Pathogenesis of Cardiac Valvular Hemangiomas: A Case Report and Literature Review
Abstract
1. Introduction
2. Case Presentation
2.1. Personal History
2.2. Clinical Assessment
2.3. Laboratory Results
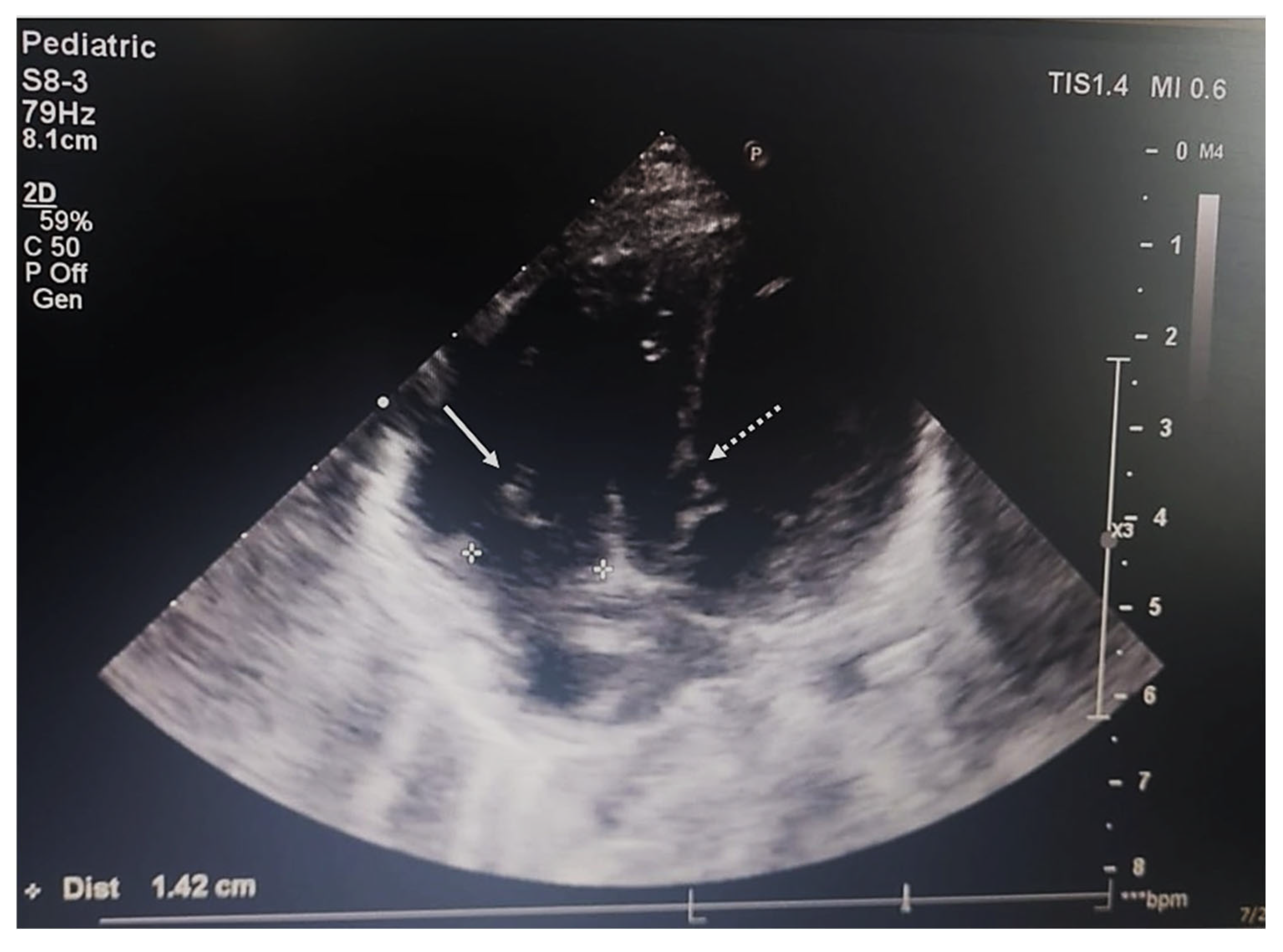
2.4. Imaging and Endoscopic Examinations
2.5. Surgery
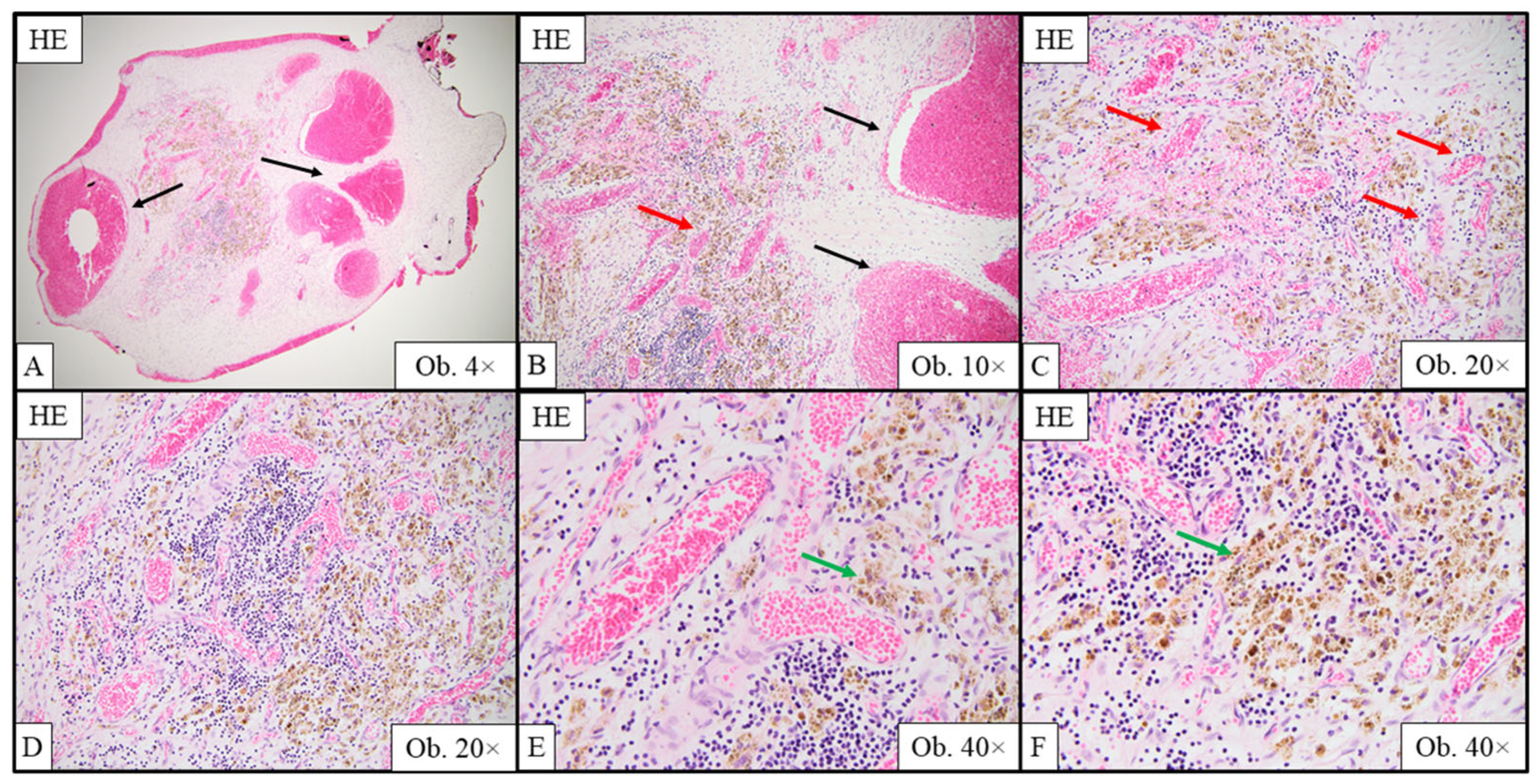
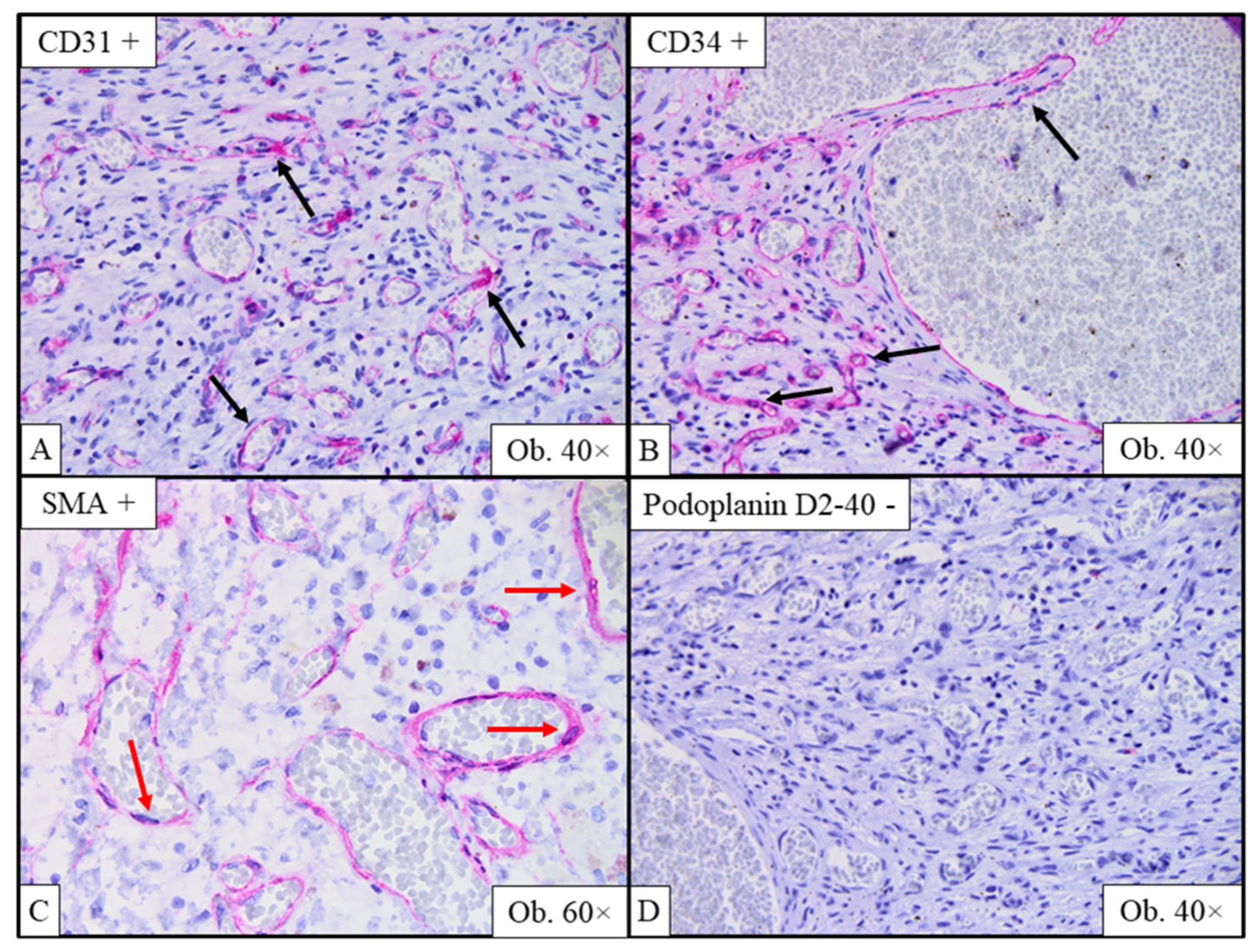
2.6. Gross and Histopathological Assessment of Surgical Specimens
2.7. Outcome and Follow-Up
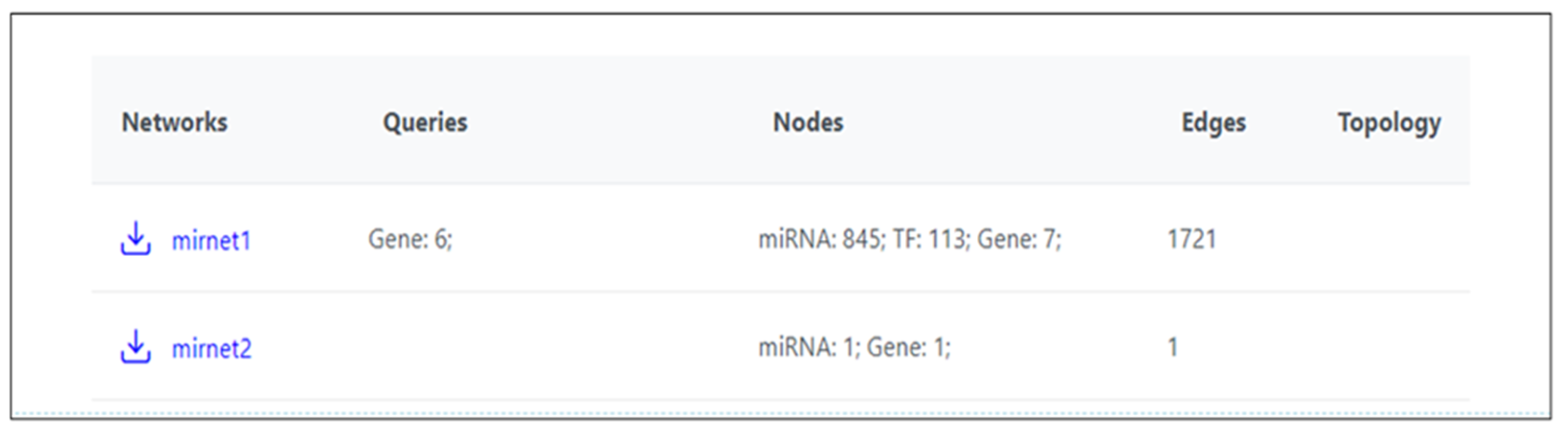
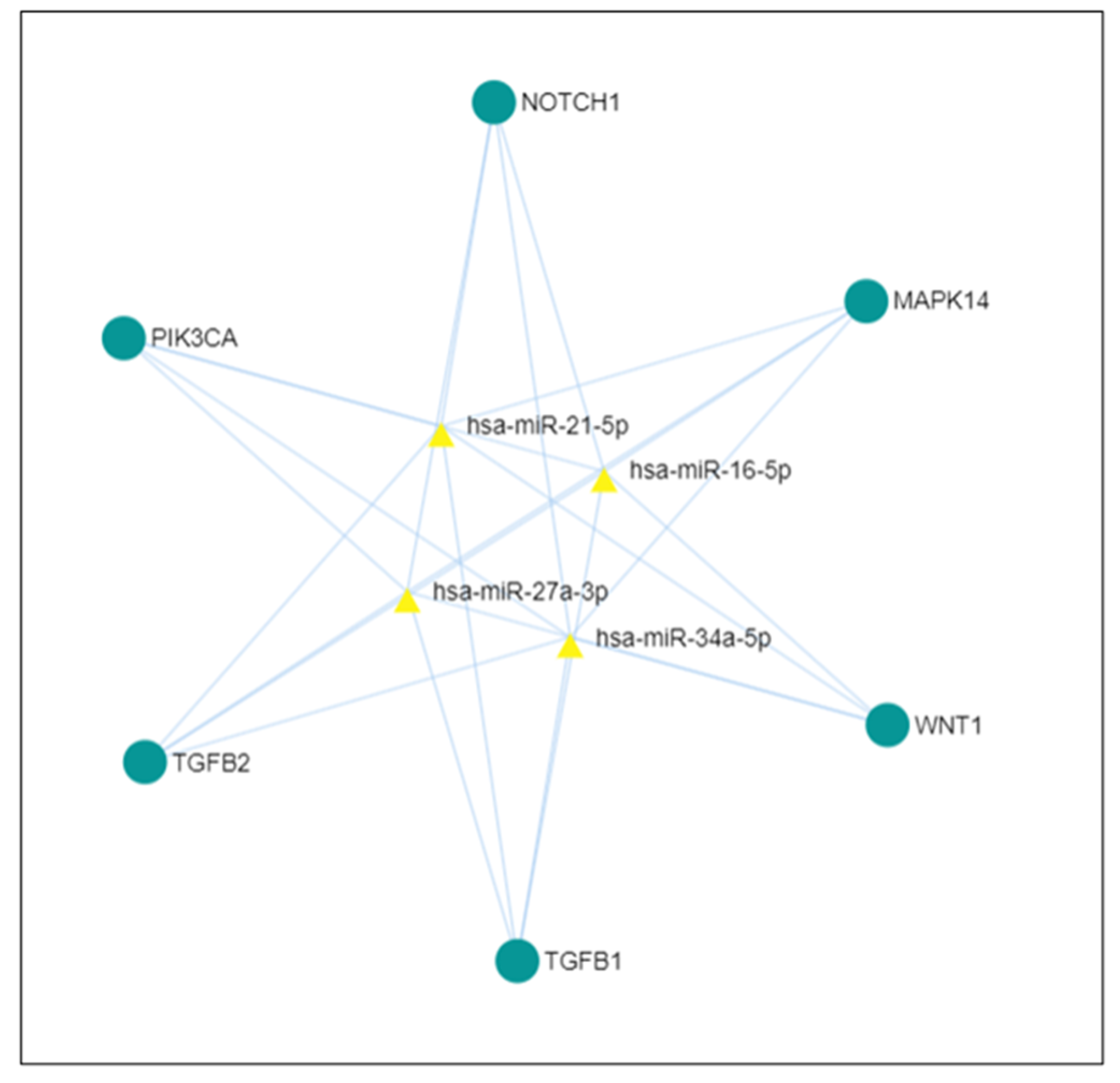
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basso, C.; Rizzo, S.; Valente, M.; Thiene, G. Cardiac masses and tumors. Heart (Br. Card. Soc.) 2016, 102, 1230–1245. [Google Scholar]
- Paraskevaidis, I.A.; Michalakeas, C.A.; Papadopoulos, C.H.; Anastasiou-Nana, M. Cardiac tumors. ISRN Oncol. 2011, 2011, 208929. [Google Scholar] [CrossRef] [PubMed]
- Maleszewski, J.J.; Basso, C.; Bois, M.C.; Glass, C.; Klarich, K.W.; Leduc, C.; Padera, R.F.; Tavora, F. The 2021 WHO Classification of Tumors of the Heart. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Hinton, R.B.; Yutzey, K.E. Heart Valve Structure and Function in Development and Disease. Annu. Rev. Physiol. 2011, 73, 29–46. [Google Scholar] [CrossRef]
- Nye, S.W.; Orsinelli, D.A.; Baker, P.B.; Brown, D.A. Surgical treatment of a hemangioma of the mitral valve. Ann. Thorac. Surg. 2001, 71, 345–347. [Google Scholar] [CrossRef]
- Lapenna, E.; De Bonis, M.; Torracca, L.; La Canna, G.; Dell’Antonio, G.; Alfieri, O. Cavernous hemangioma of the tricuspid valve: Minimally invasive surgical resection. Ann. Thorac. Surg. 2003, 76, 2097–2099. [Google Scholar] [CrossRef]
- Ray, R.; Rishi, A.; Venugopal, P.; Chopra, P. Hemangioma of the tricuspid valve: A report of two cases with review of literature. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2004, 13, 120–122. [Google Scholar] [CrossRef]
- Wong, K.K.; Sandor, G.G.S.; Sett, S.S. Isolated haemangioma of the tricuspid valve. Cardiol. Young 2004, 14, 324. [Google Scholar] [CrossRef]
- Ahmed, U.; Khurshid, S. Mitral valve hemangioma with superimposed infective endocarditis. J. Gen. Intern. Med. 2004, 19, 60–61. [Google Scholar]
- Uğraş, S.; Bayram, I. Cavernous haemangioma of the mitral valve in a child: Report of a case and review of the literature. Pathology 2005, 37, 396–398. [Google Scholar] [CrossRef]
- Vivirito, M.; Boldorini, R.; Rossi, L.; Caimmi, P.P.; Bernardi, M.; Teodori, G. Capillary hemangioma of the aortic valve: False preoperative diagnosis of endocarditis. J. Thorac. Cardiovasc. Surg. 2006, 132, 690–691. [Google Scholar] [CrossRef]
- Val-Bernal, J.F.; Cuadrado, M.; Garijo, M.F.; Revuelta, J.M. Incidental in vivo detection of an isolated hemangioma of the aortic valve in a man with a history of renal transplantation. Virchows Arch. Int. J. Pathol. 2006, 449, 121–123. [Google Scholar] [CrossRef]
- Kutay, V.; Yakut, C.; Ekim, H. Mitral Annular Tumors: Report of Two Cases in Childhood. J. Card. Surg. 2006, 21, 191–194. [Google Scholar] [CrossRef]
- Muzzi, L.; Davoli, G.; Specchia, L.; Chiavarelli, M. Primary hemangioma of the mitral valve: An unusual presentation. J. Heart Valve Dis. 2007, 16, 209–211. [Google Scholar]
- Dod, H.S.; Burri, M.V.; Hooda, D.; Sajja, V.; Qureshi, W.; Massinople, D.; Nazim, M.H.; Murray, C.; Prabhakar, G.; Williams, H.J.; et al. Two- and three-dimensional transthoracic and transesophageal echocardiographic findings in epithelioid hemangioma involving the mitral valve. Echocardiography 2008, 25, 443–445. [Google Scholar] [CrossRef]
- Yaganti, V.; Patel, S.; Yaganti, S.; Victor, M. Cavernous hemangioma of the mitral valve: A case report and review of literature. J. Cardiovasc. Med. 2009, 10, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Cannata, A.; Russo, C.F.; Merlanti, B.; Pedrotti, P.; Moreo, A.; Botta, L.; Martinelli, L. Cavernous hemangioma replacing the septal leaflet of the tricuspid valve. J. Card. Surg. 2010, 25, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Floria, M.; Guedes, A.; Buche, M.; Deperon, R.; Marchandise, B. A rare primary cardiac tumor: Cavernous hemangioma of the tricuspid valve. Eur. J. Echocardiogr. 2011, 12, 477. [Google Scholar] [PubMed][Green Version]
- Cook, A.L.; Williams, D.A.; Bergman, S.; Hines, M.H. Atypically located cardiac haemangioma of the mitral valve. Cardiol. Young 2011, 21, 598–600. [Google Scholar] [CrossRef]
- Juric, I.; Hadzibegovic, I.; Kes, P.; Biocina, B.; Milicic, D.; Basic-Jukic, N. An exceptional cause of progressive dyspnoea in a renal transplant recipient: Hemangioma of the mitral valve. Kidney Blood Press. Res. 2013, 37, 9–14. [Google Scholar] [CrossRef]
- Isbitan, A.; Shaaban, H.; Qaqa, A.; Joshi, M.; Shamoon, F. Cavernous hemangioma of the mitral valve in an adult male patient successfully treated with surgical resection: A case report. J. Heart Valve Dis. 2014, 23, 662–664. [Google Scholar]
- Gupta, B.; Ghosh, S.; Kujur, M.; Khetan, K.; Kumar, T. Tricuspid Valve Hemangioma Associated with Hypoplastic Left Heart Syndrome Presenting as Sudden Infant Death Syndrome. Turk. J. Pathol. 2016, 35, 55–57. [Google Scholar] [CrossRef]
- Val-Bernal, J.F.; Terán-Villagrá, N.; García-Diego, O.; Sarralde, J.A. Lymphocyte-rich capillary-cavernous hemangioma of the mitral valve: A case report and review of the literature. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2017, 28, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Castelein, T.; Van Praet, F.; Van Der Steen, K.; Van Camp, G. Hemangioma of the mitral valve as a rare, incidental finding in an adult female. Acta Cardiol. 2019, 74, 180–181. [Google Scholar] [CrossRef] [PubMed]
- Perez-Brandão, C.; Carvalho, R.; Pinto, F.F.; Fragata, J. Arteriovenous Hemangioma of the Mitral Valve: Successful Surgical Removal in an Infant. World J. Pediatr. Congenit. Heart Surg. 2019, 10, 355–356. [Google Scholar] [CrossRef] [PubMed]
- van Broekhoven, A.; Krijnen, P.A.J.; Niessen, H.W.M.; Vonk, A.B.A. Hemangioma of the Atherosclerotic Changed Aortic Valve. Case Rep. Cardiol. 2019, 2019, 7916298. [Google Scholar] [CrossRef]
- Parkash, O.; Ying, G.W.; Ram, A.; Vemireddy, L.P.; Zahra, F. A Rare Case of Cavernous Hemangioma of the Mitral Valve Presenting as Multifocal Embolic Brain Infarcts. Cureus 2021, 13, e17721. [Google Scholar] [CrossRef]
- Bayfield, N.; Bibo, L.; Wang, E.; Passage, J. Cavernous haemangioma of anterior mitral valve leaflet: Diagnostic utility of 3D echocardiography. BMJ Case Rep. CP 2022, 15, e247352. [Google Scholar] [CrossRef]
- Toscano, M.; Alves, A.R.; Matias, C.; Carvalho, M.; Marques, M. Hemangioma of the mitral valve: Following the murmur. Rev. Port. Cardiol. 2022, 41, 795–799. [Google Scholar] [CrossRef]
- Ranjan, A.; Agarwal, R.; Singh, D. Capillary haemangioma of the tricuspid valve annulus: A rare presentation. Int. J. Surg. Case Rep. 2023, 106, 108171. [Google Scholar] [CrossRef]
- Kesieme, E.B.; Buchan, K.G. Multiple Right Ventricular Haemangiomas. Cureus 2023, 15, e36570. [Google Scholar] [CrossRef]
- Osada, H.; Yamazaki, K.; Suzuki, T.; Tomotsuka, S.; Sugimoto, A.; Fujimoto, M.; Minatoya, K. Cardiac capillary hemangioma originating from the mitral valve. JTCVS Tech. 2023, 20, 127–129. [Google Scholar] [CrossRef]
- Rocco, R.; Daly, R.; Arghami, A. Robotic-Assisted Resection of Rare Mitral Valve Hemangioma. Mayo Clin. Proceedings. Innov. Qual. Outcomes 2024, 8, 249–252. [Google Scholar] [CrossRef]
- Kunimoto, K.; Yamamoto, Y.; Jinnin, M. ISSVA Classification of Vascular Anomalies and Molecular Biology. Int. J. Mol. Sci. 2022, 23, 2358. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.O.; Geva, T.; Kozakewich, H.; Baird, C.W.; Stout, K.K.; del Nido, P.J. Recurrent atrioventricular groove intramuscular arteriovenous malformation. Ann. Thorac. Surg. 2012, 94, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Teng, P.; Xu, H.; Ma, L.; Ni, Y. Cardiac Hemangioma: A Comprehensive Analysis of 200 Cases. Ann. Thorac. Surg. 2015, 99, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Belluschi, I.; Del Forno, B.; Lapenna, E.; Nisi, T.; Iaci, G.; Ferrara, D.; Castiglioni, A.; Alfieri, O.; De Bonis, M. Surgical Techniques for Tricuspid Valve Disease. Front. Cardiovasc. Med. 2018, 5, 118. [Google Scholar] [CrossRef]
- Anbara, T.; Sharifi, M.; Aboutaleb, N. Endothelial to Mesenchymal Transition in the Cardiogenesis and Cardiovascular Diseases. Curr. Cardiol. Rev. 2020, 16, 306–314. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to Mesenchymal Transition in Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef]
- Person, A.D.; Klewer, S.E.; Runyan, R.B. Cell biology of cardiac cushion development. Int. Rev. Cytol. 2005, 243, 287–335. [Google Scholar]
- Camenisch, T.D.; Runyan, R.B.; Markwald, R.R. Chapter 6.1—Molecular Regulation of Cushion Morphogenesis. In Heart Development and Regeneration; Rosenthal, N., Harvey, R.P., Eds.; Academic Press: Boston, MA, USA, 2010; pp. 363–387. [Google Scholar]
- Tao, G.; Kotick, J.D.; Lincoln, J. Heart valve development, maintenance, and disease: The role of endothelial cells. Curr. Top. Dev. Biol. 2012, 100, 203–232. [Google Scholar]
- Itinteang, T.; Tan, C.E.S.; van Schaijik, B.; Marsh, R.W.; Davis, P.F.; Tan, S.T. Proliferating Infantile Hemangioma Tissues and Primary Cell Lines Express Markers Associated with Endothelial-to-Mesenchymal Transition. Plast. Reconstr. Surgery. Glob. Open 2020, 8, e2598. [Google Scholar] [CrossRef]
- Takada, S.; Hojo, M.; Takebe, N.; Tanigaki, K.; Miyamoto, S. Role of Endothelial-to-Mesenchymal Transition in the Pathogenesis of Central Nervous System Hemangioblastomas. World Neurosurg. 2018, 117, e187–e193. [Google Scholar] [CrossRef]
- Takada, S.; Hojo, M.; Tanigaki, K.; Miyamoto, S. Contribution of Endothelial-to-Mesenchymal Transition to the Pathogenesis of Human Cerebral and Orbital Cavernous Malformations. Neurosurgery 2017, 81, 176–183. [Google Scholar] [CrossRef]
- miRNet. Available online: https://www.mirnet.ca/miRNet/upload/GeneUploadView.xhtml (accessed on 3 August 2024).
- Garside, V.C.; Chang, A.C.; Karsan, A.; Hoodless, P.A. Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development. Cell. Mol. Life Sci. CMLS 2013, 70, 2899–2917. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yue, L.L.; Ntsobe, T.E.; Qin, L.L.; Zeng, Y.; Xie, M.F.; Huang, H.J.; Peng, W.; Zeng, L.S.; Liu, H.J.; et al. Endothelial mesenchymal transformation and relationship with vascular abnormalities. J. Radiat. Res. Appl. Sci. 2022, 15, 198–205. [Google Scholar] [CrossRef]
- Fedele, M.; Sgarra, R.; Battista, S.; Cerchia, L.; Manfioletti, G. The Epithelial–Mesenchymal Transition at the Crossroads between Metabolism and Tumor Progression. Int. J. Mol. Sci. 2022, 23, 800. [Google Scholar] [CrossRef] [PubMed]
- Doerr, M.; Morrison, J.; Bergeron, L.; Coomber, B.L.; Viloria-Petit, A. Differential effect of hypoxia on early endothelial-mesenchymal transition response to transforming growth beta isoforms 1 and 2. Microvasc. Res. 2016, 108, 48–63. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- Ubil, E.; Duan, J.; Pillai, I.C.; Rosa-Garrido, M.; Wu, Y.; Bargiacchi, F.; Lu, Y.; Stanbouly, S.; Huang, J.; Rojas, M.; et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature 2014, 514, 585–590. [Google Scholar] [CrossRef]
- MacGrogan, D.; Luna-Zurita, L.; de la Pompa, J.L. Notch signaling in cardiac valve development and disease. Birth Defects Research. Part A Clin. Mol. Teratol. 2011, 91, 449–459. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Wu, J.K.; Kitajewski, J.K. A potential role for notch signaling in the pathogenesis and regulation of hemangiomas. J. Craniofacial Surg. 2009, 20 (Suppl. S1), 698–702. [Google Scholar] [CrossRef]
- Niessen, K.; Karsan, A. Notch Signaling in Cardiac Development. Circ. Res. 2008, 102, 1169–1181. [Google Scholar] [CrossRef]
- Dong, W.; Zhao, Y.; Wen, D.; Lin, Y.; Zeng, C.; Gu, J.; Liao, F.; Li, R.; Zhang, X.; Wang, D.; et al. Wnt4 is crucial for cardiac repair by regulating mesenchymal-endothelial transition via the phospho-JNK/JNK. Theranostics 2022, 12, 4110–4126. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Samanta, A.; Zhao, L.; Dudley, N.R.; Buehler, T.; Vincent, R.J.; Hauptman, J.; Girgis, M.; Dawn, B. Vitamin D3 induces mesenchymal-to-endothelial transition and promotes a proangiogenic niche through IGF-1 signaling. iScience 2021, 24, 102272. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Reichrath, S. Notch Signaling and Tissue Patterning in Embryology: An Introduction. Adv. Exp. Med. Biol. 2020, 1218, 1–7. [Google Scholar]
- Chang, A.C.; Garside, V.C.; Fournier, M.; Smrz, J.; Vrljicak, P.; Umlandt, P.; Fuller, M.; Robertson, G.; Zhao, Y.; Tam, A.; et al. A Notch-dependent transcriptional hierarchy promotes mesenchymal transdifferentiation in the cardiac cushion. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2014, 243, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Bischoff, J. Endothelial-to-Mesenchymal Transition. Circ. Res. 2019, 124, 1163–1165. [Google Scholar] [CrossRef]
- Yao, L.; Li, J.; Jiang, B.; Zhang, Z.; Li, X.; Ouyang, X.; Xiao, Y.; Liu, G.; Wang, Z.; Zhang, G. RNF2 inhibits E-Cadherin transcription to promote hepatocellular carcinoma metastasis via inducing histone mono-ubiquitination. Cell Death Dis. 2023, 14, 261. [Google Scholar] [CrossRef] [PubMed]
- Look, D.C.; Pelletier, M.R.; Tidwell, R.M.; Roswit, W.T.; Holtzman, M.J. Stat1 depends on transcriptional synergy with Sp1. J. Biol. Chem. 1995, 270, 30264–30267. [Google Scholar] [CrossRef] [PubMed]
- Yockell-Lelievre, J.; Spriet, C.; Cantin, P.; Malenfant, P.; Heliot, L.; De Launoit, Y.; Audette, M. Functional cooperation between Stat-1 and ets-1 to optimize icam-1 gene transcription. Biochem. Cell Biol. Biochim. Biol. Cell. 2009, 87, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.M. Soluble ICAM-1: A marker of vascular inflammation and lifestyle. Cytokine 2005, 31, 127–134. [Google Scholar] [CrossRef]
- Benedicto, A.; Herrero, A.; Romayor, I.; Marquez, J.; Smedsrød, B.; Olaso, E.; Arteta, B. Liver sinusoidal endothelial cell ICAM-1 mediated tumor/endothelial crosstalk drives the development of liver metastasis by initiating inflammatory and angiogenic responses. Sci. Rep. 2019, 9, 13111. [Google Scholar] [CrossRef]
- Filippova, G.N.; Qi, C.F.; Ulmer, J.E.; Moore, J.M.; Ward, M.D.; Hu, Y.J.; Loukinov, D.I.; Pugacheva, E.M.; Klenova, E.M.; Grundy, P.E.; et al. Tumor-associated zinc finger mutations in the CTCF transcription factor selectively alter its DNA-binding specificity. Cancer Res. 2002, 62, 48–52. [Google Scholar]
- Munteanu, I.R.; Novaconi, R.C.; Merce, A.P.; Dima, C.N.; Falnita, L.S.; Manzur, A.R.; Streian, C.G.; Feier, H.B. Cardiac Hemangiomas: A Five-Year Systematic Review of Diagnosis, Treatment, and Outcomes. Cancers 2025, 17, 1532. [Google Scholar] [CrossRef]
- Mylonas, K.S.; Ziogas, I.A.; Avgerinos, D.V. Microenvironment in Cardiac Tumor Development: What Lies Beyond the Event Horizon? Adv. Exp. Med. Biol. 2020, 1226, 51–56. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Abdullah, S.T.; Taheri, M.; Samadian, M. A review on the role of mir-16-5p in the carcinogenesis. Cancer Cell Int. 2022, 22, 342. [Google Scholar] [CrossRef]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grünert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grünert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. MicroRNA-34a-5p serves as a tumor suppressor by regulating the cell motility of bladder cancer cells through matrix metalloproteinase-2 silencing. Oncol. Rep. 2021, 45, 911–920. [Google Scholar]
- miR-21-5p|BioVendor R&D. Available online: https://www.biovendor.com/mir-21-5p (accessed on 25 July 2024).
- Qu, K.; Zhang, X.; Lin, T.; Liu, T.; Wang, Z.; Liu, S.; Zhou, L.; Wei, J.; Chang, H.; Li, K.; et al. Circulating miRNA-21-5p as a diagnostic biomarker for pancreatic cancer: Evidence from comprehensive miRNA expression profiling analysis and clinical validation. Sci. Rep. 2017, 7, 1692. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, S.; Kaur, S.; Mirza, A.H.; Brodin, B.; Pociot, F.; Kruuse, C. miRNA-27a-3p and miRNA-222-3p as Novel Modulators of Phosphodiesterase 3a (PDE3A) in Cerebral Microvascular Endothelial Cells. Mol. Neurobiol. 2019, 56, 5304–5314. [Google Scholar] [CrossRef]
- Zietzer, A.; Steffen, E.; Niepmann, S.; Düsing, P.; Hosen, M.R.; Liu, W.; Jamme, P.; Al-Kassou, B.; Goody, P.R.; Zimmer, S.; et al. MicroRNA-mediated vascular intercellular communication is altered in chronic kidney disease. Cardiovasc. Res. 2022, 118, 316–333. [Google Scholar] [CrossRef]

| Case No. | Authors, Year of Publication | Patient’s Gender | Patient’s Age (Years) | Valvular Localization | Histological Variant | Hemangioma Dimension (cm) | Clinical Manifestation |
|---|---|---|---|---|---|---|---|
| 1. | Nye et al. 2001 [5] | Female | 33 | Mitral | Cavernous | 1 | Chest tightness, palpitations |
| 2. | Lapenna et al. 2003 [6] | Female | 49 | Tricuspid | Cavernous | 3 | Syncope |
| 3. | Ray et al. 2004 [7] | Male | 47 | Tricuspid | Cavernous | 0.8 | Dyspnea |
| 4. | Male | 0 | Tricuspid | Cavernous | 0.1–0.4 (multiple tumors) | Incidental detection during autopsy | |
| 5. | Wong et al. 2004 [8] | Female | 4 | Tricuspid | - | 0.8 | Cyanosis, systolic murmur |
| 6. | Ahmed et al. 2004 [9] | - | - | Mitral | - | - | - |
| 7. | Uğraş and Bayram
2005 [10] | Male | 8 | Mitral | Cavernous | 3 | Dyspnea, palpitations |
| 8. | Vivirito et al.
2006 [11] | Male | 81 | Aortic | Capillary | - | Dyspnea, fever, thoracic pain |
| 9. | Val-Bernal et al.
2006 [12] | Male | 62 | Aortic | - | 3 | Syncope, dyspnea |
| 10. | Kutay et al.
2006 [13] | Male | 8 | Mitral | Cavernous | 3 | Dyspnea, palpitations, systolic murmur |
| 11. | Muzzi et al.
2007 [14] | - | - | Mitral | - | - | - |
| 12. | Dod et al.
2008 [15] | Female | 86 | Mitral | Epithelioid | 2.4 | Edema of lower extremities |
| 13. | Yaganti et al.
2009 [16] | Female | 33 | Mitral | Cavernous | 2 | Chest pressure, dyspnea, lightheadedness |
| 14. | Cannata et al.
2010 [17] | Male | 76 | Tricuspid | Cavernous | 4 | Incidental detection during echocardiographic evaluation |
| 15. | Floria et al. 2011 [18] | Female | 52 | Tricuspid | Cavernous | 1.6 | Chest pain, palpitations |
| 16. | Cook et al. 2011 [19] | Male | 17 | Mitral | - | 1.4 | Syncope |
| 17. | Juric et al. 2013 [20] | Female | 49 | Mitral | - | 0.9 | Chest tightness, dyspnea |
| 18. | Isbitan et al. 2014 [21] | Male | 48 | Mitral | Cavernous | - | Chest pain |
| 19. | Gupta et al. 2016 [22] | Female | 0 | Tricuspid | Cavernous | 0.8 | Incidental detection during autopsy |
| 20. | Val-Bernal et al. 2017 [23] | Male | 62 | Mitral | Capillary–cavernous | 1.3 | Edema of lower extremities |
| 21. | Castelein et al. 2019 [24] | Female | 60 | Mitral | - | 1.3 | Incidental detection during echocardiographic evaluation |
| 22. | Perez-Brandão et al. 2019 [25] | - | 1 | Mitral | Arteriovenous | 0.75 | Heart murmur |
| 23. | Van Broekhoven et al. 2019 [26] | Male | 74 | Aortic | - | - | Aortic valve insufficiency, diastolic murmur |
| 24. | Parkash et al. 2021 [27] | Female | 78 | Mitral | Cavernous | 1 0.5 (two concomitant lesions) | Multifocal embolic brain infarcts |
| 25. | Bayfield et al. 2022 [28] | Female | 44 | Mitral | Cavernous | 3 | Mitral regurgitation |
| 26. | Toscano et al. 2022 [29] | Female | 66 | Mitral | Cavernous | 2.8 | Acute heart failure, myocardial infarction |
| 27. | Ranjan et al. 2023 [30] | Male | 52 | Tricuspid | Capillary | 1 | Dyspnea, edema of lower extremities |
| 28. | Kesieme et al. 2023 [31] | Female | 49 | Tricuspid | Cavernous | - | Dizziness, palpitations, syncope |
| 29. | Osada et al. 2023 [32] | Female | 56 | Mitral | Capillary | 1 | Incidental detection during echocardiographic evaluation |
| 30. | Rocco et al. 2024 [33] | Male | 78 | Mitral | Capillary | 0.6 | Incidental detection during echocardiographic evaluation |
| 31. | Index case 2025 | Female | 0 | Tricuspid | Arteriovenous | 0.5 | Incidental detection during echocardiographic evaluation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neeter, K.-A.; Satala, C.-B.; Mihalache, D.; Neferu, A.-S.; Patrichi, G.; Opris, C.E.; Gurzu, S. Pathogenesis of Cardiac Valvular Hemangiomas: A Case Report and Literature Review. Int. J. Mol. Sci. 2025, 26, 7114. https://doi.org/10.3390/ijms26157114
Neeter K-A, Satala C-B, Mihalache D, Neferu A-S, Patrichi G, Opris CE, Gurzu S. Pathogenesis of Cardiac Valvular Hemangiomas: A Case Report and Literature Review. International Journal of Molecular Sciences. 2025; 26(15):7114. https://doi.org/10.3390/ijms26157114
Chicago/Turabian StyleNeeter, Kimberly-Allisya, Catalin-Bogdan Satala, Daniela Mihalache, Alexandru-Stefan Neferu, Gabriela Patrichi, Carmen Elena Opris, and Simona Gurzu. 2025. "Pathogenesis of Cardiac Valvular Hemangiomas: A Case Report and Literature Review" International Journal of Molecular Sciences 26, no. 15: 7114. https://doi.org/10.3390/ijms26157114
APA StyleNeeter, K.-A., Satala, C.-B., Mihalache, D., Neferu, A.-S., Patrichi, G., Opris, C. E., & Gurzu, S. (2025). Pathogenesis of Cardiac Valvular Hemangiomas: A Case Report and Literature Review. International Journal of Molecular Sciences, 26(15), 7114. https://doi.org/10.3390/ijms26157114






