HCAR3 and Kynurenic Acid in Cancer: A Promising Axis of Immunometabolic Regulation or a Scientific Mirage?
Abstract
1. Introduction
2. HCAR3 in Cancer
2.1. Colorectal Cancer
2.2. Breast Cancer
2.3. Acute Myeloid Leukemia (AML)
2.4. Skin Cancer
2.5. Other Types of Cancer
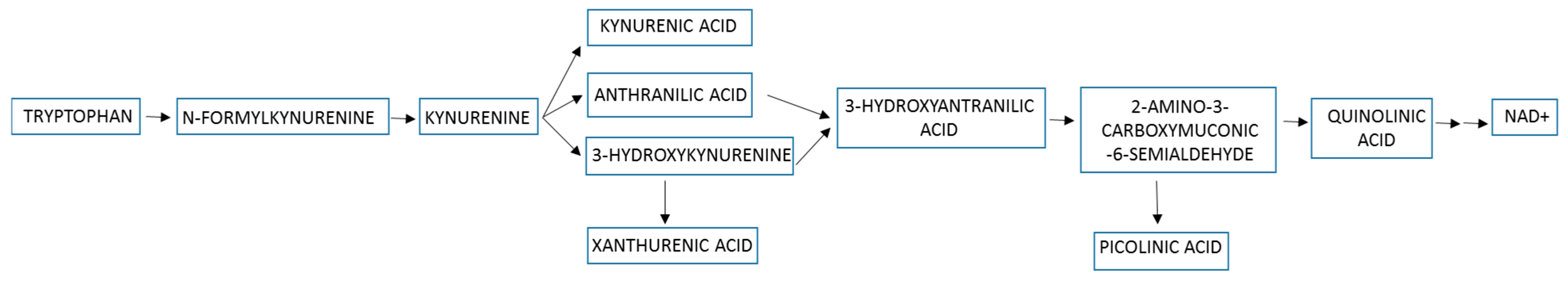
3. The Kynurenine Pathway in Cancer
4. The Possible Interactions Between Kynurenic Acid and HCAR3 in Cancer
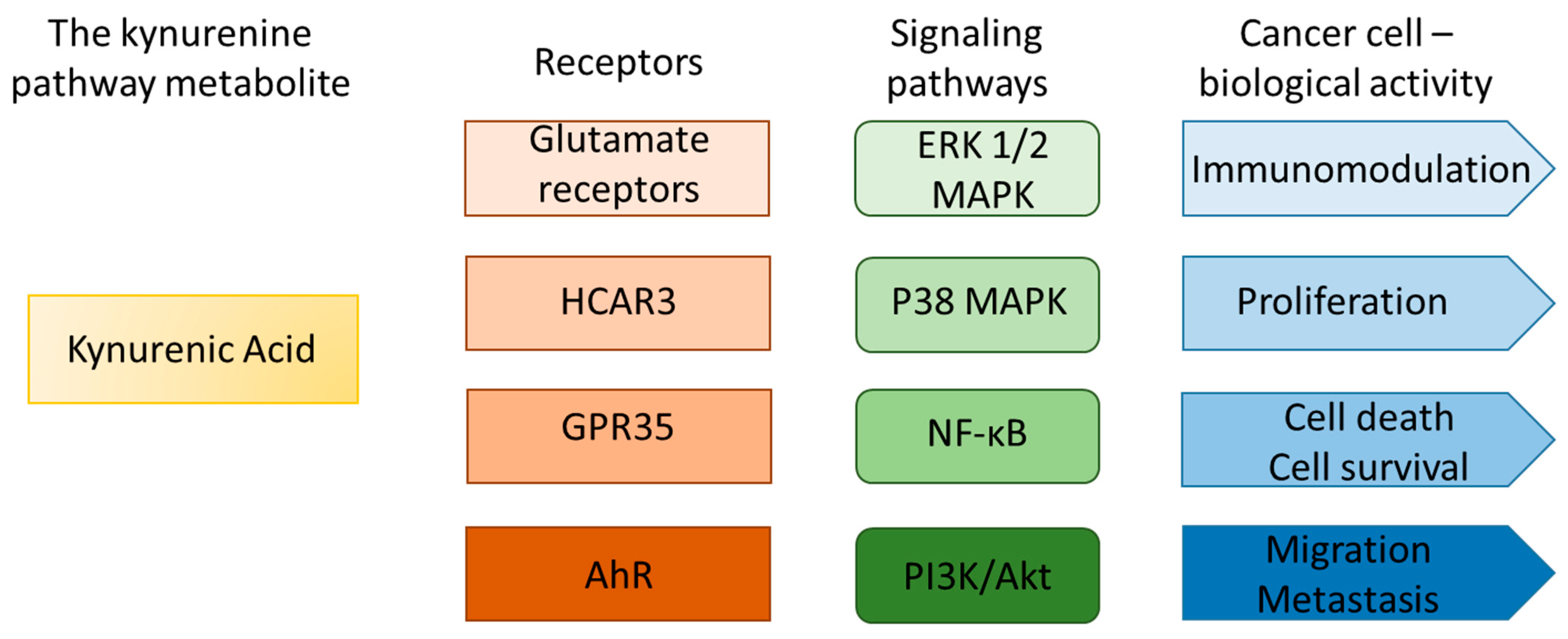
5. HCAR3 and Kynurenic Acid in Cancer: Fact or Hypothetical Interaction?
- Most studies to date have focused on HCAR3 gene expression in cancer, rather than its functional activity. Notably, overexpression of HCAR3 is not always correlated with the protein HCAR3 level; however, the regulatory mechanism of this phenomenon has not been studied. Further studies are necessary to indicate post-transcriptional or post-translational regulations involved in this process.
- To validate the hypothesis concerning the role of HCAR3 and KYNA in carcinogenesis, large-scale, multicenter studies employing standardized research protocols and statistical approaches are essential. Available data do not allow us to determine whether the modified expression level of HCAR3 in cancer enhances cancer progression or whether it is a nonspecific effect of altered metabolism of tumor cells. Is there functional compensation at the protein level between HCAR3 and other HCAR receptors?
- Previous research focused only on the interaction between HCAR3 and KYNA without considering the broader context of the kynurenine pathway and other biologically active tryptophan metabolites. It should be verified whether the suggested functional interaction concerns only HCAR3 and KYNA, or whether we should consider a broader metabolic network. Is it possible to verify the molecular and biological effects of KYNA-mediated HCAR3 activation in cancer cells, taking into account the number of KYNA-activated receptors, signaling pathways, and cell cycle regulators? Unfortunately, the lack of HCAR3 antagonists, gene silencing efficiency, occurrence of paralogs, and expression of HCAR3 only in mammalian cells are significant obstacles to carrying out further advanced research.
- The role of HCAR3 should be considered not only as a direct effect on cancer cells, but also in more complex interactions with the tumor microenvironment and the immune system.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α7 nAChR | α-7 nicotinic acetylcholine receptor |
| AhR | aryl hydrocarbon receptor |
| AML | acute myeloid leukemia |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ESCC | esophageal squamous cell carcinoma |
| ER | estrogen receptor |
| ERK | extracellular signal-regulated kinase |
| HCAR | hydroxycarboxylic acid receptor |
| GPCR | G-protein-coupled receptor |
| IDO | indoleamine 2,3-dioxygenase |
| KYNA | kynurenic acid |
| NAD | nicotinamide adenine dinucleotide |
| NMDA | N-methyl-d-aspartate receptor |
| 3-OH | 3-hydroxyoctanoic acid |
| PR | progesterone receptor |
| TCGA | The Cancer Genome Atlas |
| TDO | tryptophan 2,3-dioxygenase |
References
- Kapolka, N.J.; Isom, D.G. HCAR3: An underexplored metabolite sensor. Nat. Rev. Drug Discov. 2020, 19, 745. [Google Scholar] [CrossRef]
- Offermanns, S. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol. Sci. 2006, 27, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, Y.; Benavente, C.A.; Meyer, R.G.; Coyle, W.R.; Jacobson, M.K.; Jacobson, E.L. Nicotinic acid receptor abnormalities in human skin cancer: Implications for a role in epidermal differentiation. PLoS ONE 2011, 6, e20487. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jung, J.K.; Johnson, B.R.; Duong, T.; Decaire, M.; Uy, J.; Gharbaoui, T.; Boatman, P.D.; Sage, C.R.; Chen, R.; Richman, J.G.; et al. Analogues of Acifran: Agonists of the high and low affinity niacin receptors, GPR109a and GPR109b. J. Med. Chem. 2007, 50, 1445–1448. [Google Scholar] [CrossRef]
- Ye, F.; Pan, X.; Zhang, Z.; Xiang, X.; Li, X.; Zhang, B.; Ning, P.; Liu, A.; Wang, Q.; Gong, K.; et al. Structural basis for ligand recognition of the human hydroxycarboxylic acid receptor HCAR3. Cell Rep. 2024, 43, 114895. [Google Scholar] [CrossRef] [PubMed]
- Irukayama-Tomobe, Y.; Tanaka, H.; Yokomizo, T.; Hashidate-Yoshida, T.; Yanagisawa, M.; Sakurai, T. Aromatic D-amino acids act as chemoattractant factors for human leukocytes through a G protein-coupled receptor, GPR109B. Proc. Natl. Acad. Sci. USA 2009, 106, 3930–3934. [Google Scholar] [CrossRef]
- Skinner, P.J.; Cherrier, M.C.; Webb, P.J.; Sage, C.R.; Dang, H.T.; Pride, C.C.; Chen, R.; Tamura, S.Y.; Richman, J.G.; Connolly, D.T.; et al. 3-Nitro-4-amino benzoic acids and 6-amino nicotinic acids are highly selective agonists of GPR109b. Bioorg. Med. Chem. Lett. 2007, 17, 6619–6622. [Google Scholar] [CrossRef]
- Skinner, P.J.; Webb, P.J.; Sage, C.R.; Dang, T.H.; Pride, C.C.; Chen, R.; Tamura, S.Y.; Richman, J.G.; Connolly, D.T.; Semple, G. 5-N,N-Disubstituted 5-aminopyrazole-3-carboxylic acids are highly potent agonists of GPR109b. Bioorg. Med. Chem. Lett. 2009, 19, 4207–4209. [Google Scholar] [CrossRef]
- Kapolka, N.J.; Taghon, G.J.; Rowe, J.B.; Morgan, W.M.; Enten, J.F.; Lambert, N.A.; Isom, D.G. DCyFIR: A high-throughput CRISPR platform for multiplexed G protein-coupled receptor profiling and ligand discovery. Proc. Natl. Acad. Sci. USA 2020, 117, 13117–13126. [Google Scholar] [CrossRef]
- Stäubert, C.; Broom, O.J.; Nordström, A. Hydroxycarboxylic acid receptors are essential for breast cancer cells to control their lipid/fatty acid metabolism. Oncotarget 2015, 6, 19706–19720. [Google Scholar] [CrossRef]
- Pedro, M.P.; Lund, K.; Kang, S.W.S.; Chen, T.; Stuelten, C.H.; Porat-Shliom, N.; Iglesias-Bartolome, R. GPCR Screening Reveals that the Metabolite Receptor HCAR3 Regulates Epithelial Proliferation, Migration, and Cellular Respiration. J. Investig. Dermatol. 2024, 144, 1311–1321.e1317. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Tunaru, S.; Langhans, C.D.; Hanson, J.; Michalski, C.W.; Kölker, S.; Jones, P.M.; Okun, J.G.; Offermanns, S. Deorphanization of GPR109B as a receptor for the beta-oxidation intermediate 3-OH-octanoic acid and its role in the regulation of lipolysis. J. Biol. Chem. 2009, 284, 21928–21933. [Google Scholar] [CrossRef]
- Wnorowski, A.; Wnorowska, S.; Kurzepa, J.; Parada-Turska, J. Alterations in Kynurenine and NAD(+) Salvage Pathways during the Successful Treatment of Inflammatory Bowel Disease Suggest HCAR3 and NNMT as Potential Drug Targets. Int. J. Mol. Sci. 2021, 22, 13497. [Google Scholar] [CrossRef]
- Walczak, K.; Wnorowski, A.; Turski, W.A.; Plech, T. Kynurenic acid and cancer: Facts and controversies. Cell. Mol. Life Sci. 2020, 77, 1531–1550. [Google Scholar] [CrossRef]
- Walczak, K.; Zurawska, M.; Kiś, J.; Starownik, R.; Zgrajka, W.; Bar, K.; Turski, W.A.; Rzeski, W. Kynurenic acid in human renal cell carcinoma: Its antiproliferative and antimigrative action on Caki-2 cells. Amino Acids 2012, 43, 1663–1670. [Google Scholar] [CrossRef]
- Walczak, K.; Turski, W.A.; Rajtar, G. Kynurenic acid inhibits colon cancer proliferation in vitro: Effects on signaling pathways. Amino Acids 2014, 46, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Dąbrowski, W.; Langner, E.; Zgrajka, W.; Piłat, J.; Kocki, T.; Rzeski, W.; Turski, W.A. Kynurenic acid synthesis and kynurenine aminotransferases expression in colon derived normal and cancer cells. Scand. J. Gastroenterol. 2011, 46, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Kazimierczak, P.; Szalast, K.; Plech, T. UVB Radiation and Selected Tryptophan-Derived AhR Ligands-Potential Biological Interactions in Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 7500. [Google Scholar] [CrossRef]
- Yang, X.; Wei, W.; Tan, S.; Guo, L.; Qiao, S.; Yao, B.; Wang, Z. Identification and verification of HCAR3 and INSL5 as new potential therapeutic targets of colorectal cancer. World J. Surg. Oncol. 2021, 19, 248. [Google Scholar] [CrossRef]
- Ding, H.; Xiong, X.X.; Fan, G.L.; Yi, Y.X.; Chen, Y.R.; Wang, J.T.; Zhang, W. The New Biomarker for Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma (CESC) Based on Public Database Mining. BioMed Res. Int. 2020, 2020, 5478574. [Google Scholar] [CrossRef]
- Gutiu, A.G.; Zhao, L.; Marrah, A.J.; Maher, A.J.; Voss, B.B.; Mayberry, T.G.; Cowan, B.C.; Wakefield, M.R.; Fang, Y. Promising immunotherapeutic treatments for colon cancer. Med. Oncol. 2025, 42, 175. [Google Scholar] [CrossRef]
- Santolla, M.F.; De Francesco, E.M.; Lappano, R.; Rosano, C.; Abonante, S.; Maggiolini, M. Niacin activates the G protein estrogen receptor (GPER)-mediated signalling. Cell. Signal. 2014, 26, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.E.; Viana-Errasti, J.; Buchanan, D.D.; Valle, L. Genetics, genomics and clinical features of adenomatous polyposis. Fam. Cancer 2025, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Y.; Hu, Z.; Wang, D.; Sun, X.; Ren, C. Differential effects of NOD2 polymorphisms on colorectal cancer risk: A meta-analysis. Int. J. Color. Dis. 2010, 25, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Michaels, E.; Worthington, R.O.; Rusiecki, J. Breast Cancer: Risk Assessment, Screening, and Primary Prevention. Med. Clin. N. Am. 2023, 107, 271–284. [Google Scholar] [CrossRef]
- McGuire Sams, C.; Shepp, K.; Pugh, J.; Bishop, M.R.; Merner, N.D. Rare and potentially pathogenic variants in hydroxycarboxylic acid receptor genes identified in breast cancer cases. BMC Med. Genom. 2021, 14, 284. [Google Scholar] [CrossRef]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef]
- Zellner, C.; Pullinger, C.R.; Aouizerat, B.E.; Frost, P.H.; Kwok, P.Y.; Malloy, M.J.; Kane, J.P. Variations in human HM74 (GPR109B) and HM74A (GPR109A) niacin receptors. Hum. Mutat. 2005, 25, 18–21. [Google Scholar] [CrossRef]
- Shultz, C.; Gates, C.; Petros, W.; Ross, K.; Veltri, L.; Craig, M.; Wen, S.; Primerano, D.A.; Hazlehurst, L.; Denvir, J.; et al. Association of genetic variants and survival in patients with acute myeloid leukemia in rural Appalachia. Cancer Rep. 2023, 6, e1746. [Google Scholar] [CrossRef]
- Zhou, L.; Tang, Y.; Cryan, E.V.; Demarest, K.T. Human epidermoid A431 cells express functional nicotinic acid receptor HM74a. Mol. Cell. Biochem. 2007, 294, 243–248. [Google Scholar] [CrossRef]
- Scatozza, F.; Moschella, F.; D’Arcangelo, D.; Rossi, S.; Tabolacci, C.; Giampietri, C.; Proietti, E.; Facchiano, F.; Facchiano, A. Nicotinamide inhibits melanoma in vitro and in vivo. J. Exp. Clin. Cancer Res. 2020, 39, 211. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Cai, Y.; Shrestha, S.M.; Shen, D.; Zhao, W.; Shi, R. Construction and Validation of an Immune-Related Gene Prognostic Index for Esophageal Squamous Cell Carcinoma. BioMed Res. Int. 2021, 2021, 7430315. [Google Scholar] [CrossRef]
- Li, T.; Liu, Q.; Zhang, R.; Liao, Q.; Zhao, Y. Identification of prognosis-related genes and construction of multi-regulatory networks in pancreatic cancer microenvironment by bioinformatics analysis. Cancer Cell Int. 2020, 20, 341. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Basson, C.; Serem, J.C.; Hlophe, Y.N.; Bipath, P. The tryptophan-kynurenine pathway in immunomodulation and cancer metastasis. Cancer Med. 2023, 12, 18691–18701. [Google Scholar] [CrossRef] [PubMed]
- León-Letelier, R.A.; Dou, R.; Vykoukal, J.; Sater, A.H.A.; Ostrin, E.; Hanash, S.; Fahrmann, J.F. The kynurenine pathway presents multi-faceted metabolic vulnerabilities in cancer. Front. Oncol. 2023, 13, 1256769. [Google Scholar] [CrossRef]
- Gouasmi, R.; Ferraro-Peyret, C.; Nancey, S.; Coste, I.; Renno, T.; Chaveroux, C.; Aznar, N.; Ansieau, S. The Kynurenine Pathway and Cancer: Why Keep It Simple When You Can Make It Complicated. Cancers 2022, 14, 2793. [Google Scholar] [CrossRef]
- Corm, S.; Berthon, C.; Imbenotte, M.; Biggio, V.; Lhermitte, M.; Dupont, C.; Briche, I.; Quesnel, B. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients’ sera by HPLC and is inducible by IFN-gamma. Leuk. Res. 2009, 33, 490–494. [Google Scholar] [CrossRef]
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine emerges from the shadows—Current knowledge on its fate and function. Pharmacol. Ther. 2021, 225, 107845. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Ciudad, M.T.; McGaha, T.L. New insights into tryptophan metabolism in cancer. Trends Cancer 2025. [Google Scholar] [CrossRef] [PubMed]
- Kaszaki, J.; Palásthy, Z.; Erczes, D.; Rácz, A.; Torday, C.; Varga, G.; Vécsei, L.; Boros, M. Kynurenic acid inhibits intestinal hypermotility and xanthine oxidase activity during experimental colon obstruction in dogs. Neurogastroenterol. Motil. 2008, 20, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Varga, G.; Erces, D.; Fazekas, B.; Fülöp, M.; Kovács, T.; Kaszaki, J.; Fülöp, F.; Vécsei, L.; Boros, M. N-Methyl-D-aspartate receptor antagonism decreases motility and inflammatory activation in the early phase of acute experimental colitis in the rat. Neurogastroenterol. Motil. 2010, 22, 217–225, e268. [Google Scholar] [CrossRef]
- Cosi, C.; Mannaioni, G.; Cozzi, A.; Carlà, V.; Sili, M.; Cavone, L.; Maratea, D.; Moroni, F. G-protein coupled receptor 35 (GPR35) activation and inflammatory pain: Studies on the antinociceptive effects of kynurenic acid and zaprinast. Neuropharmacology 2011, 60, 1227–1231. [Google Scholar] [CrossRef]
- Glavin, G.B.; Pinsky, C. Kynurenic acid attenuates experimental ulcer formation and basal gastric acid secretion in rats. Res. Commun. Chem. Pathol. Pharmacol. 1989, 64, 111–119. [Google Scholar] [PubMed]
- Pawlak, K.; Myśliwiec, M.; Pawlak, D. Kynurenine pathway—A new link between endothelial dysfunction and carotid atherosclerosis in chronic kidney disease patients. Adv. Med. Sci. 2010, 55, 196–203. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef]
- Marciniak, S.; Wnorowski, A.; Smolińska, K.; Walczyna, B.; Turski, W.; Kocki, T.; Paluszkiewicz, P.; Parada-Turska, J. Kynurenic Acid Protects against Thioacetamide-Induced Liver Injury in Rats. Anal. Cell. Pathol. 2018, 2018, 1270483. [Google Scholar] [CrossRef]
- Zdzisińska, B.; Wejksza, K.; Walter-Croneck, A.; Turski, W.A.; Kandefer-Szerszeń, M. Kynurenic acid in blood and bone marrow plasma of monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma (MM) patients. Leuk. Res. 2010, 34, 38–45. [Google Scholar] [CrossRef]
- Vezzani, A.; Gramsbergen, J.B.; Versari, P.; Stasi, M.A.; Procaccio, F.; Schwarcz, R. Kynurenic acid synthesis by human glioma. J. Neurol. Sci. 1990, 99, 51–57. [Google Scholar] [CrossRef]
- Grishanova, A.Y.; Perepechaeva, M.L. Kynurenic Acid/AhR Signaling at the Junction of Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 6933. [Google Scholar] [CrossRef]
- Sun, T.; Xie, R.; He, H.; Xie, Q.; Zhao, X.; Kang, G.; Cheng, C.; Yin, W.; Cong, J.; Li, J.; et al. Kynurenic acid ameliorates NLRP3 inflammasome activation by blocking calcium mobilization via GPR35. Front. Immunol. 2022, 13, 1019365. [Google Scholar] [CrossRef] [PubMed]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2017, 8, 1957. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.N.; Stone, T.W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982, 247, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988, 154, 85–87. [Google Scholar] [CrossRef]
- Hilmas, C.; Pereira, E.F.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef]
- Krupa, A.; Kowalska, I. The Kynurenine Pathway-New Linkage between Innate and Adaptive Immunity in Autoimmune Endocrinopathies. Int. J. Mol. Sci. 2021, 22, 9879. [Google Scholar] [CrossRef]
- Retamal, I.; Pezoa, I.; Silva, I.; Gómez-Valenzuela, F.; Godoy, J.; Garrido, M.; Martin, A. 1272 HCAR3 and AhR a novel regulatory axis for immune checkpoint pathway in gastric cancer: Role of Fusobacterium nucleatum. J. Immunother. Cancer 2024, 12 (Suppl. S2), A1426. [Google Scholar] [CrossRef]
- Mandrika, I.; Tilgase, A.; Petrovska, R.; Klovins, J. Hydroxycarboxylic Acid Receptor Ligands Modulate Proinflammatory Cytokine Expression in Human Macrophages and Adipocytes without Affecting Adipose Differentiation. Biol. Pharm. Bull. 2018, 41, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mohammed, A.; Wong, H.R.; Palaniyar, N.; Kamaleswaran, R. Machine Learning Identifies Complicated Sepsis Course and Subsequent Mortality Based on 20 Genes in Peripheral Blood Immune Cells at 24 H Post-ICU Admission. Front. Immunol. 2021, 12, 592303. [Google Scholar] [CrossRef] [PubMed]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases. Front. Immunol. 2016, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, C.; Zhang, J.; Su, W.; Wang, G.; Wang, Z. The Kynurenine Pathway and Indole Pathway in Tryptophan Metabolism Influence Tumor Progression. Cancer Med. 2025, 14, e70703. [Google Scholar] [CrossRef]
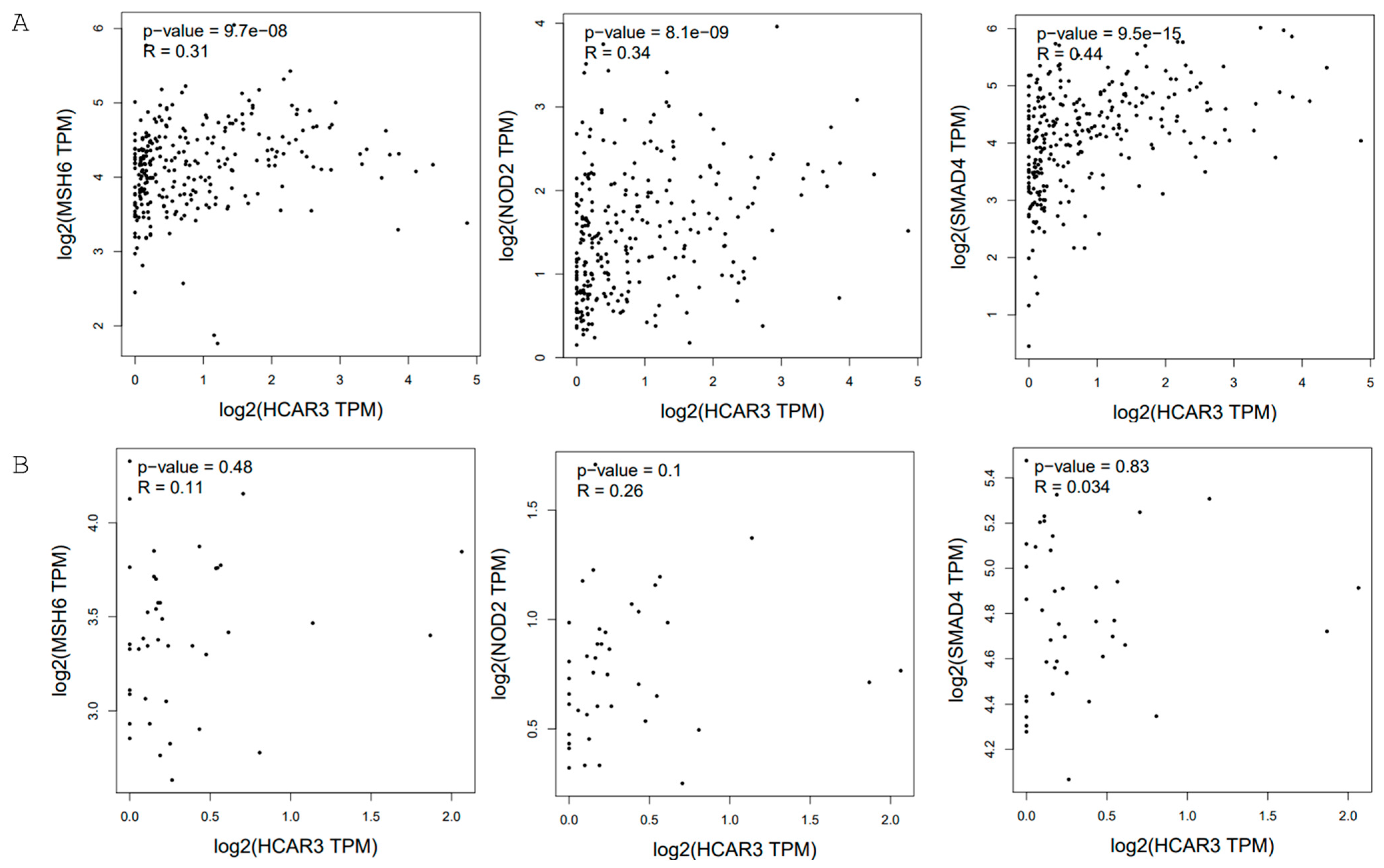
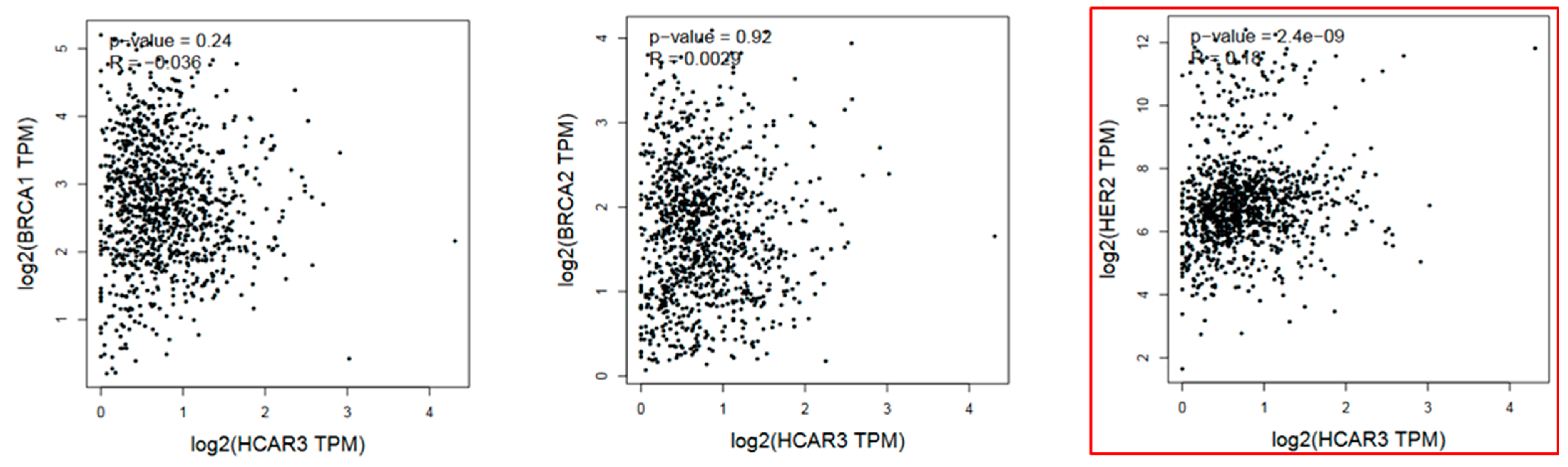
| Type of Cancer | pTPM Cancer | pTPM Validation | |
|---|---|---|---|
| Central nervous system cancer | Glioblastoma multiforme | 0.2 (N = 141) | 0.4 (N = 58) |
| Lung cancer | Lung adenocarcinoma | 1.5 (N = 497) | 2.8 (N = 105) |
| Lung squamous cell carcinoma | 6.5 (N = 489) | 11.3 (N = 68) | |
| Gastrointestinal cancer | Colon adenocarcinoma | 0.5 (N = 254) | 3.6 (N = 486) |
| Rectum adenocarcinoma | 0.4 (N = 88) | 2.6 (N = 207) | |
| Stomach adenocarcinoma | 0.5 (N = 346) | No data available | |
| Liver hepatocellular carcinoma | 0.2 (N = 362) | 0.5 (N = 231) | |
| Pancreatic adenocarcinoma | 0.5 (N = 176) | 1.5 (N = 80) | |
| Urinary tract cancers | Bladder urothelial carcinoma | 8.1 (N = 169) | No data available |
| Kidney chromophobe | 1.2 (N = 64) | No data available | |
| Kidney renal clear cell carcinoma | 0.2 (N = 521) | 0.4 (N = 100) | |
| Kidney renal papillary cell carcinoma | 0.1 (N = 282) | No data available | |
| Cancers of the male reproductive system | Prostate adenocarcinoma | 0.4 (N = 480) | No data available |
| Testicular germ cell tumor | 0.9 (N = 133) | No data available | |
| Cancers of the female reproductive system | Cervical squamous cell carcinoma and endocervical adenocarcinoma | 7.3 (N = 283) | No data available |
| Ovary serous cystadenocarcinoma | 0.2 (N = 349) | 0.2 (N = 81) | |
| Uterine corpus endometrial carcinoma | 0.1 (N = 176) | No data available | |
| Skin cutaneous melanoma | 0.1 (N = 99) | No data available | |
| Breast invasive carcinoma | 0.7 (N = 1022) | No data available | |
| Head and neck squamous cell carcinoma | 10.5 (N = 492) | No data available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walczak, K.; Krasowska, D. HCAR3 and Kynurenic Acid in Cancer: A Promising Axis of Immunometabolic Regulation or a Scientific Mirage? Int. J. Mol. Sci. 2025, 26, 6269. https://doi.org/10.3390/ijms26136269
Walczak K, Krasowska D. HCAR3 and Kynurenic Acid in Cancer: A Promising Axis of Immunometabolic Regulation or a Scientific Mirage? International Journal of Molecular Sciences. 2025; 26(13):6269. https://doi.org/10.3390/ijms26136269
Chicago/Turabian StyleWalczak, Katarzyna, and Dorota Krasowska. 2025. "HCAR3 and Kynurenic Acid in Cancer: A Promising Axis of Immunometabolic Regulation or a Scientific Mirage?" International Journal of Molecular Sciences 26, no. 13: 6269. https://doi.org/10.3390/ijms26136269
APA StyleWalczak, K., & Krasowska, D. (2025). HCAR3 and Kynurenic Acid in Cancer: A Promising Axis of Immunometabolic Regulation or a Scientific Mirage? International Journal of Molecular Sciences, 26(13), 6269. https://doi.org/10.3390/ijms26136269







