induced DNA damage and apoptosis in HaCaT keratinocytes. Gen. Physiol. Biophys. 2019, 38, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Jaiswal, A.K. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 2012, 287, 9873–9886. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Bin Emran, T.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Xu, J.; Lian, L.-J.; Wu, C.; Wang, X.-F.; Fu, W.-Y.; Xu, L.-H. Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice. Food Chem. Toxicol. 2008, 46, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Branco, V.; Aschner, M.; Carvalho, C. Neurotoxicity of mercury: An old issue with contemporary significance. Adv. Neurotoxicol. 2021, 5, 239–262. [Google Scholar] [CrossRef]
- dos Santos, A.A.; Ferrer, B.; Gonçalves, F.M.; Tsatsakis, A.M.; Renieri, E.A.; Skalny, A.V.; Farina, M.; Rocha, J.B.T.; Aschner, M. Oxidative stress in methylmercury-induced cell toxicity. Toxics 2018, 6, 47. [Google Scholar] [CrossRef]
- Ni, M.; Li, X.; Yin, Z.; Sidoryk-Weogonekgrzynowicz, M.; Jiang, H.; Farina, M.; Rocha, J.B.T.; Syversen, T.; Aschner, M. Comparative study on the response of rat primary astrocytes and microglia to methylmercury toxicity. Glia 2011, 59, 810–820. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Shon, M.Y.; Park, S.K.; Nam, S.H. Antioxidant activity of theaflavin and thearubigin separated from Korean microbially fermented tea. J. Food Sci. Nutr. 2007, 12, 7–10. [Google Scholar] [CrossRef]
- Mukherjee, P.; Poddar, S.; Talukder, G.; Sharma, A. Protection by black tea extract against chromosome damage induced by two heavy metals in mice. Pharm. Biol. 1999, 37, 243–247. [Google Scholar] [CrossRef]
- Areba, G.O.; Khalid, R.; Ngure, R.M.; Maloba, F.; Nyaga, N.; Moseti, K.O.; Ngotho, M.; Wanyoko, J.K.; Karori, S.M.; Wachira, F.N. Neuroprotective effects of tea against cadmium toxicity. Bioact. Compd. Health Dis. 2019, 2, 230–246. [Google Scholar] [CrossRef]
| Concentration [%] | ||
|---|---|---|
| Compounds | Green Tea | Black Tea |
| Catechins | 30–35 | 3–10 |
| Simple polyphenols | 2 | 3 |
| Oxidised polyphenols | 6 | 23–25 |
| Flavonols | 2 | 1 |
| Theanine | 3 | |
| Aminoacids | 3 | |
| Peptides | 6–16 | |
| Lipids | 2–8 | |
| Carbohydrates | 10–15 | |
| Caffeine | 3–6 | |
| Minerals | 4–10 | |
| Pectins | 3–4 | |
| Chlorophyll/other pigments | 0.5 | |
| Volatile compounds | 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wnuk, E. The Antioxidant Potential of Black Tea Polyphenols in Heavy Metal Toxicity: An In Vitro Perspective. Int. J. Mol. Sci. 2025, 26, 7926. https://doi.org/10.3390/ijms26167926
Wnuk E. The Antioxidant Potential of Black Tea Polyphenols in Heavy Metal Toxicity: An In Vitro Perspective. International Journal of Molecular Sciences. 2025; 26(16):7926. https://doi.org/10.3390/ijms26167926
Chicago/Turabian StyleWnuk, Ewa. 2025. "The Antioxidant Potential of Black Tea Polyphenols in Heavy Metal Toxicity: An In Vitro Perspective" International Journal of Molecular Sciences 26, no. 16: 7926. https://doi.org/10.3390/ijms26167926
APA StyleWnuk, E. (2025). The Antioxidant Potential of Black Tea Polyphenols in Heavy Metal Toxicity: An In Vitro Perspective. International Journal of Molecular Sciences, 26(16), 7926. https://doi.org/10.3390/ijms26167926





