Protective Effects of Regular Physical Activity: Differential Expression of FGF21, GDF15, and Their Receptors in Trained and Untrained Individuals
Abstract
1. Introduction
2. Results
2.1. Comparison Gene Expression Between Trained and Non-Trained Group
2.2. Comparison Gene Expression Between Time Points in Trained and Non-Trained Group
2.3. Analysis of ELISA Results
2.4. Correlation Analysis
3. Discussion
Limitations
4. Materials and Methods
4.1. Participants
4.2. Physical Activity
4.3. Sample Collection
4.4. Isolation of the RNA and cDNA Synthesis
4.5. Real-Time PCR
4.6. ELISA
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FGF21 | fibroblast growth factor 21 |
| GDF15 | growth differentiation factor 15 |
| FGFR | fibroblast growth factor receptors |
| KLB | β-klotho |
| MIC-1 | macrophage inhibitory cytokine 1 |
| TGF-β | transforming growth factor-β |
| GFRAL | the glial cell line-derived neurotrophic factor receptor alpha-like |
| GAPDH | human glyceraldehyde 3-phosphate dehydrogenase |
| Ct | cycle threshold |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| ELISA | enzyme-linked immunosorbent assay |
References
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Letizia Mauro, G. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. BioMed Res. Int. 2018, 2018, 4840531. [Google Scholar] [CrossRef] [PubMed]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Conn, V.S.; Koopman, R.J.; Ruppar, T.M.; Phillips, L.J.; Mehr, D.R.; Hafdahl, A.R. Insulin Sensitivity Following Exercise Interventions: Systematic Review and Meta-Analysis of Outcomes Among Healthy Adults. J. Prim. Care Community Health 2014, 5, 211–222. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Diabetes Prevention Program Research Group Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Schuch, F.B.; Stubbs, B.; Meyer, J.; Heissel, A.; Zech, P.; Vancampfort, D.; Rosenbaum, S.; Deenik, J.; Firth, J.; Ward, P.B.; et al. Physical Activity Protects from Incident Anxiety: A Meta-Analysis of Prospective Cohort Studies. Depress. Anxiety 2019, 36, 846–858. [Google Scholar] [CrossRef]
- Maciejewska-Skrendo, A.; Tarnowski, M.; Kopytko, P.; Kochanowicz, A.; Mieszkowski, J.; Stankiewicz, B.; Sawczuk, M. CCL2 Gene Expression and Protein Level Changes Observed in Response to Wingate Anaerobic Test in High-Trained Athletes and Non-Trained Controls. Int. J. Environ. Res. Public Health 2022, 19, 9947. [Google Scholar] [CrossRef]
- Małkowska, P.; Sawczuk, M. Cytokines as Biomarkers for Evaluating Physical Exercise in Trained and Non-Trained Individuals: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 11156. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in Health, Resilience and Disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Thyfault, J.P.; Bergouignan, A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia 2020, 63, 1464. [Google Scholar] [CrossRef]
- Izumiya, Y.; Bina, H.A.; Ouchi, N.; Akasaki, Y.; Kharitonenkov, A.; Walsh, K. FGF21 Is an Akt-Regulated Myokine. FEBS Lett. 2008, 582, 3805–3810. [Google Scholar] [CrossRef] [PubMed]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARalpha and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine Regulation of the Fasting Response by PPARalpha-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Adya, R.; Chen, J.; Ramanjaneya, M.; Bari, M.F.; Bhudia, S.K.; Hillhouse, E.W.; Tan, B.K.; Randeva, H.S. Novel Insights into the Cardio-Protective Effects of FGF21 in Lean and Obese Rat Hearts. PLoS ONE 2014, 9, e87102. [Google Scholar] [CrossRef]
- Muise, E.S.; Azzolina, B.; Kuo, D.W.; El-Sherbeini, M.; Tan, Y.; Yuan, X.; Mu, J.; Thompson, J.R.; Berger, J.P.; Wong, K.K. Adipose Fibroblast Growth Factor 21 Is Up-Regulated by Peroxisome Proliferator-Activated Receptor Gamma and Altered Metabolic States. Mol. Pharmacol. 2008, 74, 403–412. [Google Scholar] [CrossRef]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic Activation Induces FGF21 Expression and Release in Brown Adipose Tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Inagaki, T.; Satapati, S.; Ding, X.; He, T.; Goetz, R.; Mohammadi, M.; Finck, B.N.; Mangelsdorf, D.J.; Kliewer, S.A.; et al. FGF21 Induces PGC-1α and Regulates Carbohydrate and Fatty Acid Metabolism during the Adaptive Starvation Response. Proc. Natl. Acad. Sci. USA 2009, 106, 10853. [Google Scholar] [CrossRef]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast Growth Factor 21 Corrects Obesity in Mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Mangelsdorf, D.J. Fibroblast Growth Factor 21: From Pharmacology to Physiology1. Am. J. Clin. Nutr. 2009, 91, 254S–257S. [Google Scholar] [CrossRef]
- Oost, L.J.; Kustermann, M.; Armani, A.; Blaauw, B.; Romanello, V. Fibroblast Growth Factor 21 Controls Mitophagy and Muscle Mass. J. Cachexia Sarcopenia Muscle 2019, 10, 630–642. [Google Scholar] [CrossRef]
- Suomalainen, A.; Elo, J.M.; Pietiläinen, K.H.; Hakonen, A.H.; Sevastianova, K.; Korpela, M.; Isohanni, P.; Marjavaara, S.K.; Tyni, T.; Kiuru-Enari, S.; et al. FGF-21 as a Biomarker for Muscle-Manifesting Mitochondrial Respiratory Chain Deficiencies: A Diagnostic Study. Lancet Neurol. 2011, 10, 806–818. [Google Scholar] [CrossRef]
- Lehtonen, J.M.; Forsström, S.; Bottani, E.; Viscomi, C.; Baris, O.R.; Isoniemi, H.; Höckerstedt, K.; Österlund, P.; Hurme, M.; Jylhävä, J.; et al. FGF21 Is a Biomarker for Mitochondrial Translation and mtDNA Maintenance Disorders. Neurology 2016, 87, 2290–2299. [Google Scholar] [CrossRef]
- Ming, A.Y.K.; Yoo, E.; Vorontsov, E.N.; Altamentova, S.M.; Kilkenny, D.M.; Rocheleau, J.V. Dynamics and Distribution of Klothoβ (KLB) and Fibroblast Growth Factor Receptor-1 (FGFR1) in Living Cells Reveal the Fibroblast Growth Factor-21 (FGF21)-Induced Receptor Complex. J. Biol. Chem. 2012, 287, 19997–20006. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a Novel Macrophage Inhibitory Cytokine, Is a Divergent Member of the TGF-β Superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Taniguchi, Y.; Shinkai, S.; Tanaka, M.; Ito, M. Secreted Growth Differentiation Factor 15 as a Potential Biomarker for Mitochondrial Dysfunctions in Aging and Age-Related Disorders. Geriatr. Gerontol. Int. 2016, 16, 17–29. [Google Scholar] [CrossRef]
- Johann, K.; Kleinert, M.; Klaus, S. The Role of GDF15 as a Myomitokine. Cells 2021, 10, 2990. [Google Scholar] [CrossRef]
- Conte, M.; Martucci, M.; Mosconi, G.; Chiariello, A.; Cappuccilli, M.; Totti, V.; Santoro, A.; Franceschi, C.; Salvioli, S. GDF15 Plasma Level Is Inversely Associated With Level of Physical Activity and Correlates with Markers of Inflammation and Muscle Weakness. Front. Immunol. 2020, 11, 915. [Google Scholar] [CrossRef]
- Huang, J.; Ding, X.; Dong, Y.; Zhu, H. Growth Differentiation Factor-15 Orchestrates Inflammation-Related Diseases via Macrophage Polarization. Discov. Med. 2024, 36, 248–255. [Google Scholar] [CrossRef]
- Ito, T.; Nakanishi, Y.; Yamaji, N.; Murakami, S.; Schaffer, S.W. Induction of Growth Differentiation Factor 15 in Skeletal Muscle of Old Taurine Transporter Knockout Mouse. Biol. Pharm. Bull. 2018, 41, 435–439. [Google Scholar] [CrossRef]
- Tang, H.; Inoki, K.; Brooks, S.V.; Okazawa, H.; Lee, M.; Wang, J.; Kim, M.; Kennedy, C.L.; Macpherson, P.C.D.; Ji, X.; et al. mTORC1 Underlies Age-Related Muscle Fiber Damage and Loss by Inducing Oxidative Stress and Catabolism. Aging Cell 2019, 18, e12943. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.I.; Ost, M.; Kasch, J.; Schumann, S.; Heider, S.; Klaus, S. Role of GDF15 in Active Lifestyle Induced Metabolic Adaptations and Acute Exercise Response in Mice. Sci. Rep. 2019, 9, 20120. [Google Scholar] [CrossRef]
- Patel, M.S.; Lee, J.; Baz, M.; Wells, C.E.; Bloch, S.; Lewis, A.; Donaldson, A.V.; Garfield, B.E.; Hopkinson, N.S.; Natanek, A.; et al. Growth Differentiation Factor-15 Is Associated with Muscle Mass in Chronic Obstructive Pulmonary Disease and Promotes Muscle Wasting in Vivo. J. Cachexia Sarcopenia Muscle 2016, 7, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a Novel Metabolic Regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, S.; Barari, A.; Abdi, A. The Effect of Special Training Exercise on FGF21 Expression and FGFR-1 among CABG Patients. Res. Bull. Med. Sci. 2021, 26, e16. [Google Scholar]
- Tang, S.; Hao, Y.; Yuan, Y.; Liu, R.; Chen, Q. Role of Fibroblast Growth Factor Receptor 4 in Cancer. Cancer Sci. 2018, 109, 3024–3031. [Google Scholar] [CrossRef]
- Wen, X.; Li, X.; Tang, Y.; Tang, J.; Zhou, S.; Xie, Y.; Guo, J.; Yang, J.; Du, X.; Su, N.; et al. Chondrocyte FGFR3 Regulates Bone Mass by Inhibiting Osteogenesis. J. Biol. Chem. 2016, 291, 24912–24921. [Google Scholar] [CrossRef]
- Kozhemyakina, E.; Lassar, A.B.; Zelzer, E. A Pathway to Bone: Signaling Molecules and Transcription Factors Involved in Chondrocyte Development and Maturation. Development 2015, 142, 817. [Google Scholar] [CrossRef]
- Jena, J.; García-Peña, L.M.; Pereira, R.O. The Roles of FGF21 and GDF15 in Mediating the Mitochondrial Integrated Stress Response. Front. Endocrinol. 2023, 14, 1264530. [Google Scholar] [CrossRef]
- Campderrós, L.; Sánchez-Infantes, D.; Villarroya, J.; Nescolarde, L.; Bayès-Genis, A.; Cereijo, R.; Roca, E.; Villarroya, F. Altered GDF15 and FGF21 Levels in Response to Strenuous Exercise: A Study in Marathon Runners. Front. Physiol. 2020, 11, 550102. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Higuchi, M. Endurance Exercise Reduces Hepatic Fat Content and Serum Fibroblast Growth Factor 21 Levels in Elderly Men. J. Clin. Endocrinol. Metab. 2016, 101, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Clemmensen, C.; Sjøberg, K.A.; Carl, C.S.; Jeppesen, J.F.; Wojtaszewski, J.F.P.; Kiens, B.; Richter, E.A. Exercise Increases Circulating GDF15 in Humans. Mol. Metab. 2018, 9, 187–191. [Google Scholar] [CrossRef]
- Klein, A.B.; Nicolaisen, T.S.; Ørtenblad, N.; Gejl, K.D.; Jensen, R.; Fritzen, A.M.; Larsen, E.L.; Karstoft, K.; Poulsen, H.E.; Morville, T.; et al. Pharmacological but Not Physiological GDF15 Suppresses Feeding and the Motivation to Exercise. Nat. Commun. 2021, 12, 1041. [Google Scholar] [CrossRef]
- Vecchiatto, B.; de Castro, T.L.; Muller, C.R.; Azevedo-Martins, A.K.; Evangelista, F.S. Physical Exercise-Induced FGF-21 to Fight Obesity: An Update Review. Obesities 2022, 2, 372–379. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscles and Their Myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctôt, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The Effects of Physical Activity and Exercise on Brain-Derived Neurotrophic Factor in Healthy Humans: A Review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
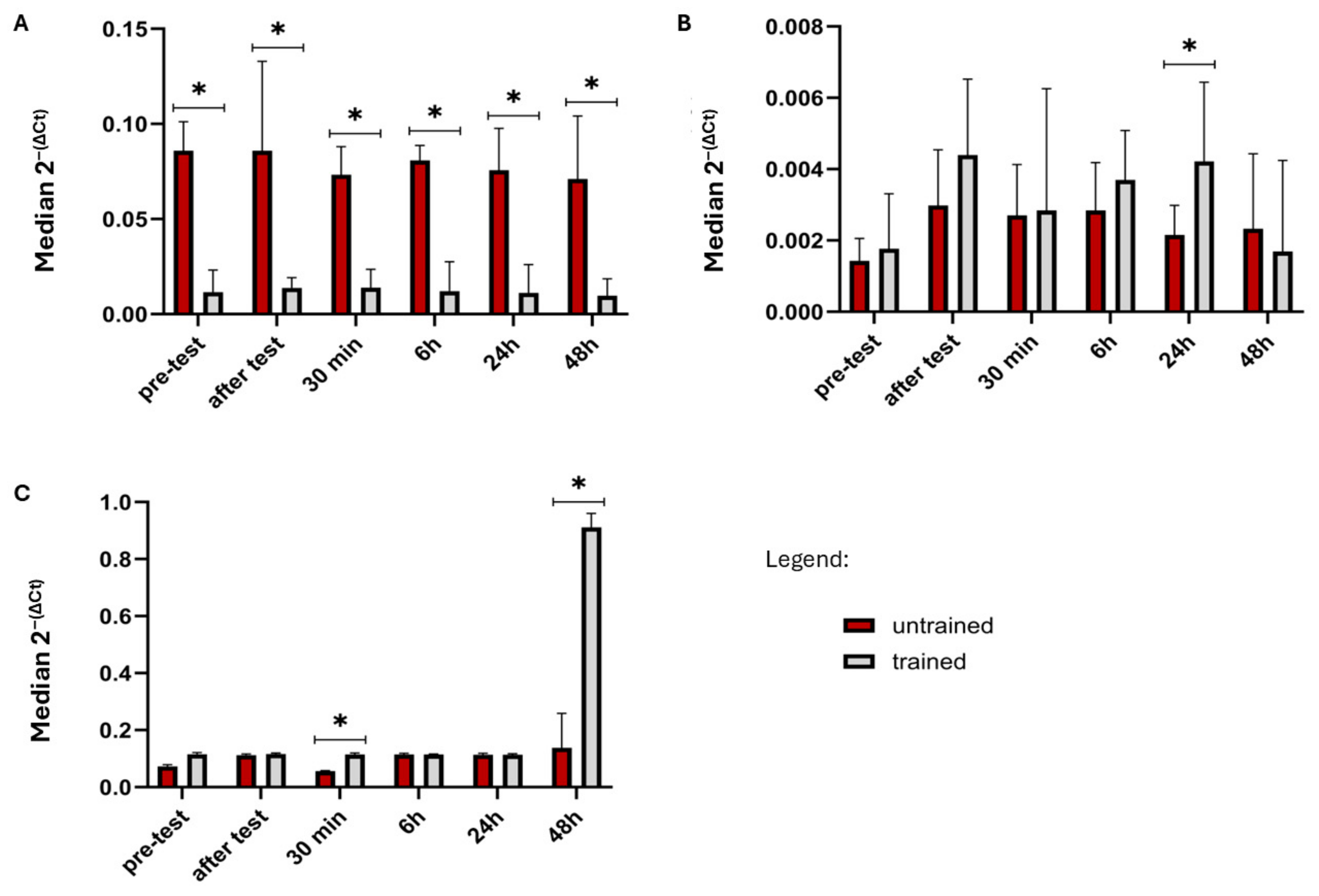
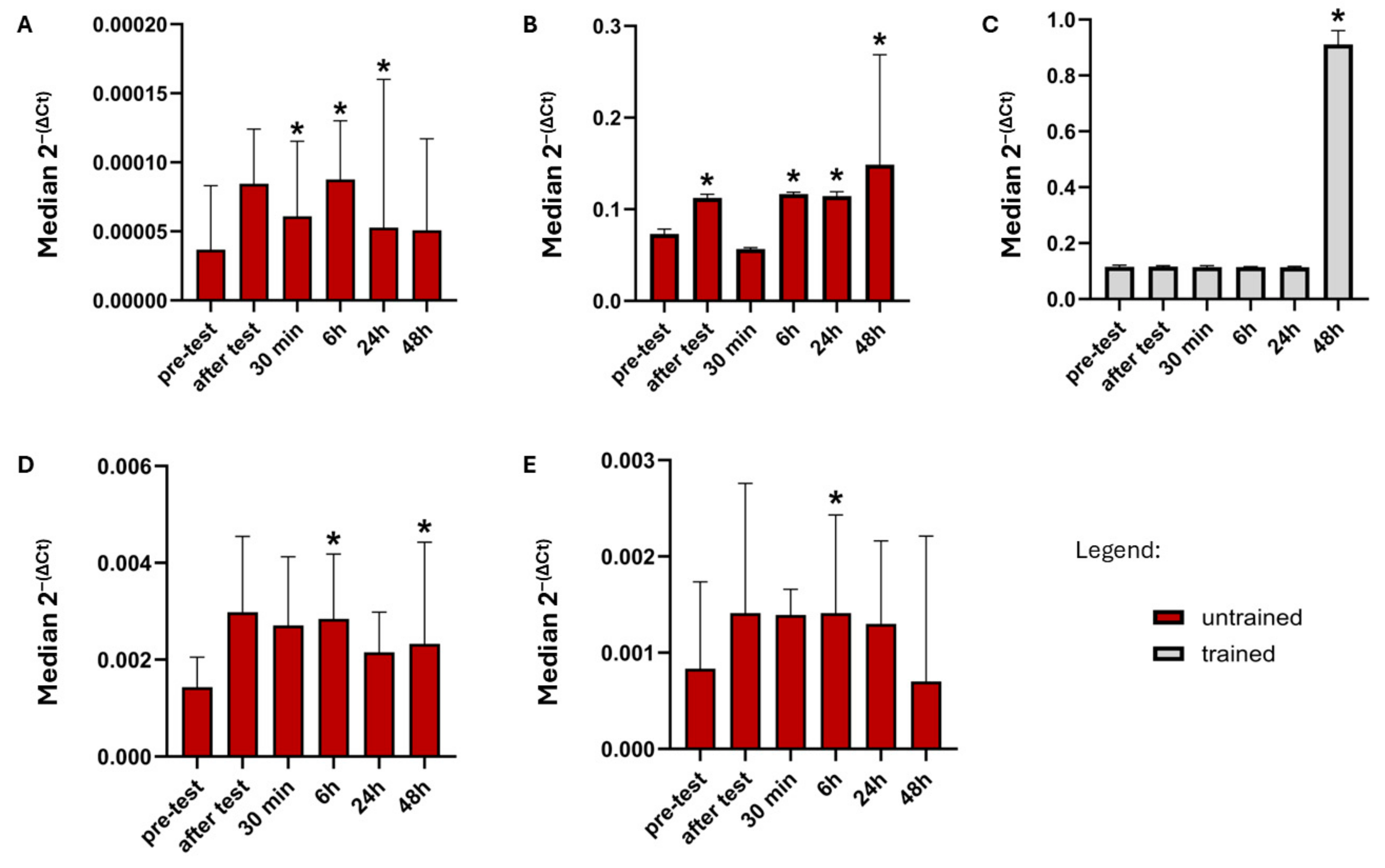
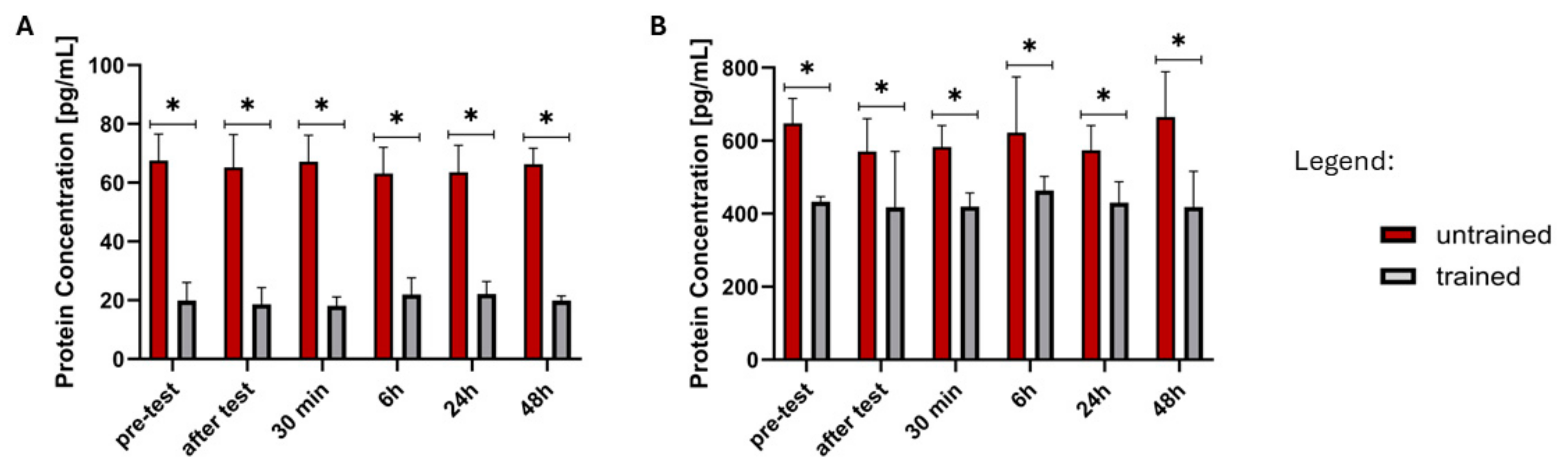
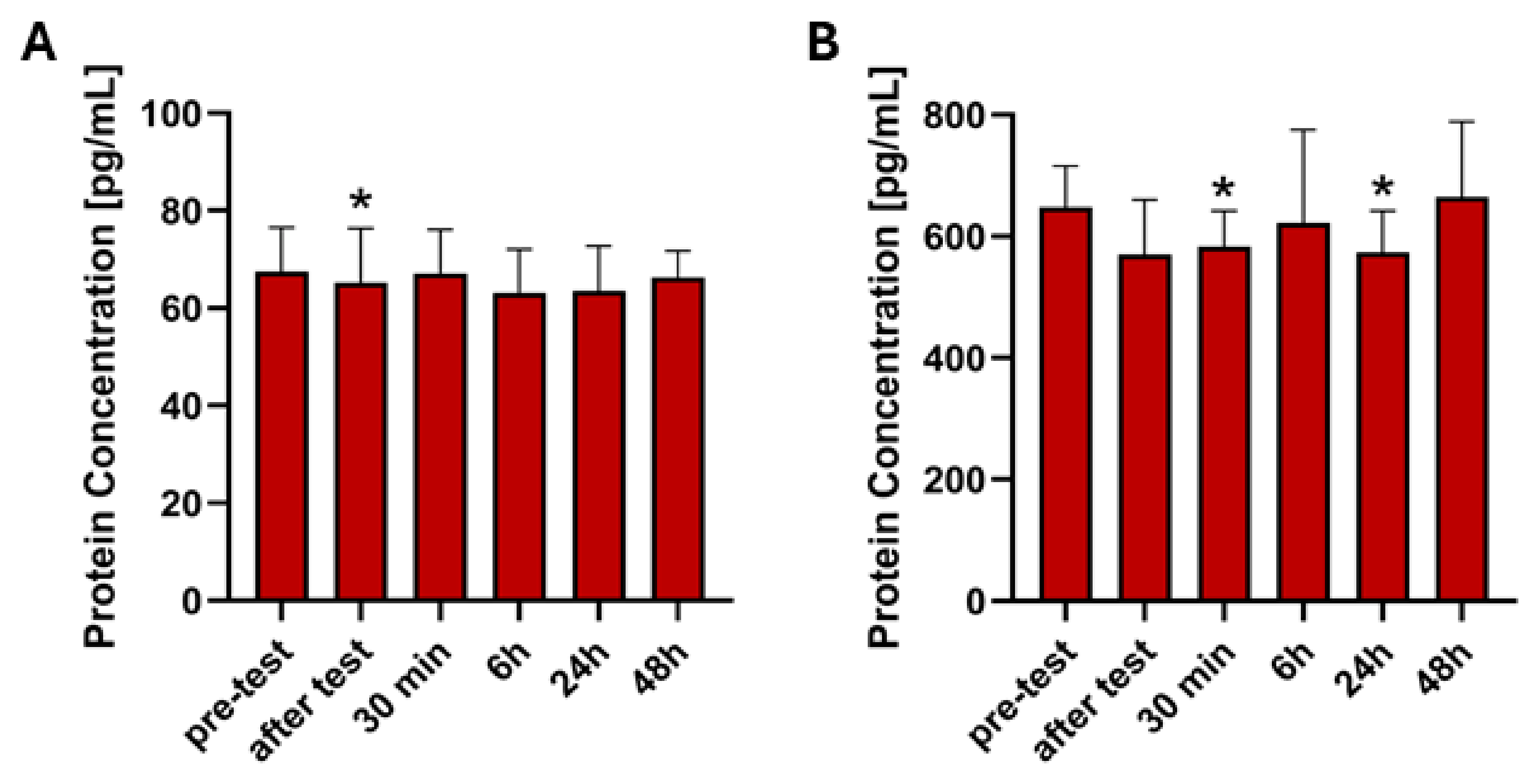
| Gene | Pre-Exercise | Immediately Post-Exercise | 0.5 h Post- Exercise | 6 h Post- Exercise | 24 h Post- Exercise | 48 h Post- Exercise |
|---|---|---|---|---|---|---|
| FGF21 | UT < T (Z = −1.198, p = 0.231) | UT < T (Z = −0.415, p = 0.678) | UT < T (Z = −0.967, p = 0.333) | UT < T (Z = −1.267, p = 0.205) | UT < T (Z = −0.760, p = 0.447) | UT < T (Z = −0.184, p = 0.854) |
| GDF15 | UT < T (Z = −1.059, p = 0.289) | UT < T (Z = −0.829, p = 0.407) | UT < T (Z = −1.152, p = 0.249) | UT < T (Z = −1.013, p = 0.311) | UT < T (Z = −1.106, p = 0.269) | UT < T (Z = −0.553, p = 0.580) |
| FGFR1 | UT > T (Z = 3.869, p = 0.0001) | UT > T (Z = 3.824, p = 0.0001) | UT > T (Z = 3.869, p = 0.0001) | UT > T (Z = 3.271, p = 0.0011) | UT > T (Z = 3.685, p = 0.0002) | UT > T (Z = 3.962, p = 0.00007) |
| FGFR3 | UT < T (Z = −1.013, p = 0.311) | UT < T (Z = −1.289, p = 0.197) | UT < T (Z = −1.013, p = 0.311) | UT < T (Z = −0.829, p = 0.407) | UT < T (Z = −2.257, p = 0.024) | UT > T (Z = 0.253, p = 0.800) |
| FGFR4 | UT < T (Z = −1.843, p = 0.065) | UT < T (Z = −0.576, p = 0.565) | UT < T (Z = −2.303, p = 0.021) | UT < T (Z = 0.345, p = 0.730) | UT < T (Z = −1.681, p = 0.093) | UT < T (Z = −2.879, p = 0.004) |
| GFRAL | UT < T (Z = −1.474, p = 0.140) | UT < T (Z = −0.875, p = 0.381) | UT < T (Z = −1.036, p = 0.300) | UT < T (Z = −1.566, p = 0.117) | UT < T (Z = −1.152, p = 0.249) | UT < T (Z = 1.244, p = 0.214) |
| KLB | UT < T (Z = −0.921, p = 0.357) | UT < T (Z = −0.553, p = 0.580) | UT < T (Z = −0.645, p = 0.519) | UT < T (Z = −1.129, p = 0.259) | UT < T (Z = −0.345, p = 0.730) | UT < T (Z = −0.184, p = 0.854) |
| Gene | Group | After Test | 0.5 h | 6 h | 24 h | 48 h |
|---|---|---|---|---|---|---|
| FGF21 | UT | nd | nd | nd | nd | nd |
| T | nd | nd | nd | nd | nd | |
| GDF15 | UT | nd | nd | nd | nd | nd |
| T | nd | nd | nd | nd | nd | |
| FGFR1 | UT | nd | nd | nd | nd | nd |
| T | nd | nd | nd | nd | nd | |
| FGFR3 | UT | nd | nd | ↑ (Z = 2.132, p = 0.0330) | nd | ↑ (Z = 1.992, p = 0.0464) |
| T | nd | nd | nd | nd | nd | |
| FGFR4 | UT | ↑ (Z = 2.411, p = 0.0159) | nd | ↑ (Z = 2.691, p = 0.0071) | ↑ (Z = 2.481, p = 0.0131) | ↑ (Z = 2.760, p = 0.0058) |
| T | nd | nd | nd | nd | ↑ (Z = 3.408, p = 0.0007) | |
| KLB | UT | nd | ↑ (Z = 2.510, p = 0.0121) | ↑ (Z = 2.621, p = 0.0088) | ↑ (Z = 1.992, p = 0.0464) | nd |
| T | nd | nd | nd | nd | nd | |
| GFRAL | UT | nd | nd | ↑ (Z = 2.341, p = 0.0192) | nd | nd |
| T | nd | nd | nd | nd | nd |
| Protein | Group | After Test | 0.5 h | 6 h | 24 h | 48 h |
|---|---|---|---|---|---|---|
| FGF21 | UT | ↓ (Z = 2.062, p = 0.0392) | nd | nd | nd | nd |
| T | nd | nd | nd | nd | nd | |
| GDF15 | UT | nd | ↓ (Z = 2.481, p = 0.0131) | nd | ↓ (Z = 1.992, p = 0.0464) | nd |
| T | nd | nd | nd | nd | nd |
| Time Point | Gene/Protein | Spearman’s R (Untrained) | p-Value (Untrained) | Spearman’s R (Trained) | p-Value (Trained) |
|---|---|---|---|---|---|
| Pre-test | FGF21 | 0.0604 | 0.8445 | −0.0268 | 0.9244 |
| GDF15 | −0.0604 | 0.8445 | 0.2250 | 0.4201 | |
| After test | FGF21 | −0.2641 | 0.3833 | 0.2390 | 0.3909 |
| GDF15 | −0.2473 | 0.4154 | 0.2750 | 0.3212 | |
| 0.5 h | FGF21 | 0.0385 | 0.9006 | 0.0947 | 0.7370 |
| GDF15 | −0.4505 | 0.1223 | 0.3000 | 0.2773 | |
| 6 h | FGF21 | 0.2393 | 0.4310 | −0.1107 | 0.6945 |
| GDF15 | −0.1099 | 0.7208 | 0.3643 | 0.1819 | |
| 24 h | FGF21 | 0.3269 | 0.2757 | 0.2182 | 0.4346 |
| GDF15 | −0.2143 | 0.4821 | 0.4701 | 0.0770 | |
| 48 h | FGF21 | 0.0934 | 0.7615 | 0.0206 | 0.9419 |
| GDF15 | −0.2967 | 0.3249 | 0.0876 | 0.7563 |
| Gene | Name | Forward | Reverse |
|---|---|---|---|
| hGAPDH | glyceraldehyde 3-phosphate dehydrogenase | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
| hFGF21 | fibroblast growth factor 21 | CTGCAGCTGAAAGCCTTGAAGC | GTATCCGTCCTCAAGAAGCAGC |
| hFGFR1 | fibroblast growth factor receptor 1 | TAATGGACTCTGTGGTGCCCTC | ATGTGTGGTTGATGCTGCCG |
| hFGFR3 | fibroblast growth factor receptor 3 | TCCATCTCCTGGCTGAAGAACG | TGTTCTCCACGACGCAGGTGTA |
| hFGFR4 | fibroblast growth factor receptor 4 | AACACCGTCAAGTTCCGCTGTC | CATCACGAGACTCCAGTGCTGA |
| hKLB | β-Klotho | AAGAGTCCACGCCAGATGTGCA | CCACACGTACAGATGAGGATCG |
| hGDF15 | growth differentiation factor 15 | CAACCAGAGCTGGGAAGATTCG | CCCGAGAGATACGCAGGTGCA |
| hGFRAL | glial cell-derived neurotrophic factor (GDNF) family receptor α-like | GGAGAGTAATGGAAGATGCCTGC | GAAGTCATCAGTGCAAAGACACTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małkowska, P.; Tomasiak, P.; Tkacz, M.; Zgutka, K.; Tarnowski, M.; Maciejewska-Skrendo, A.; Buryta, R.; Rosiński, Ł.; Sawczuk, M. Protective Effects of Regular Physical Activity: Differential Expression of FGF21, GDF15, and Their Receptors in Trained and Untrained Individuals. Int. J. Mol. Sci. 2025, 26, 7115. https://doi.org/10.3390/ijms26157115
Małkowska P, Tomasiak P, Tkacz M, Zgutka K, Tarnowski M, Maciejewska-Skrendo A, Buryta R, Rosiński Ł, Sawczuk M. Protective Effects of Regular Physical Activity: Differential Expression of FGF21, GDF15, and Their Receptors in Trained and Untrained Individuals. International Journal of Molecular Sciences. 2025; 26(15):7115. https://doi.org/10.3390/ijms26157115
Chicago/Turabian StyleMałkowska, Paulina, Patrycja Tomasiak, Marta Tkacz, Katarzyna Zgutka, Maciej Tarnowski, Agnieszka Maciejewska-Skrendo, Rafał Buryta, Łukasz Rosiński, and Marek Sawczuk. 2025. "Protective Effects of Regular Physical Activity: Differential Expression of FGF21, GDF15, and Their Receptors in Trained and Untrained Individuals" International Journal of Molecular Sciences 26, no. 15: 7115. https://doi.org/10.3390/ijms26157115
APA StyleMałkowska, P., Tomasiak, P., Tkacz, M., Zgutka, K., Tarnowski, M., Maciejewska-Skrendo, A., Buryta, R., Rosiński, Ł., & Sawczuk, M. (2025). Protective Effects of Regular Physical Activity: Differential Expression of FGF21, GDF15, and Their Receptors in Trained and Untrained Individuals. International Journal of Molecular Sciences, 26(15), 7115. https://doi.org/10.3390/ijms26157115





