Sperm DNA Fragmentation Impairs Early Embryo Development but Is Not Predictive of Pregnancy Outcomes: Insights from 870 ICSI Cycles
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics and Clinical Outcomes
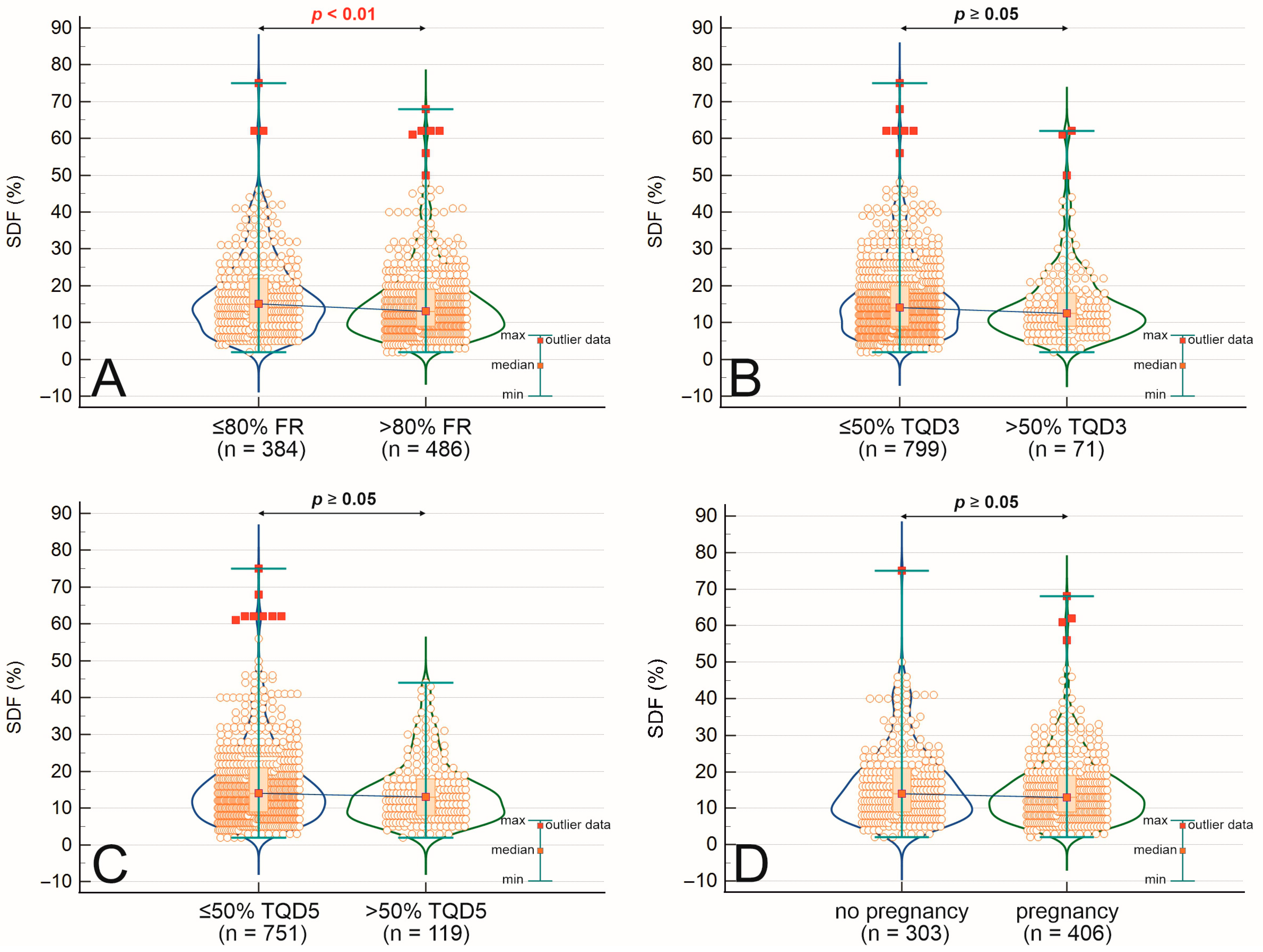
2.2. Comparison of Mean SDF Values Across Fertilization, Embryo Quality, and Pregnancy Outcomes
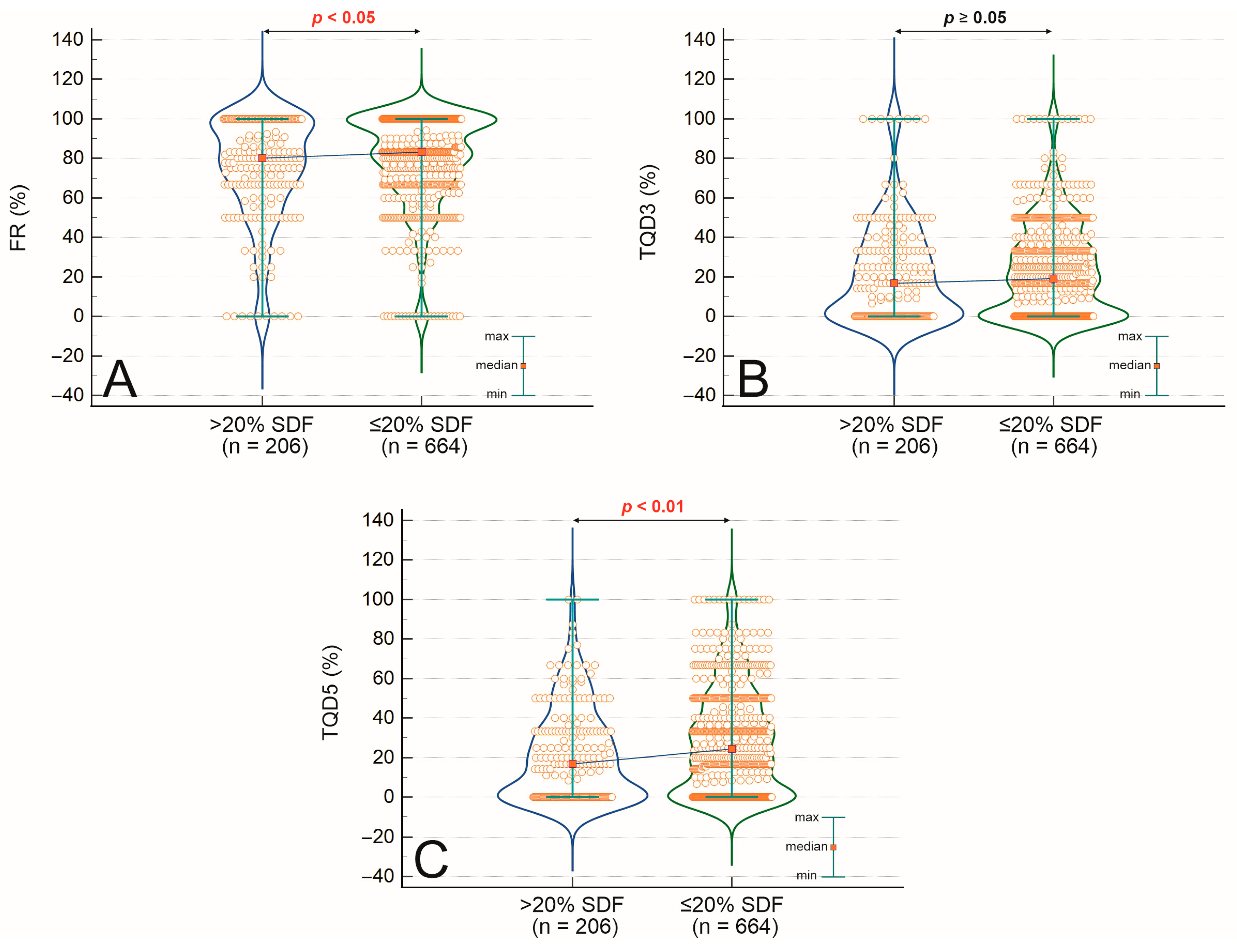
2.3. Associations and Correlations Between Sperm DNA Fragmentation, Semen Quality, Embryo Development, and Clinical Outcomes
2.4. Logistic Regression Analysis
3. Discussion
3.1. SDF and Sperm Quality
3.2. SDF and Fertilization Efficiency
3.3. SDF and Embryo Development
3.4. SDF and Clinical Pregnancy: A Multifactorial Equation
3.5. Practical Implications for ART
3.6. Strengths, Limitations, and Future Directions
4. Materials and Methods
4.1. Study Design and Participants
4.2. Assessment of Sperm DNA Fragmentation
4.3. Controlled Ovarian Stimulation and ICSI Procedure
4.4. Embryo Culture and Transfer
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Selvam, M.K.P.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J. Men’s Health 2020, 38, 412–471. [Google Scholar] [CrossRef]
- Khalafalla, K.; Majzoub, A.; Elbardisi, H.; Bhathella, A.; Chaudhari, A.; Agarwal, A.; Henkel, R.; AlMarzooki, T.; Burjaq, H.; Arafa, M. The Effect of Sperm DNA Fragmentation on Intracytoplasmic Sperm Injection Outcome. Andrologia 2021, 53, e14180. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Agarwal, A.; Esteves, S.C. Clinical Utility of Sperm DNA Damage in Male Infertility. Panminerva Med. 2019, 61, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Zini, A.; Coward, R.M.; Evenson, D.P.; Gosálvez, J.; Lewis, S.E.M.; Sharma, R.; Humaidan, P. Sperm DNA Fragmentation Testing: Summary Evidence and Clinical Practice Recommendations. Andrologia 2021, 53, e13874. [Google Scholar] [CrossRef]
- Calogero, A.E.; Cannarella, R.; Agarwal, A.; Hamoda, T.A.A. The Renaissance of Male Infertility Management in the Golden Age of Andrology. World J. Men’s Health 2023, 41, 237–254. [Google Scholar] [CrossRef]
- Casasus, P.; Luongo, F.P.; Haxhiu, A.; Orini, M.; Scupoli, G.; Governini, L.; Piomboni, P.; Buratini, J.; Canto, M.D.; Luddi, A. Paternal Age Amplifies Cryopreservation-Induced Stress in Human Spermatozoa. Cells 2024, 13, 625. [Google Scholar] [CrossRef]
- Gisbert Iranzo, A.; Cano-Extremera, M.; Hervás, I.; Falquet Guillem, M.; Gil Juliá, M.; Navarro-Gomezlechon, A.; Pacheco-Rendón, R.M.; Garrido, N. Sperm Selection Using Microfluidic Techniques Significantly Decreases Sperm DNA Fragmentation (SDF), Enhancing Reproductive Outcomes: A Systematic Review and Meta-Analysis. Biology 2025, 14, 792. [Google Scholar] [CrossRef] [PubMed]
- Luongo, F.P.; Perez Casasus, S.; Haxhiu, A.; Barbarulo, F.; Scarcella, M.; Governini, L.; Piomboni, P.; Scarica, C.; Luddi, A. Exposure to Cumulus Cell Secretome Improves Sperm Function: New Perspectives for Sperm Selection In Vitro. Cells 2023, 12, 2349. [Google Scholar] [CrossRef]
- Gill, K.; Machalowski, T.; Harasny, P.; Kups, M.; Grabowska, M.; Duchnik, E.; Sipak, O.; Fraczek, M.; Kurpisz, M.; Kurzawa, R.; et al. Male Infertility Coexists with Decreased Sperm Genomic Integrity and Oxidative Stress in Semen Irrespective of Leukocytospermia. Antioxidants 2022, 11, 1987. [Google Scholar] [CrossRef]
- Aitken, R.J.; Lewis, S.E.M. REVIEW ARTICLE DNA Damage in Testicular Germ Cells and Spermatozoa. When and How Is It Induced? How Should We Measure It? What Does It Mean? Andrology 2023, 11, 1545–1557. [Google Scholar] [CrossRef]
- Fernández, J.L.; Muriel, L.; Goyanes, V.; Segrelles, E.; Gosálvez, J.; Enciso, M.; LaFromboise, M.; De Jonge, C. Simple Determination of Human Sperm DNA Fragmentation with an Improved Sperm Chromatin Dispersion Test. Fertil. Steril. 2005, 84, 833–842. [Google Scholar] [CrossRef]
- Zini, A.; Sigman, M. Are Tests of Sperm DNA Damage Clinically Useful? Pros. and Cons. J. Androl. 2009, 30, 219–229. [Google Scholar] [CrossRef]
- Gill, K.; Rosiak, A.; Gaczarzewicz, D.; Jakubik, J.; Kurzawa, R.; Kazienko, A.; Rymaszewska, A.; Laszczynska, M.; Grochans, E.; Piasecka, M. The Effect of Human Sperm Chromatin Maturity on ICSI Outcomes. Hum. Cell 2018, 31, 220–231. [Google Scholar] [CrossRef]
- Gill, K.; Jakubik, J.; Rosiak-Gill, A.; Kups, M.; Lukaszuk, M.; Kurpisz, M.; Fraczek, M.; Piasecka, M. Utility and Predictive Value of Human Standard Semen Parameters and Sperm Dna Dispersion for Fertility Potential. Int. J. Environ. Res. Public Health 2019, 16, 2004. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Novo, S.; Torres, M.; Salas-Huetos, A.; Rovira, S.; Antich, M.; Yeste, M. Sperm DNA Integrity Does Play a Crucial Role for Embryo Development after ICSI, Notably When Good-Quality Oocytes from Young Donors Are Used. Biol. Res. 2022, 55, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cissen, M.; Van Wely, M.; Scholten, I.; Mansell, S.; De Bruin, J.P.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. Measuring Sperm DNA Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta Analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, L.; Lin, Y.; Liu, Y. The Effect of Sperm DNA Fragmentation on Clinical Pregnancy and Miscarriage Following Intrauterine Insemination: Updated Systematic Review and Meta-Analysis. Urology 2025, 1–8. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Ambar, R.F.; Agarwal, A.; Henkel, R. Etiologies of Sperm DNA Damage and Its Impact on Male Infertility. Andrologia 2021, 53, e13706. [Google Scholar] [CrossRef] [PubMed]
- Farkouh, A.; Salvio, G.; Kuroda, S.; Saleh, R.; Vogiatzi, P. Sperm DNA Integrity and Male Infertility: A Narrative Review and Guide for the Reproductive Physicians. Transl. Androl. Urol. 2022, 11, 1023–1044. [Google Scholar] [CrossRef]
- Agarwal, A.; Farkouh, A.; Parekh, N.; Zini, A.; Arafa, M. Sperm DNA Fragmentation: A Critical Assessment of Clinical Practice Guidelines. World J. Men’s Health 2022, 40, 30–37. [Google Scholar] [CrossRef]
- Al Omrani, B.; Al Eisa, N.; Javed, M.; Al Ghedan, M.; Al Matrafi, H.; Al Sufyan, H. Associations of Sperm DNA Fragmentation with Lifestyle Factors and Semen Parameters of Saudi Men and Its Impact on ICSI Outcome. Reprod. Biol. Endocrinol. 2018, 16, 49. [Google Scholar] [CrossRef]
- Osman, A.; Alsomait, H.; Seshadri, S.; El-Toukhy, T.; Khalaf, Y. The Effect of Sperm DNA Fragmentation on Live Birth Rate after IVF or ICSI: A Systematic Review and Meta-Analysis. Reprod. Biomed. Online 2015, 30, 120–127. [Google Scholar] [CrossRef]
- Choi, H.Y.; Kim, S.K.; Kim, S.H.; Choi, Y.M.; Jee, B.C. Impact of Sperm DNA Fragmentation on Clinical in Vitro Fertilization Outcomes. Clin. Exp. Reprod. Med. 2017, 44, 224–231. [Google Scholar] [CrossRef]
- Bounartzi, T.; Dafopoulos, K.; Anifandis, G.; Messini, C.I.; Koutsonikou, C.; Kouris, S.; Satra, M.; Sotiriou, S.; Vamvakopoulos, N.; Messinis, I.E. Pregnancy Prediction by Free Sperm DNA and Sperm DNA Fragmentation in Semen Specimens of IVF/ICSI-ET Patients. Hum. Fertil. 2016, 19, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Green, K.A.; Patounakis, G.; Dougherty, M.P.; Werner, M.D.; Scott, R.T.; Franasiak, J.M. Sperm DNA Fragmentation on the Day of Fertilization Is Not Associated with Embryologic or Clinical Outcomes after IVF/ICSI. J. Assist. Reprod. Genet. 2020, 37, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Liang, Z.; Wu, J.; Li, L.; Chen, C.; Jin, F.; Tian, Y. Sperm DNA Fragmentation and Male Fertility: A Retrospective Study of 5114 Men Attending a Reproductive Center. J. Assist. Reprod. Genet. 2021, 38, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Morris, A.; Vogiatzi, P.; Saleh, R.; Sallam, H.; Boitrelle, F.; Garrido, N.; Arafa, M.; Gül, M.; Rambhatla, A.; et al. Predictive Value of Seminal Oxidation-Reduction Potential Analysis for Reproductive Outcomes of ICSI. Reprod. Biomed. Online 2022, 45, 1007–1020. [Google Scholar] [CrossRef]
- Ribeiro, S.; Sousa, M. In Vitro Fertilisation and Intracytoplasmic Sperm Injection Predictive Factors: A Review of the Effect of Female Age, Ovarian Reserve, Male Age, and Male Factor on IVF/ICSI Treatment Outcomes. J. Bras. Reprod. Assist. 2023, 27, 97–111. [Google Scholar] [CrossRef]
- Kaiyal, R.S.; Karna, K.K.; Kuroda, S.; Sgayer, I.; Shlush, E.; Vij, S.C.; Lundy, S.D.; Cannarella, R. Sperm Chromatin Dispersion Assay Reliability and Assisted Reproductive Technology Outcomes: Systematic Review and Meta-Analysis. Andrology 2024, 13, 718–730. [Google Scholar] [CrossRef]
- Stringer, J.M.; Winship, A.; Liew, S.H.; Hutt, K. The Capacity of Oocytes for DNA Repair. Cell. Mol. Life Sci. 2018, 75, 2777–2792. [Google Scholar] [CrossRef]
- Sakkas, D.; Alvarez, J.G. Sperm DNA Fragmentation: Mechanisms of Origin, Impact on Reproductive Outcome, and Analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Yeste, M.; Becerra-Tomás, N.; Aston, K.I.; James, E.R.; Salas-Huetos, A. Clinical Implications of Sperm DNA Damage in IVF and ICSI: Updated Systematic Review and Meta-Analysis. Biol. Rev. 2021, 96, 1284–1300. [Google Scholar] [CrossRef]
- Sedó, C.A.; Bilinski, M.; Lorenzi, D.; Uriondo, H.; Noblía, F.; Longobucco, V.; Lagar, E.V.; Nodar, F. Effect of Sperm DNA Fragmentation on Embryo Development: Clinical and Biological Aspects. J. Bras. Reprod. Assist. 2017, 21, 343–350. [Google Scholar] [CrossRef]
- El-Ela, A.A.; Kandeel, A.; Hassan, E.; Nasr, M.; Esam, Y. Utility of Magnetic Activated Cell Sorting (MACS) in Assisted Reproduction. Al-Azhar Int. Med. J. 2022, 3, 4. [Google Scholar] [CrossRef]
- Wang, Q.; Gu, X.; Chen, Y.; Wang, X.; Lv, J.; Yu, M. The Effect of Sperm DNA Fragmentation on in Vitro Fertilization Outcomes of Unexplained Infertility. Clinics 2023, 78, 100261. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Xiao, S.; Qiu, X.; Jin, J.; Pan, C.; Li, Y.; Fei, Q.; Yang, X.; Zhang, L.; Huang, X. Effect of Sperm DNA Fragmentation on Clinical Outcome of Frozen-Thawed Embryo Transfer and on Blastocyst Formation. PLoS ONE 2014, 9, e94956. [Google Scholar] [CrossRef] [PubMed]
- Ješeta, M.; MyškovÃi, M.; ÁkovÃi, J.Z.; Crha, I.; Crha, K.; Chmelikova, E.; Kistanova, E.; Ventruba, P. Can Oocytes Repair Fragmented DNA of Spermatozoa? Med. J. Cell Biol. 2020, 8, 73–77. [Google Scholar] [CrossRef]
- Meseguer, M.; Santiso, R.; Garrido, N.; García-Herrero, S.; Remohí, J.; Fernandez, J.L. Effect of Sperm DNA Fragmentation on Pregnancy Outcome Depends on Oocyte Quality. Fertil. Steril. 2011, 95, 124–128. [Google Scholar] [CrossRef]
- Ruiz-Díaz, S.; Mazzarella, R.; Navarrete-López, P.; Fernández-González, R.; de Frutos, C.; Maroto, M.; Cucala, C.; Beltrán-Breña, P.; Lombó, M.; Rizos, D.; et al. Bull Spermatozoa Selected by Thermotaxis Exhibit High DNA Integrity, Specific Head Morphometry, and Improve ICSI Outcome. J. Anim. Sci. Biotechnol. 2023, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dehghanpour, F.; Khalili, M.A.; Mangoli, E.; Talebi, A.R.; Anbari, F.; Shamsi, F.; Woodward, B.; Doostabadi, M.R. Free Centrifuge Sorting Method for High-Count Sperm Preparation Improves Biological Characteristics of Human Spermatozoa and Clinical Outcome: A Sibling Oocytes Study. Andrologia 2022, 54, e14554. [Google Scholar] [CrossRef]
- Sharma, S.; Kabir, M.A.; Asghar, W. Selection of Healthy Sperm Based on Positive Rheotaxis Using a Microfluidic Device. Analyst 2022, 147, 1589–1597. [Google Scholar] [CrossRef]
- Doostabadi, M.R.; Mangoli, E.; Marvast, L.D.; Dehghanpour, F.; Maleki, B.; Torkashvand, H.; Talebi, A.R. Microfluidic Devices Employing Chemo- and Thermotaxis for Sperm Selection Can Improve Sperm Parameters and Function in Patients with High DNA Fragmentation. Andrologia 2022, 54, e14623. [Google Scholar] [CrossRef]
- Ali, A.H.; Ajina, T.; Ben Ali, M.; Mehdi, M. Efficacy of Density Gradient Centrifugation Technique (DGC) in Enhancing Sperm Cell DNA Quality for Assisted Reproductive Technique. Middle East Fertil. Soc. J. 2022, 27, 1–9. [Google Scholar] [CrossRef]
- Sarbandi, I.R.; Lesani, A.; Moghimi Zand, M.; Nosrati, R. Rheotaxis-Based Sperm Separation Using a Biomimicry Microfluidic Device. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, S.A.; Ding, L.; Parast, F.Y.; Nosrati, R.; Warkiani, M.E. Sperm Quality Metrics Were Improved by a Biomimetic Microfluidic Selection Platform Compared to Swim-up Methods. Microsystems Nanoeng. 2023, 9, 1–14. [Google Scholar] [CrossRef]
- Ahmadi, H.; Aghebati-Maleki, L.; Rashidiani, S.; Csabai, T.; Nnaemeka, O.B.; Szekeres-Bartho, J. Long-Term Effects of ART on the Health of the Offspring. Int. J. Mol. Sci. 2023, 24, 13564. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Simoni, M. Sperm DNA Fragmentation Index as a Promising Predictive Tool for Male Infertility Diagnosis and Treatment Management—Meta-Analyses. Reprod. Biomed. Online 2018, 37, 315–326. [Google Scholar] [CrossRef]
- Datta, A.K.; Campbell, S.; Diaz-Fernandez, R.; Nargund, G. Livebirth Rates Are Influenced by an Interaction between Male and Female Partners’ Age: Analysis of 59 951 Fresh IVF/ICSI Cycles with and without Male Infertility. Hum. Reprod. 2024, 39, 2491–2500. [Google Scholar] [CrossRef]
- Vaegter, K.K.; Lakic, T.G.; Olovsson, M.; Berglund, L.; Brodin, T.; Holte, J. Which Factors Are Most Predictive for Live Birth after in Vitro Fertilization and Intracytoplasmic Sperm Injection (IVF/ICSI) Treatments? Analysis of 100 Prospectively Recorded Variables in 8,400 IVF/ICSI Single-Embryo Transfers. Fertil. Steril. 2017, 107, 641–648.e2. [Google Scholar] [CrossRef] [PubMed]
- Repalle, D.; Saritha, K.V.; Bhandari, S.; Chittora, M.; Choudhary, J. Role of Female Age in Regulating the Effect of Sperm DNA Fragmentation on the Live Birth Rates in Intracytoplasmic Sperm Injection Cycles with Own and Donor Oocytes. J. Hum. Reprod. Sci. 2022, 15, 64–71. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Stavros, S.; Potiris, A.; Molopodi, E.; Mavrogianni, D.; Zikopoulos, A.; Louis, K.; Karampitsakos, T.; Nazou, E.; Sioutis, D.; Christodoulaki, C.; et al. Sperm DNA Fragmentation: Unraveling Its Imperative Impact on Male Infertility Based on Recent Evidence. Int. J. Mol. Sci. 2024, 25, 10167. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G. Pregnancies after Intracytoplasmic Injection of Single Spermatozoon into an Oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Tannus, S.; Son, W.Y.; Gilman, A.; Younes, G.; Shavit, T.; Dahan, M.H. The Role of Intracytoplasmic Sperm Injection in Non-Male Factor Infertility in Advanced Maternal Age. Hum. Reprod. 2017, 32, 119–124. [Google Scholar] [CrossRef]
- Amirjannati, N.; Mohazzab, A.; Fathalian, M.; Akhavizadegan, H. Comparison of Embryological Results of Microinjection in Two Groups of Men with and without Requesting Sperm DNA Fragmentation Index Measurement. Bio. Med. Res. Int. 2024, 2024, 1–6. [Google Scholar] [CrossRef]
- Siddhartha, N.; Reddy, N.S.; Pandurangi, M.; Muthusamy, T.; Vembu, R.; Kasinathan, K. The Effect of Sperm DNA Fragmentation Index on the Outcome of Intrauterine Insemination and Intracytoplasmic Sperm Injection. J. Hum. Reprod. Sci. 2019, 12, 189–198. [Google Scholar] [CrossRef]
- Sun, T.C.; Zhang, Y.; Li, H.T.; Liu, X.M.; Yi, D.X.; Tian, L.; Liu, Y.X. Sperm DNA Fragmentation Index, as Measured by Sperm Chromatin Dispersion, Might Not Predict Assisted Reproductive Outcome. Taiwan. J. Obstet. Gynecol. 2018, 57, 493–498. [Google Scholar] [CrossRef] [PubMed]

| Parameter | No. of Cycles | Mean ± SD | Rate [%] |
|---|---|---|---|
| Female age [years] | 870 | 36.10 ± 4.72 | — |
| Male age [years] | 870 | 38.12 ± 5.88 | — |
| Duration of infertility [years] | 429 * | 3.82 ± 2.39 | — |
| Sperm concentration [×106/mL] | 870 | 52.30 ± 56.44 | — |
| Fast progressive motility [%] | 870 | 11.85 ± 9.71 | — |
| Normal sperm morphology [%] | 870 | 2.56 ± 1.12 | — |
| Sperm DNA fragmentation (SDF) [%] | 870 | 16.17 ± 10.38 | — |
| Fertilization rate [% per oocyte injected] | 870 | 78.25 ± 24.42 | 83.33 (median) |
| Blastocyst development rate [% per fertilized MII oocyte] | 870 | — | 57.41 (mean); 60 (median) |
| Good-quality blastocyst [%] | 870 | 26.28 ± 25.57 | — |
| Implantation rate [% per transfer] | 870 | — | 49.06 |
| Clinical pregnancy rate [% per transfer] | 377/659 | — | 57.21 |
| Miscarriage rate [% per clinical pregnancy] | 49/377 | — | 12.99 |
| Comparison Group | Group A | Group B | SDF [%] (Mean ± SD) | p-Value * |
|---|---|---|---|---|
| Fertilization Rate (FR) | FR < 80% (n = 384) | FR ≥ 80% (n = 486) | 17.04 ± 10.50 vs. 15.47 ± 10.24 | 0.009 |
| Top-quality embryos day 3 (TQ3) | TQD3 < 50% (n = 799) | TQD3 ≥ 50% (n = 71) | 16.26 ± 10.30 vs. 15.13 ± 11.20 | 0.184 |
| Top-quality blastocysts (TQ5) | TQD5 < 50% (n = 751) | TQD5 ≥ 50% (n = 119) | 16.41 ± 10.56 vs. 14.60 ± 9.05 | 0.056 |
| Pregnancy Outcome | No pregnancy (n = 303) | Pregnancy (n = 406) | 16.47 ± 10.33 vs. 15.40 ± 9.56 | 0.210 |
| Pregnancy Result | Clinical Pregnancy (n = 377) | Miscarriage (n = 49) | 16.39 ± 10.39 vs. 13.33 ± 7.29 | 0.037 |
| Parameter | SDF > 20% (n = 206) | SDF ≤ 20% (n = 664) | p-Value (Mann–Whitney U/χ2) | Correlation with SDF (Spearman’s r) | p-Value (Spearman) |
|---|---|---|---|---|---|
| Male age [years] | 39.80 ± 6.60 | 37.60 ± 5.54 | <0.001 | 0.162 | <0.001 |
| Male BMI [kg/m2] | 26.8 ± 4.71 | 26.55 ± 4.32 | 0.755 | −0.043 | 0.287 |
| Sperm concentration [×106/mL] | 46.71 ± 62.59 | 53.93 ± 54.46 | <0.001 | −0.179 | <0.001 |
| Fast progressive motility [%] | 8.91 ± 9.06 | 12.71 ± 9.73 | <0.001 | −0.271 | <0.001 |
| Slow progressive motility [%] | 14.90 ± 10.94 | 21.47 ± 11.70 | <0.001 | −0.332 | <0.001 |
| Immotile sperm cells [%] | 15.02 ± 6.75 | 18.01 ± 7.04 | <0.001 | 0.398 | <0.001 |
| Normal sperm morphology [%] | 2.00 ± 1.92 | 2.00 ± 1.88 | 0.083 | −0.137 | <0.001 |
| Teratozoospermia Index (TZI) | 2.84 ± 1.73 | 2.56 ± 1.68 | 0.020 | – | – |
| Fertilization rate [%] | 74.48 ± 26.72 | 79.42 ± 23.57 | 0.019 | −0.084 | 0.012 |
| Top-quality embryos (day 3) [%] | 16.67 ± 25.51 | 19.09 ± 23.38 | 0.187 | −0.069 | 0.040 |
| Top-quality blastocysts [%] | 21.30 ± 23.08 | 27.83 ± 26.12 | <0.001 | −0.110 | 0.001 |
| Clinical pregnancy rate [%] | 51.85% (84/162) | 58.86% (322/547) | 0.113 | – | – |
| Parameter | OR | 95% CI | Coefficient (β) | Std. Error | Wald χ2 | p-Value |
|---|---|---|---|---|---|---|
| Fertilization rate > 80% | 0.984 | 0.971–0.997 | −0.0162 | 0.0067 | 5.92 | 0.015 |
| Top-quality embryos (Day 3) | 0.983 | 0.966–1.001 | −0.0169 | 0.0092 | 3.33 | 0.068 |
| Top-quality blastocysts (Day 5) | 0.975 | 0.958–0.992 | −0.0255 | 0.0088 | 8.35 | 0.003 |
| Clinical pregnancy (n = 377/659) | 0.989 | 0.974–1.004 | −0.0109 | 0.0077 | 2.02 | 0.154 |
| Miscarriage (n = 49/377) | 0.961 | 0.924–1.000 | −0.0396 | 0.0204 | 3.76 | 0.052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machałowski, T.; Machałowska, J.; Gill, K.; Ziętek, M.; Piasecka, M.; Mrugacz, G.; Ciepiela, P. Sperm DNA Fragmentation Impairs Early Embryo Development but Is Not Predictive of Pregnancy Outcomes: Insights from 870 ICSI Cycles. Int. J. Mol. Sci. 2025, 26, 7923. https://doi.org/10.3390/ijms26167923
Machałowski T, Machałowska J, Gill K, Ziętek M, Piasecka M, Mrugacz G, Ciepiela P. Sperm DNA Fragmentation Impairs Early Embryo Development but Is Not Predictive of Pregnancy Outcomes: Insights from 870 ICSI Cycles. International Journal of Molecular Sciences. 2025; 26(16):7923. https://doi.org/10.3390/ijms26167923
Chicago/Turabian StyleMachałowski, Tomasz, Julita Machałowska, Kamil Gill, Maciej Ziętek, Małgorzata Piasecka, Grzegorz Mrugacz, and Przemysław Ciepiela. 2025. "Sperm DNA Fragmentation Impairs Early Embryo Development but Is Not Predictive of Pregnancy Outcomes: Insights from 870 ICSI Cycles" International Journal of Molecular Sciences 26, no. 16: 7923. https://doi.org/10.3390/ijms26167923
APA StyleMachałowski, T., Machałowska, J., Gill, K., Ziętek, M., Piasecka, M., Mrugacz, G., & Ciepiela, P. (2025). Sperm DNA Fragmentation Impairs Early Embryo Development but Is Not Predictive of Pregnancy Outcomes: Insights from 870 ICSI Cycles. International Journal of Molecular Sciences, 26(16), 7923. https://doi.org/10.3390/ijms26167923







